Abstract
This study characterizes Hsp70 induction in human smooth muscle cells (SMC) by herbimycin A and cyclopentenone prostaglandins. The magnitude of Hsp70 induction by cyclopentenone prostaglandins was 8- to 10-fold higher than induction by herbimycin A. Hsp70 induction by Δ12PGJ2 was first observed at 10 μM, rose to 4000–5000 ng/mL within one log unit and a maximum response was not observed; concentrations of Δ12PGJ2 higher than 30 μM were toxic to the cells. A maximum response with herbimycin A (500 ng/mL) was reached at 0.05 μM and maintained to 1 μM without toxicity. Both, Δ12PGJ2 and herbimycin A, were inhibited by dithiothreitol (DTT, 100 μM) at lower concentrations and became less sensitive to inhibition at higher concentrations. Hsp70 induction after incubation of SMC with Δ12PGJ2 followed by addition of herbimycin A was significantly higher than Hsp70 induction after incubation with herbimycin A followed by addition of Δ12PGJ2. When cells were incubated with [3H]-PGJ2, followed by protein denaturation, substantial radioactivity remained protein-bound suggesting that the prostaglandin must be covalently bound. Covalent binding was largely insensitive to DTT. Maximal Hsp70 induction was observed after 5 minutes of exposure of the cells to herbimycin A followed by a 20 hour recovery period in agent-free medium. Cells required 3–4 hours of exposure to Δ12PGJ2 followed by a 20 hour recovery period in order to see high Hsp70 induction. Binding of the heat shock factor (HSF) to the heat shock element (HSE) in the presence of herbimycin A or Δ12PGJ2, and the effects of DTT, mirrored the results of Hsp70 induction. The results suggest that probable differences between the 2 agents are at the level of the signal transduction prior to HSF activation.
INTRODUCTION
Organisms under stress synthesize increased levels of a set of proteins known as stress proteins or heat shock proteins (Hsp). Hsp are classified into families with inducible as well as constitutive members that exhibit similar molecular weights and share similar physiological functions (Lindquist 1986; Hendrick and Hartl 1993; Morimoto and Santoro 1998; Latchman 1999). Hsp play protective roles that help the cell survive the immediate stress and build increased tolerance to further insult. The inducible member of the Hsp70 family, Hsp70, has been studied extensively, and its protective effects have been documented. In addition, members from other Hsp families play overlapping or supportive roles which enhance the stress response (Feige and Polla 1994; Yellon and Marber 1994; Mestril and Dillmann 1995; Benjamin and McMillan 1998). Hsp70 induction occurs after the heat shock transcription factor (HSF) translocates to the nucleus and binds to the heat shock element (HSE), a specific region in the promoter of heat shock inducible genes (Fernandes et al 1994; Morimoto et al 1996).
One major question is whether it is possible to induce Hsp through pharmacological agents or gene therapy without stress to the organism, so that it can be beneficial for the treatment of disease. Several Hsp70 inducers have been reported in the literature. However, most agents induce Hsp70 concomitant with toxicity. On the other hand, herbimicin A and cyclopentenone prostaglandins have been reported to induce Hsp70 and provide protection against insult at nontoxic concentrations (Santoro 1994; Hegde et al 1995).
Herbimycin A is a benzoquinoid ansamycin antibiotic that acts as a tyrosine kinase inhibitor and also induces Hsp70 in various cell lines (Murakami et al 1991). Hsp70 induction by herbimycin A is thought to protect rat cardiomyocytes and myogenic cells against simulated ischemia (Morris et al 1996; Conde et al 1997). HSF activation was observed in herbimycin A-treated primate cells but not in rodent cells, suggesting that herbimycin A could bypass regulatory pathways of stress protein expression (Hegde et al 1995). Mechanistic studies of kinase inhibition suggest that herbimycin A inhibits access of ATP to the kinase by binding irreversibly to reactive sulfhydryl groups in the kinase domain of v-Src and BCR-ABL tyrosine kinases (Uehara et al 1989; Fukazawa et al 1991; Fukazawa et al 1994). Herbimycin A also promotes the degradation of transmembrane tyrosine kinase receptors by the 20S proteasome (Sepp-Lorenzino et al 1995). The functional connection between tyrosine kinase inhibition by herbimycin A and heat shock protein induction has been questioned (Morris et al 1996). However, at present, there are no conclusive studies on this issue.
