Abstract
Rationale
Normal cardiac physiology requires highly regulated cytosolic Ca2+ concentrations and abnormalities in Ca2+ handling are associated with heart failure. The majority of approaches to identify the components that regulate intracellular Ca2+ dynamics rely on cells in culture, mouse models, and human samples. However, a genetically robust system for unbiased screens of mutations that affect Ca2+ handling remains a challenge.
Objective
We sought to develop a new method to measure myocardial Ca2+ cycling in adult Drosophila and determine whether cardiomyopathic fly hearts recapitulate aspects of diseased mammalian myocardium.
Methods and Results
Using engineered transgenic Drosophila that have cardiac-specific expression of Ca2+ sensing fluorescent protein, GCaMP2, we developed methods to measure parameters associated with myocardial Ca2+ handling. The following key observations were identified: (1) Control w1118 Drosophila hearts have readily measureable Ca2+-dependent fluorescent signals that are dependent on L-type Ca2+ channels and SR Ca2+ stores and originate from rostral and caudal pacemakers; (2) A fly mutant, held-up2 (hdp2) that has a point mutation in Troponin I and has a dilated cardiomyopathic phenotype demonstrates abnormalities in myocardial Ca2+ handling that include increases in the duration of the 50% rise in intensity to peak intensity, the half-time of fluorescence decline from peak, the full duration at half maximal intensity (FDHM) and decreases in the linear slope of decay from 80% to 20% intensity decay; and (3) Hearts from hdp2 mutants had reductions in caffeine-induced Ca2+ increases and reductions in ryanodine receptor (RyR) without changes in L-type Ca2+ channel transcripts compared to w1118.
Conclusions
Our results show that the cardiac-specific expression of GCaMP2 provides a means to characterize propagating Ca2+ transients in adult fly hearts. Moreover, the adult fruit fly heart recapitulates several aspects of Ca2+ regulation observed in mammalian myocardium. A mutation in Drosophila that causes an enlarged cardiac chamber and impaired contractile function is associated with abnormalities in the cytosolic Ca2+ transient as well as changes in transcript levels of proteins associated with Ca2+ handling. This new methodology has the potential to permit an examination of evolutionarily conserved myocardial Ca2+ handing mechanisms by applying the vast resources available in the fly genomics community to conduct genetic screens to identify new genes involved in generated Ca2+ transients and arrhythmias.
Keywords: Drosophila heart, myocardial calcium, GCaMP2
Introduction
Cardiomyocytes require highly regulated Ca2+ handling to maintain normal contractile function and intracellular signaling.1, 2 Abnormalities in Ca2+ handling are associated with cardiac hypertrophy, cardiomyopathies, and heart failure.3–6 Moreover, altered Ca2+ regulation can lead to loss of myocytes, dysregulated contractile function, and arrhythmias that lead to sudden cardiac death.1, 7–9
While the major components involved in myocardial Ca2+ regulation are known, the ability to identify new molecules in Ca2+ cycling is limited to the availability of genetic models systems that allow for genetic screens. The fruit fly, Drosophila melanogaster, is an excellent genetic model that has vast resources to facilitate the identification and mapping of new genes that cause disease.10, 11 Previously, we and others have demonstrated that the fly heart can recapitulate aspects associated with mammalian cardiac contractile dysfunction.1, 4, 5, 12–19 Moreover, since many of the genes and signaling pathways are evolutionarily conserved among flies and mammals, studies of the fly cardiac system can provide insight into human heart disease.17, 20–25 For example, receptor tyrosine kinase signaling pathways, Notch pathways, and many transcription factors have been identified in the fly, thus providing insights into how corresponding orthologs function in mammals.13, 19, 23, 26–28 Therefore, we sought to develop a method to measure Ca2+ handling in the adult fly heart. This approach establishes the means to potentially identify new mutants previously not known to be involved in myocardial Ca2+ signaling.