Cyclopentenone prostaglandins are antiviral agents that induce Hsp70 synthesis in different mammalian cells. The antiviral effect is most likely mediated by Hsp induction (Santoro et al 1980; Santoro 1994). Cyclopentenone prostaglandins induce the expression of heat shock genes through HSF1 activation at noncytotoxic concentrations (Amici et al 1992; Santoro et al 1989). The common structural feature of cyclopentenone prostaglandins is the presence of an α, β- unsaturated carbonyl group in the cyclopentane ring. This structure alone, 2-cyclopenten-1-one, induces Hsp70 synthesis and exhibits antiviral activity with lower potency than cyclopentenone prostaglandin (Rossi et al 1996).
Although herbimycin A and cyclopentenone prostaglandins have been shown to induce Hsp70 and afford different types of protection in various cell lines (Morris et al 1996, Santoro 1996), a direct comparison between the two is not available. This study presents a mechanistic comparison between Hsp70 induction by herbimycin A and cyclopentenone prostaglandins (PGJ2 and Δ12PGJ2) in human smooth muscle cells (SMC).
MATERIALS AND METHODS
Cell culture
Cells were grown in basal medium (Clonetics, Walkersville, MD, USA) containing 5% fetal bovine serum, human endothelial growth factor (0.5 ng/mL), insulin (5 ng/mL), human fibroblast growth factor (2 ng/mL), gentamicin sulfate (50 μg/mL) and amphotericin-B (0.05 μg/mL).
Western analysis
Samples of cell lysates in Laemmli buffer containing similar amounts of protein were subjected to 10% SDS-PAGE and transferred to a nitrocellulose membrane (Novex, Carlsbad, CA, USA). Western analysis was carried out with mouse monoclonal anti-hsp70 (0.1 μg/mL, Stressgen, British Columbia, Canada) as primary antibody and goat anti-mouse immunoglobulin G conjugated to horseradish peroxidase (1:2500-fold dilution; Biosource, Camarillo, CA, USA) as secondary antibody. Renaissance™ (NEN) was used for chemiluminescence detection.
Enzyme-Linked Immunosorbent Assay (ELISA)
Primary human SMC (Clonetics, Walkersville, MD, USA) were plated in 96-well cell culture plates (8000 cells/well). The next day, cells underwent different treatments and were allowed to recover in normal growth medium for 20 hours. Cells were washed with phosphate buffer saline (PBS) and lysed with sodium bicarbonate (pH 9.5, 0.1 M, 200 μL/well). Several dilutions of each lysate were carried through ELISA to ensure absorbance readings in the 0.1–1 range.
Aliquots (100 μL) of each dilution were transferred to ELISA plates (Corning, Corning, NY, USA) and incubated for 2 hours at 37°C to allow for protein binding. Excess lysate was discarded and plates were treated with Superblock (Pierce, New York, NY, USA) according to the manufacturer's instructions. Lysate was incubated with rabbit anti-hsp70 polyclonal antibodies (1: 1000-fold dilution in PBS containing 0.1% bovine serum albumin (BSA), 5% goat serum and 0.05% Tween) for 2 hours at 37°C. The secondary antibody was goat anti-rabbit IgG conjugated to horseradish peroxidase (Biosource, 1:2500 dilution). Color development was carried out by addition of orthophenyl diamine (OPD, Sigma, St Louis, MO, USA) according to manufacturer's instructions. Absorbance measurements were made in an automated plate reader at 490 nm. Hsp70 concentration in the lysate was determined from a standard curve obtained from 5 or 6 different concentrations (0–120 ng/mL range) of Hsp70 (Stressgen) which gave absorbance values in the range of 0 to 1. The standard curve was determined with every ELISA plate and a comparison made with the average of standard curves accumulated in previous experiments to ensure the validity of results.
Order of drug addition
During this experiment only, cells were incubated for 48 hours in serum-free adapting medium before treatment with the reagents. Serum starvation alone did not cause Hsp70 induction; when cells were grown in serum-containing medium, the results were similar but differences were more marked after serum starvation. Serum-containing medium was added after treatment during all experiments involving a recovery period.
Toxicity assay
Cell viability was quantitated by the CellTiter 96®AQueous One Solution Assay (Promega, Madison, WI, USA) according to the manufacturer's instructions. In this assay, metabollically active cells contain dehydogenase enzymes that reduce an oxidized chromophore precursor (MTS), to formazan. The highest absorbance is observed in control cells; cells exposed to toxic agents exhibit lower absorbance due to loss of dehydrogenase activity. Human SMC in 96-well plates were treated with herbimycin A or Δ12PGJ2 at various concentrations in triplicate for 4 hours. Medium was then replaced with 100 μL of agent-free medium plus 20 μL of MTS solution. Plate was incubated for an additional hour and formazan product was detected by absorbance at 490 nm. Control cells had a mean absorbance value of 0.59. Background absorbance value (ie, no cells in the well) was 0.15. Percent toxicity is the absorbance difference between absorbance for control cells and the absorbance for cells incubated with compounds expressed as percent of absorbance of control cells. Toxicity values greater than 20% were considered toxic.
Protein synthesis
Human SMC were incubated with herbimycin A or Δ12-PGJ2 or DTT, or combinations of the 2 agents and DTT. After treatment, cells were washed with PBS and further incubated for 2 hours in medium containing 7.5 μCi/well of 35S methionine and cysteine (specific activity 14.3 μCi/μL). Cells were then washed with PBS, cell lysates prepared, and SDS-PAGE carried out. The level of radioactivity on each lane was quantitated with a phosphorImager after exposure to a phosphor screen. Cycloheximide (50 mg/mL) was used as a positive control.
Preparation of cell extracts and gel shift assay
Rat A10 smooth muscle or human primary SMC were subjected to heat shock (1 hour at 43°C) in a water bath or to different drug treatments for various times with or without recovery time (3 hours at 37°C). Whole cell extracts were prepared as previously described with minor modifications (Mosser et al 1988). Briefly, cells were harvested in cold PBS followed by reconstitution in ice-cold HEPES buffer (20 mM, pH 7.5, 25% glycerol, 50 mM NaCl, 1 mM PMSF, 1 mM DTT, 1.5 mM MgCl2, 0.1 mM EGTA, 0.1 mM EDTA and 5 μg/mL leupeptin) and lysed by gradual addition of NaCl (5 M) to a final concentration of 0.42 M. Incubation of resuspended lysates was carried out at 0°C for 30 minutes with periodic agitation. The lysate was centrifuged (12 000 × g, 15 minutes, 4°C), and the supernatant was aliquoted and frozen at −70°C. Protein concentrations were determined using a protein assay kit (BioRad, Hercules, CA, USA).
HSE, GATCTCGGCTGGAATATTCCCGACCTGGCAGCCGA (Mosser et al 1988), was end-labeled with γ-[32P]-ATP in the presence of T4 Kinase as described by the manufacturer (Gibco/BRL, Rockville, MD, USA). The probe was purified by gel electrophoresis (8% polyacrylamide). The gel was exposed to X-ray film, the band corresponding to the double stranded HSE was excised, and the DNA was eluted from the gel with 300 mM NaCl-TE buffer at 37°C. DNA was precipitated with ice-cold ethanol and resuspended in water (20 μL). Whole cell extracts (10 μg) were mixed with [32P]-HSE (20 fmol, 30 000 dpm/μL) double-stranded synthetic oligonucleotide in binding buffer (10 mM Tris, pH 7.6, 1 mM EDTA, 5 mM DTT, 5% glycerol, 0.5 μg poly dI-dC, 5 ug BSA) to a final volume of 25 μL. Incubation of the mixture was carried out at 25°C for 30 minutes.
Reaction mixtures were run on a 4% polyacrylamide gel in 0.5 X TBE buffer (NOVEX) for 2.5 hours at 200 Volts. Gels were dried, exposed to a phosphor screen (16 hours), and scanned on a PhosphorImager (STORM 840, Molecular Dynamics, Sunnyvale, CA, USA).
Heat shocked cells showed a band, assigned to the HSF-[32P]-HSE complex based on the following: (1) the band disappeared when using 50-fold excess unlabeled HSE over [32P]-HSE; (2) The band was unaffected when using 100-fold excess of unrelated NFκB DNA probe or when using pre-immune serum; and (3) the signal corresponding to the complex disappeared when binding was carried out in the presence of anti-HSF-1. The addition of anti-HSF-1 must prevent complex formation by binding to an HSF domain important for binding to HSE. Untreated cells did not exhibit the band corresponding to the HSF-[32P]- HSE complex.
Protein denaturation
Human SMC were incubated overnight with [3H]-PGJ2 (189 Ci/mmol, Amersham, Buckinghamshire, UK, custom synthesis), washed, harvested by trypsinization, and cell protein was precipitated with ice-cold acetone (0.5 mL). Protein precipitate was then washed 15 times with Acetone/H20 (75/25, 0.5 mL). The radioactivity in each wash and the radioactivity left in the protein precipitate were determined with a scintillation counter.
Statistical analysis
Values are expressed as mean (±) SEM. Analysis of variance (ANOVA), was used to identify significant differences between groups.