The fly circulatory system consists of a single layer of tinC expressing myocytes arranged as an open linear tube with the main cardiac chamber located directly underneath the dorsal cuticle in the proximal abdominal region.29–31 Additionally, a strap of non-tinC expressing striated muscle cells, referred to as the ventral longitudinal muscle or dorsal diaphragm, is tightly associated with the ventral aspect of the abdominal circulatory system.32 Despite a simple circulatory system, many genes that are critical for cardiac function in the fly are conserved among mammals, including humans. For example, transcription factors such as NKX2.5, structural proteins such as delta-sarcoglycan, dystrophin, myosins, and troponins, and receptor-mediated signaling pathways are required for normal cardiac function in the fly heart.13, 15, 18, 19, 33–40
Based on the concept that genes and pathways necessary for Drosophila heart function are conserved among species, we developed a method to measure Ca2+ handling in the intact adult fly heart. Transgenic flies in a w1118 genetic background that expressed the Ca2+-dependent fluorescent reporter GCaMP2, under the direct control of the 304 base pair cardiac-specific tinC genomic element, allowed us to measure myocardial propagating Ca2+ transients with high fidelity in adult Drosophila. We characterized myocardial Ca2+ transients in control w1118 flies and in a fly mutant, hdp2 that has an enlarged cardiac chamber and impaired contractile function similar to the functional abnormalities observed in mammalian dilated cardiomyopathy.17 Our results demonstrate that the adult fly heart has similar Ca2+ handling properties as compared to mammalian hearts and establish new methods to potentially identify fly mutants that have abnormalities in genes affecting the regulation of myocardial Ca2+.
Methods
Materials
w1118 flies were obtained from Bloomington Stock Center. The hdp2 was provided by Dr. James Vigoreaux. GCaMP2 vector was obtained from Addgene. pCasper5 and pGreenHPelican plasmids were obtained from the Drosophila Genome Resource Center. Rhod2-AM and Fluo-4 Ca2+ sensitive dyes were obtained from Invitrogen. Cytochalasin D and cadmium chloride were purchased from Sigma. Diltiazem, caffeine, thapsigargin, and tetrodotoxin (TTX) were purchased from EMD chemicals. Probes used for qPCR were obtained from Applied Biosystems, Inc.
Transgenic fly engineering
Transgenic flies harboring cardiac-specific GCaMP2 were generated in a w1118 genetic background as described in the Online Data Supplement. To examine myocardial Ca2+ handling in hdp2, the transgenic GCaMP2 line was introduced into the hdp2 line that was previously backcrossed into the w1118 genetic background. Therefore, the differences in fluorescence were attributed to the troponin I mutation since the w1118 genetic background was used for all experiments.
Optical Coherence Tomography
The cardiac chamber sizes in awake, adult Drosophila were measured using optical coherence tomography as previously described.17 Adult female flies were collected between five and seven days after eclosion, briefly anesthetized using carbon dioxide exposure, gently placed on soft gel plates, and allowed to awaken before imaging using a 1310 nm OCT microscopy system (Bioptigen, Inc.). M-modes obtained from transverse oriented B-mode through the A1 segment of the Drosophila heart were used to calculate end-diastolic dimensions (EDD), end-systolic dimension (ESD), and heart rate. Fractional shortening was calculated as (EDD-ESD)/EDD × 100.
Heart dissections
Female flies were collected 48 hours after eclosion for myocardial Ca2+ fluorescence measurements. Hearts were prepared according to previously described methods.41, 42 Detailed methods are described in the Online Data Supplement. The heart remained attached to the dorsal cuticle and was readily identified as a beating structure along the midline from abdominal segments A1 to A4 (Figure 1). Dissected fly hearts remained beating for up to 2 hours at room temperature. Fly heart contractions were stopped by incubation in hemolymph buffer that contained 40 uM cytochalasin-D. Multiple trials to stop fly heart contractions with blebbistatin were unsuccessful. In order to image fly hearts on an inverted microscope, each heart preparation was transferred to a confocal dish containing 100 ul of oxygenated hemolymph buffer. The heart specimen was oriented with the dorsal side up and a glass cover slip was gently applied to avoid movement during imaging.