RESULTS
Hsp70 induction by herbimycin A and Δ12 PGJ2 in human SMC exhibits different characteristics
Both herbimycin A and Δ12PGJ2 induced Hsp70 in human SMC; however, the magnitude of Hsp70 induction was markedly greater when using the cyclopentenone prostaglandin. In this case, Hsp70 induction was first observed at 10 μM and rapidly rose to 4000–5000 ng/mL levels within less than one log unit (Fig 1). A maximum response was not observed. Hsp70 induction was similar in magnitude and sensitivity of response when using two other cyclopentenone prostaglandins: PGJ2 or 15-deoxy-Δ12,14-PGJ2 (not shown).
Fig. 1.

Hsp70 induction by herbimycin A and the cyclopentenone prostaglandin Δ12PGJ2. Determination of Hsp70 induction by herbimycin A and Δ12PGJ2 by Western Analysis (inset). Human primary SMC were preincubated with various concentrations of herbimycin A or Δ12PGJ2 for 4 hours. Cells were then allowed to recover in drug-free medium for 20 hours. Western analysis was carried as specified in materials and methods using mouse monoclonal anti-hsp70 as a primary antibody and goat anti-mouse immunoglobulin G conjugated to horseradish peroxidase as secondary antibody. Cell lysates from cells treated with prostaglandin were diluted 5-fold. C, control cells; V, DMSO vehicle 0.5%; S, Hsp70 standard (10 ng). Determination of Hsp70 induction by herbimycin A and Δ12PGJ2 by ELISA. Human primary SMC were preincubated with various concentrations of herbimycin A (n = 4) or Δ12PGJ2 (n = 4), for 4 hours in the presence or absence of DTT (100 μM). After preincubation, cells were allowed to recover in drug-free medium for 20 hours. Cell lysates were prepared and Hsp70 levels were determined by ELISA as specified in materials and methods. Error bars are standard error of the mean; they are shown only for the dose response when DTT is absent for clarity. Error bars in the presence of DTT were similar.
When using herbimycin A, Hsp70 induction was observed at concentrations in the range of 0.05–1 μM, but the magnitude of Hsp70 induction was about 8- to 10-fold lower than with Δ12PGJ2: around 500 ng/mL. Contrary to Δ12PGJ2, a maximum response with herbimycin A was reached at a low concentration and maintained over a large concentration range (0.05–1 μM). The results of the ELISA were corroborated by Western analysis (Fig 1 inset).
Different Hsp70 induction levels could indicate different sensitivities of the cells to the 2 agents within the same path of induction or alternatively, different mechanisms of induction.
Either herbimycin A, Δ12PGJ2, or both could be protein alkylators; proteins with nucleophilic residues could react with the quinone in herbimycin A or the cyclopentenone in prostaglandin to form covalent adducts (scheme 1). Herbimycin A has the potential of reacting with protein nucleophiles of certain tyrosine kinases (Fukazawa et al 1991). The α, β- unsaturated carbonyl group in cyclopentenone prostaglandins is required for Hsp70 induction. Further, the cyclopentenone ring alone can induce Hsp70 expression and exhibit antiviral activity (Rossi et al 1996)
Scheme 1
In order to assess the relevance of protein alkylation to Hsp70 induction by herbimycin A and Δ12PGJ2, we studied the effect of DTT on Hsp70 induction by these agents in SMC. Both, Δ12PGJ2 and herbimycin A, were inhibited by DTT (100 μM) at the lower concentrations and became less sensitive to inhibition at the higher concentrations (Fig 1).
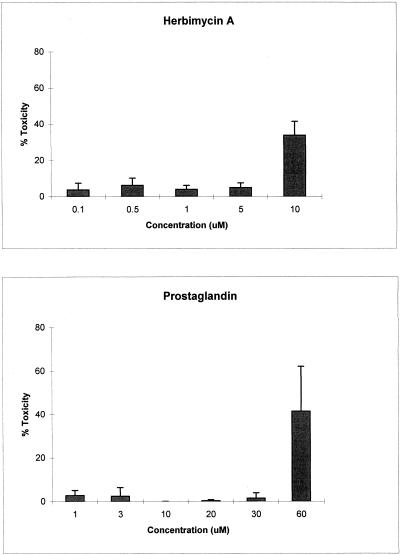
Under the experimental conditions for Hsp70 induction, the prostaglandins were toxic to the cells between 30 and 60 μM whereas herbimycin A was toxic to the cells between 5 and 10 μM (Fig 2).
Fig. 2.
Cytotoxicity. Human primary SMC were incubated with various concentrations of herbimycin A or Δ12-PGJ2 for 4 hours. Drugs were removed and cytotoxicity, measured by MTS (materials and methods), was determined. Triplicate runs for each concentration were carried out in each of 3 independent experiments. Percent toxicity is the average of the results for the 3 experiments; error bars represent the standard error of the mean.