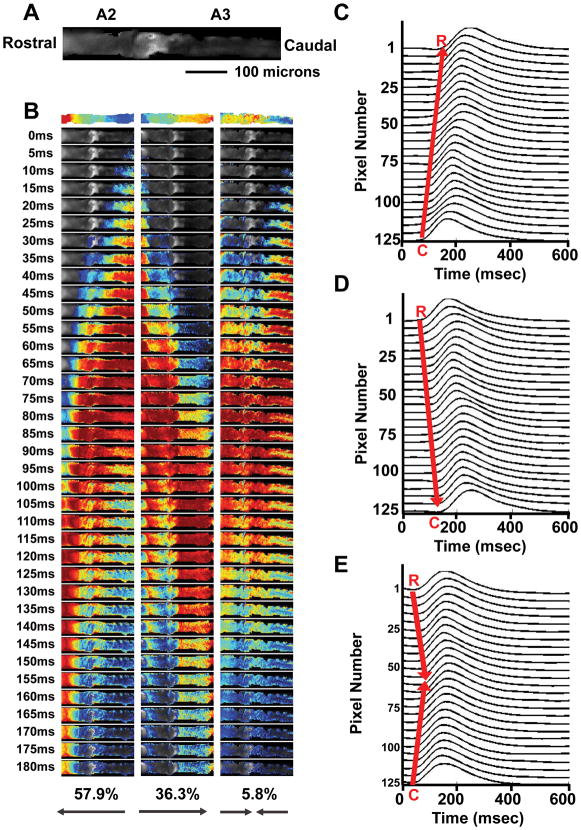
Figure 1. Measurement of Ca2+-dependent fluorescence in adult hearts from w1118; tinC-GCaMP2.
A. Representative bright field image (top) and fluorescence image (bottom) of the heart prepared from an adult w1118; tinC-GCaMP2. The heart preparation includes the dorsal cuticle and is oriented with abdominal segments (A1, A2, A3, and A4) as shown. B. Representative pseuodocolored isochrone map from a w1118; tinC-GCaMP2 heart showing caudal to rostral Ca2+ wave propagation. Blue and red pixels indicate the earliest and latest time points during the recording, respectively. The pseudocolored line represents the pixel intensities averaged along the vertical columns. C. Fluorescence intensity traces for three individual pixels representing the earliest (blue), middle (green), and latest (red) times during the Ca2+ wave propagation and the superimposed traces are shown. A 100 msec time bar is shown.
A Leica 165FC stereomicroscope equipped with an Andor iXon 860 EMCCD camera was used for experiments employing cadmium, EGTA, diltiazem, TTX, caffeine, thapsigargin, and low [Na+]o buffer. Images were analyzed using Andor Solis software (Andor technologies, Inc.).
For experiments to determine the effects of motion artifact caused by cardiac contractions, Ca2+ dependent fluorescence was measured at baseline and after abolishing contraction by treatment with 40 uM cytochalasin-D for 10 minutes at room temperature. Hearts from flies expressing GFP under the direct control of tinC (tinC-GFP) were also prepared in the same manner but without cytochalasin-D treatment.
Fluorescence Ca2+ imaging
Fly hearts were imaged using a Nikon TE2000-U inverted fluorescence microscope equipped with an Andor iXon 860 EMCCD camera at a rate of 200 frames per second. The spatiotemporal analysis of Ca2+ signals was performed using Matlab software as follows. Ca2+ signals were detrended by fitting and subtracting a 2nd-order polynomial and temporally filtered using 12-sample median and mean filters. Non-myocardial pixels inside the recording field-of-view were excluded from further analysis by imposing a threshold on the peak-to-peak range of each pixel’s fluorescence intensity. Ca2+ transient activation times were defined along each upstroke when the pixel intensity reached 50% of its peak. These “50% rise” times were expressed relative to the earliest activation time and used to construct isochrone maps of propagation. In addition, the temporal characteristics of each Ca2+ transient were assessed using dF/dtmax (the maximum slope of the transient’s upstroke), the time between 50% rise and peak, the half-time of fluorescence decline from peak, the full duration half maximum (FDHM, or time between 50% rise and 50% decay), and the slope of the transient’s decay calculated using a linear fit of the transient from 20% to 80% decay. Since the parameters of the Ca2+ transients at the rostral, middle, and caudal regions of the heart were similar and super imposable, all pixels in the recoding over the entire heart were corrected for the time-offset and used to generate an average Ca2+ wave for comparison among groups of recordings. The conduction velocity (CV) of each propagating Ca2+ transient was calculated using a manually-selected rostral and caudal subregion by dividing the distance between their centers-of-mass by the difference between their mean activation times.
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA samples from the dissected hearts from groups of 60 female w1118 or hdp2 flies 48–72 hours after eclosion were prepared and used for quantitative (real time) PCR as described in detail in the Online Data Supplement.
Statistical analyses
Statistical calculations employed t-tests or analyses of variances (ANOVAs) with Bonferroni corrections for multiple comparisons using GraphPad Prism statistical software (GraphPad Software, Inc.).