The difference in Hsp70 levels when using the 2 different inducers is not due to inhibition of protein synthesis. Neither of these reagents by itself or in the presence of DTT inhibited protein synthesis (Fig 3).
Fig. 3.
Protein synthesis in SMC after treatment with herbimycin A, prostaglandin, and DTT. Human primary SMC were incubated for 4 hours with various concentrations (μM) of herbimycin A (HA), Δ12-PGJ2 (PG), DTT, and combinations of herbimycin A (1 μM) or Δ12-PGJ2 (30 μM) with DTT (100 μM). Cells were then washed twice with PBS and further incubated for 2 hours in medium containing 35S methionine and cysteine. After incubation with the radiolabeled amino acids, cells were washed with PBS (3 ×), cell lysates prepared and SDS-PAGE carried out. The level of radioactivity on each lane was quantitated with a phosphorImager after 3-day exposure to a phosphoscreen. The radioactivity level obtained with untreated cells was set to 100% and other readings were normalized to this value. U, untreated; D, DMSO vehicle (0.5%); CH, cycloheximide (50 mg/mL) positive control.
In order to explore the possibility that the stress response occurs through different pathways when using herbimycin A and Δ12PGJ2, cells were incubated with the prostaglandin followed by addition of herbimycin A and vice-versa. When cells were first incubated with Δ12PGJ2 followed by the addition of herbimycin A, the level of Hsp70 induction was twice the value of Hsp70 induction obtained when cells were incubated with herbimycin A followed by Δ12PGJ2 (Fig 4). This observation, that the order of addition makes a difference for the level of Hsp70 induction, suggests the presence of 2 pathways of induction.
Fig. 4.

Effect of order of drug addition on Hsp70 induction. Human primary SMC were incubated for 48 hours in serum free adapting medium and then incubated with Δ12PGJ2 (25 μM, 4 hours) followed by addition of herbimycin A (1 μM, last hour) (PG then HA) or with herbimycin A (1 μM, 1 hour) followed by addition of Δ12PGJ2 (25 μM, 4 hours) (HA then PG). Control assays included herbimycin A alone (1 μM, 1 hour) and Δ12PGJ2 alone (25 μM, 4 hours). Times of exposure to the 2 agents were determined based on the results shown in Figure 5. After drug exposure, cells were allowed to recover for 20 hours in serum-containing medium. Hsp70 protein levels were determined by ELISA. Results were normalized to the hsp level when cells were incubated with prostaglandin only. Error bars represent standard error of the mean from 5 independent experiments. * P < 0.001 vs PG then HA
[3H]-PGJ2 binds covalently to protein
Radioactive cyclopentenone prostaglandin ([3H]-PGJ2) was used to assess the extent of covalent binding of prostaglandin to protein. Cells were incubated with [3H]-PGJ2 and proteins were subsequently precipitated and extensively washed with acetone. Protein denaturation should allow release of prostaglandin that is not covalently bound so that any radioactivity associated with protein after denaturation must be covalently bound. Radioactivity bound to protein after denaturation was substantial even in the presence of DTT (Table 1) indicating significant covalent binding largely insensitive to DTT.
Table 1.
Radioactivity bound to protein after protein denaturation
Maximal Hsp70 induction in SMC by herbimycin A and Δ12PGJ2 requires different drug exposure times
Maximal induction of Hsp70 in SMC by Δ12PGJ2 or herbimycin A required strikingly different drug exposure times. Incubation of SMC with Δ12PGJ2 for 5 minutes or 1 hour did not result in significant Hsp70 induction 20 hours post-treatment. Exposure of cells to Δ12PGJ2 for 4 hours, followed by 20 hour incubation in agent-free medium, resulted in maximal levels of induction. Half-maximal induction required 2 hours of Δ12PGJ2 treatment. In contrast, 5 minutes exposure to herbimycin A followed by a 20-hour incubation in agent-free medium was sufficient for maximal induction observed with this agent (Fig 5). One possible interpretation of this result is that a limited amount of herbimycin A could bind to a receptor, alkylate it, and trigger a response in the cells which is independent of additional herbimycin A once the response is initiated. Δ12PGJ2 on the other hand, may need to cross the cell membrane before it can trigger the stress response. This could be a slow process and removal of the prostaglandin, before it reaches an equilibrium point somewhere between 3 and 4 hours, would minimize Hsp70 induction. It is also possible that there is a slow-binding membrane-bound or cytosolic receptor during Δ12PGJ2 mediated Hsp70 induction.
Fig. 5.
Time of drug exposure required for Hsp70 induction. Human primary SMC were incubated for the indicated times with herbimycin A (1 μM, n = 5) or Δ12PGJ2 (20 μM, n = 4). Cells were then allowed to recover in drug-free medium for an additional 20 hours. Levels of Hsp70 in cell lysates were determined by ELISA. Data were normalized to maximal level of Hsp70 observed for each drug in each experiment respectively. Error bars represent standard error of the mean
In the case of herbimycin A, it is possible that it binds and gets into the cells within 5 minutes so that it is present after removal of the medium. This behavior would constitute a significant difference with respect to prostaglandin. In any case, the drug removal procedure included removal of drug-containing medium, cell washing with 200 μL of PBS followed by addition of 200 μL drug-free medium. The concentration of drug left over, if any, would be expected to be well below 10 nM, a concentration at which Hsp70 induction was not observed with either agent.
Binding kinetics of HSF to HSE
In order to determine the time at which HSF binding to HSE begins to occur, herbimycin A or Δ12PGJ2 were incubated for various times and HSF-HSE binding was monitored at the end of each time. Binding began to appear at 15–30 minutes, was prominent at 1 hour, and became maximal at 2–3 hours regardless of the agent used (Fig 6).
Fig. 6.

Kinetics of binding of HSF to [32P]-HSE after cell exposure to herbimycin A or Δ12PGJ2. (a) Rat SMC were heat shocked 1 hour at 43°C (heat shock) or incubated with herbimycin A (1 μM) or Δ12PGJ2 (20 μM) for the times indicated. At the end of each time point, a cell lysate was prepared and a binding experiment with [32P]-HSE carried out. After electrophoresis, results were quantitated with a PhosphorImager (STORM 840). A representative gel shift assay is shown. HSE, 50-fold excess HSE over [32P]-HSE. NFκB, 100-fold excess of NFκB DNA probe. Serum, preimmune rabbit serum. anti-HSF1 ab, antibody to HSF1. (b) Densitometry analysis of the [HSE-HSF] signals from 3 independent experiments. Data were normalized to the most intense signal in each treatment. Error bars represent the standard error of the mean
In additional experiments, when cells were exposed to herbimycin A for 5 minutes and allowed to recover for 3 hours, maximal HSF-HSE binding was observed. In contrast, when cells were exposed to Δ12PGJ2 for 5 minutes, and allowed to recover for 3 hours, HSF-HSE binding was not observed (Fig 7). These results were in agreement with the time of exposure required for Hsp70 induction (Fig 5). Complex was observed with either agent when cells were exposed continuously for 3 hours and cell lysates prepared without a recovery period (Fig 7).
Fig. 7.

Time of cell exposure to drugs required for HSF activation. Rat SMC were heat shocked for 1 hour at 43°C (heat shock) or incubated with herbimycin A (1 μM) or Δ12PGJ2 (20 μM) for 5 minutes and allowed to recover for 3 hours without drug (5’ + 3 h recov.) or 3 hours without recovery (3h). After treatment, cell lysates were prepared and a gel shift assay was carried out as in the experiment illustrated in Figure 6.
DTT effect on HSF binding to [32P]-HSE
HSF binding to HSE when using herbimycin A or Δ12PGJ2 in the presence or absence of DTT (Fig 8) closely correlated to Hsp70 induction (Fig 1). Binding of HSF to HSE was inhibited by DTT when using herbimycin A at 0.1 μM but not at 1 μM. Similarly, HSF binding to HSE was reduced but not abolished by DTT when using Δ12PGJ2 at 20 μM. The inhibition was largely overcome when Δ12PGJ2 was 30 μM (Fig 8). The results indicate that DTT inhibition of Hsp70 induction by herbimycin A, when present, must come as a result of an event prior to HSF activation. This is consistent with DTT preventing protein alkylation, oxidative stress, or both, events that must occur prior to HSF activation.
Fig. 8.

Effect of DTT on HSF binding to [32P]-HSE. (a) Rat SMC were incubated with herbimycin A (0.1 or 1 μM) or prostaglandin Δ12PGJ2 (20 or 30 μM) in the presence (+) or absence (-) of DTT (100 μM), for 3 hours. Cell lysates were then prepared and incubated with [32P]-HSE followed by gel electrophoresis. Binding was quantitated with a PhosphorImager (STORM 840). A representative gel shift assay is shown. Heat Shock, heat shocked cells (1 hour at 43°C). HSE, 50-fold excess HSE over [32P]-HSE. Free, unbound γ-[32P]HSE probe (b) Densitometry analysis of the signals from 3 independent experiments similar to that shown in (a). Data were normalized to the most intense HSF-HSE band. Error bars are the standard error of the mean. ** P < 0.05 vs (-) DTT. * P < 0.05 vs (-) DTT
DISCUSSION
The present work compares Hsp70 induction in SMC by herbimycin A and Δ12PGJ2, a cyclopentenone prostaglandin. The 2 agents most likely induce Hsp70 through protein alkylation since Hsp70 induction can be inhibited by DTT in both cases (Fig 1). Treatments with herbimycin A or prostaglandin were sensitive at the lower concentration and less sensitive at higher drug concentrations. Protein alkylation by prostaglandin was demonstrated by covalent binding between prostaglandin and protein (Table 1). The results are also in agreement with the expected chemical reactivity of the 2 compounds (scheme 1). Inhibition of HSF binding to HSE in the presence of DTT (Fig 8), closely correlated to Hsp70 induction (Fig 1). Further, the kinetics of binding of HSF to HSE were similar for both compounds (Fig 6).
Three major differences were observed: first, the level of Hsp70 induction by Δ12PGJ2 was substantially greater than by herbimycin A (Fig 1). Second, when using both compounds, the order of addition affected the level of Hsp70 induction (Fig 4). And third, maximal Hsp70 induction by the 2 agents required strikingly different drug exposure times (Fig 5).
Taken together, the results suggest that differences in levels of Hsp70 induction could be accounted for during events prior to HSF activation. HSF activation could be the point of convergence for different Hsp inducers.
Hsp70 induction by herbimycin A is low compared to Hsp70 induction by Δ12PGJ2; this could be due to a negative regulator. Morimoto and coworkers recently identified a negative regulator of the heat shock response: a heat shock binding protein 1 (HSBP1). This protein is localized in the nucleus and interacts with active trimeric HSF1 in addition to associating with HSP70 (Satyal et al 1998). In the case of prostaglandin mediated Hsp70 induction, it is conceivable that this protein or a related negative regulator could be alkylated thus preventing the regulation of the heat shock transcriptional response. In this regard, studies of cultured cells with Δ12PGJ2 have shown that the prostaglandin can accumulate in the nucleus where it binds covalently to proteins of chromatin and nuclear matrix (Narumiya et al 1987).
The results obtained when the 2 agents are added in different order could be explained in light of alkylation of a negative regulator by prostaglandin but not by herbimycin A. This is reasonable since alkylation by the 2 agents could have different specificities. If prostaglandin is added first and it alkylates the regulator, regulation would be lost and subsequent addition of herbimycin A would increase Hsp70 induction (Fig 4). If herbimycin A is added first and does not alkylate the regulator, the stress response becomes regulated, the regulator would be less available for alkylation, and increase of Hsp70 induction upon further addition of prostaglandin would be marginal (Fig 4).
A direct signaling mechanism for HSF activation by heat and oxidative stress has been suggested by Wu and coworkers. On the other hand, other inducers of the heat stress response like salicylate, dinitrophenol, ethanol, and arsenite do not directly activate HSF, suggesting that they must act through different mechanisms (Zhong et al 1998). It is not possible from the results of this work to determine if direct or indirect mechanisms are at play during Hsp70 induction by herbimycin A and prostaglandins.
The present work indicates that even though the effect of pharmacological agents is ultimately funneled through HSF activation, there are differences in the signaling pathway that could be more or less desirable to therapeutic treatments where Hsp70 induction is of interest.
REFERENCES
- Amici C, Sistonen L, Santoro MG, Morimoto RI. Antiproliferative prostaglandins activate heat shock transcription factor. Proc Natl Acad Sci U S A. 1992;89:6227–6231. doi: 10.1073/pnas.89.14.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- Conde AG, Lau SS, Dillmann WH, Mestril R. Induction of heat shock proteins by tyrosine kinase inhibitors in rat cardiomyocytes and myogenic cells confers protection against simulated ischemia. J Mol Cell Cardiol. 1997;26:1927–1938. doi: 10.1006/jmcc.1997.0431. [DOI] [PubMed] [Google Scholar]
- Feige U, Polla BS. Hsp70—a multi-gene, multi-structure, muti-function family with potential clinical applications. Experientia. 1994;50:979–986. doi: 10.1007/BF01923452. [DOI] [PubMed] [Google Scholar]
- Fernandes M, O'Brien T, and Lis JT 1994 Structure and Regulation of Heat Shock Gene Promoters. In: The Biology of Heat Shock Proteins and Molecular Chaperones, eds Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY , 375–393. [Google Scholar]
- Fukazawa H, Li PM, Yamamoto C, Murakami Y, Mizuno S, Uehara Y. Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro. Biochem Pharmacol. 1991;42:1661–1671. doi: 10.1016/0006-2952(91)90500-5. [DOI] [PubMed] [Google Scholar]
- Fukazawa H, Uehara Y, Murakami Y, Mizuno S, Hamada M, Takeuchi T. Labeling of v-Src and BCR-ABL tyrosine kinases with [ 14C]herbimycin A and its use in the elucidation of the kinase inactivation mechanism. FEBS Lett. 1994;340:155–158. doi: 10.1016/0014-5793(94)80127-4. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Zuo J, Voellmy R, Welch WJ. Short circuiting stress protein expression via a tyrosine kinase inhibitor, herbimycin A. J Cell Physiol. 1995;165:186–200. doi: 10.1002/jcp.1041650122. [DOI] [PubMed] [Google Scholar]
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Latchman DS 1999 Stress Proteins: An Overview. In: Stress Proteins, ed Latchman DS. Springer, Berlin, 1 –7. [Google Scholar]
- Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mestril R, Dillmann WH. Heat Shock Proteins and Protection against Myocardial Ischemia. J Mol Cell Cardiol. 1995;27:45–52. doi: 10.1016/s0022-2828(08)80006-5. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kroeger PE, Cotto JJ. The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. EXS. 1996:139–163. doi: 10.1007/978-3-0348-9088-5_10. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Morris SD, Cumming DV, Latchman DS, Yellon DM. Specific induction of the 70-kD heat stress proteins by the tyrosine kinase inhibitor herbimycin-A protects rat neonatal cardiomyocytes. A new pharmacological route to stress protein expression? J Clin Invest. 1996;97:706–712. doi: 10.1172/JCI118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Theodorakis NG, Morimoto RI. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988;8:4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Uehara Y, Yamamoto C, Fukazawa H, Mizuno S. Induction of hsp72/73 by herbimycin A, an inhibitor of transformation by tyrosine kinase oncogenes. Exp Cell Res. 1991;195:338–344. doi: 10.1016/0014-4827(91)90382-5. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ohno K, Fukushima M, Fujiwara M. Site and mechanism of growth inhibition by prostaglandins. III. Distribution and binding of prostaglandin A2 and Δ 12-Prostaglandin J2 in Nuclei. J Pharmacol Exp Ther. 1987;242:306–311. [PubMed] [Google Scholar]
- Rossi A, Elia G, Santoro GM. 2-Cyclopenten-1-one, a new inducer of heat shock protein 70 with antiviral activity. J Biol Chem. 1996;271:32192–32196. doi: 10.1074/jbc.271.50.32192. [DOI] [PubMed] [Google Scholar]
- Santoro MG. Heat shock proteins and virus replication: hsp70s as mediators of the antiviral effects of prostaglandins. Experientia. 1994;50:1039–1047. doi: 10.1007/BF01923459. [DOI] [PubMed] [Google Scholar]
- Santoro MG. Viral infection. EXS. 1996:337–357. doi: 10.1007/978-3-0348-9088-5_23. [DOI] [PubMed] [Google Scholar]
- Santoro MG, Benedetto A, Carruba G, Garaci E, Jaffe BM. Prostaglandin A compounds as antiviral agents. Science. 1980;209:1032–1034. doi: 10.1126/science.6157190. [DOI] [PubMed] [Google Scholar]
- Santoro MG, Garaci E, Amici C. Prostaglandins with antiproliferative activity induce the synthesis of a heat shock protein in human cells. Proc Natl Acad Sci U S A. 1989;86:8407–8411. doi: 10.1073/pnas.86.21.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyal SH, Chen D, Fox SG, Kramer JM, Morimoto RI. Negative regulation of the heat shock transcriptional response by HSBP1. Genes Dev. 1998;12:1962–1974. doi: 10.1101/gad.12.13.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp-Lorenzino L, Ma Z, Lebwohl DE, Vinitsky A, Rosen N. Herbimycin A induces the 20 S proteasome- and ubiquitin-dependent degradation of receptor tyrosine kinases. J Biol Chem. 1995;270:16580–16587. doi: 10.1074/jbc.270.28.16580. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Fukazawa H, Murakami Y, Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulhydryl compounds. Biochem Biophys Res Comm. 1989;163:803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Marber MS. Hsp70 in myocardial ischemia. Experientia. 1994;50:1075–1084. doi: 10.1007/BF01923464. [DOI] [PubMed] [Google Scholar]
- Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol Cell. 1998;2:101–108. doi: 10.1016/s1097-2765(00)80118-5. [DOI] [PubMed] [Google Scholar]