Results
To measure Ca2+ handling properties in the adult fly heart, we engineered transgenic flies that harbored a non-His-tagged GCaMP2 construct under the control of tinC in a w1118 background. We designated this fly line as w1118; tinC-GCaMP2. GCaMP2 is a circularly permuted enhanced GFP that contains a calmodulin Ca2+ binding domain and the M13 helix of myosin light chain kinase and has been previously used as a Ca2+ sensing fluorescence indicator.43–45 Adult hearts isolated from w1118; tinC-GCaMP2 had robust, cardiac-specific fluorescence (Figure 1 and Movie 1). High-speed imaging (200 fps) of fluorescence signals along the heart tube in abdominal segments A2 and A3 were measured for all subsequent studies. Cytochalasin-D was used during the preparation of heart samples to stop cardiac contraction while maintaining Ca2+-dependent fluorescence. Therefore, the measured fluorescence signals reflected changes in cytosolic Ca2+ and unlikely the result of motion artifact. Heart preparations that were not treated with cytochalasin D demonstrated significant motion artifact during imaging that accounted for at least 50% of the observed changes in fluorescence intensity (Online Figure I). As an additional control, experiments using tinC-GFP fly hearts demonstrated a significant increase in fluorescence intensity during systole (Online Figure I). Of note, we also conducted experiments that employed the application of Ca2+-sensitive dyes but observed poor incorporation of dyes into the fly heart with significant non-specific accumulation in non-cardiac tissue rendering us unable to acquire reproducible Ca2+ transients (data not shown).
We processed the raw image data from recordings of the fluorescence signals from the adult heart preparations using Matlab software to measure changes in pixel intensities. Each cardiac Ca2+ wave was represented by ~5000 pixels per frame for 500 frames of image data corresponding to the abdominal A2 and A3 segments of the fly heart. This region of the heart tube is a single cell layer thick and contains approximately eight cardiomyocytes on each side of the heart.46 Since the level of GCaMP2 that was present in each heart can vary and GCaMP2 is not a ratiometric indicator, we normalized fluorescence intensity of each pixel to the maximum intensity at each pixel over all frames obtained during each recording. The changes in pixel fluorescence intensities in the hearts were analyzed from pseudocolored movies that represented changes at each pixel from 50% rise to 50% decay in normalized intensity (Online Movie II and corresponding isochrone map in figure 1). Additionally, the isochrone pixel intensities for each recoding were averaged along the vertical columns and represented as a line (Figure 1B). The vertical column averaging was performed because this processing served as an additional control for motion artifact due to possible subtle fly heart contraction after cytochalasin-D treatment. Analyses of time-offset adjusted pixel intensities from different areas of the heart were similar and super imposable. We therefore used the average pixel intensity traces for each heart beat to quantify the Ca2+ transient in each heart (Figure 1C).
We examined 714 propagating Ca2+ transients from 32 w1118 flies and show that 57.9% of generated Ca2+ transients were directed anterograde (from the caudal to the rostral direction) while 36.6% were directed retrograde (from the rostral to the caudal direction). Bidirectional generated Ca2+ transients occurred in 5.8% of heart preparations (Figure 2). These findings were consistent with the previously description of rostral and caudal pacemakers in the adult fly heart.31, 47, 48
Figure 2. Determination of Ca2+ propagation in adult hearts from w1118; tinC-GCaMP2.
A. The abdominal A2 and A3 segments from a representative w1118; tinC-GCaMP2 heart in the rostral to caudal orientation is shown. B. Three different representative temporal isochrones with blue and red pixels indicating the earliest and latest times of activation, respectively, are shown (top). Series of consecutive 5 msec frames from temporal isochrone movies are shown. Caudal to rostral (left column), rostral to caudal (middle column), and bidirectional (right column) Ca2+ propagation with the corresponding percentages from 714 measures in 32 w1118; tinC-GCaMP2 hearts are shown. C. Individual pixel intensity traces along the heart from w1118; tinC-GCaMP2 showing caudal to rostral Ca2+ propagation during a 600 msec recording. D. Individual pixel intensity traces along the heart from w1118; tinC-GCaMP2 showing rostral to caudal Ca2+ propagation during a 600 msec recording. E. Individual pixel intensity traces along the heart from w1118; tinC-GCaMP2 showing bidirectional Ca2+ propagation originating from both the rostral and caudal regions during a 600 msec recording.
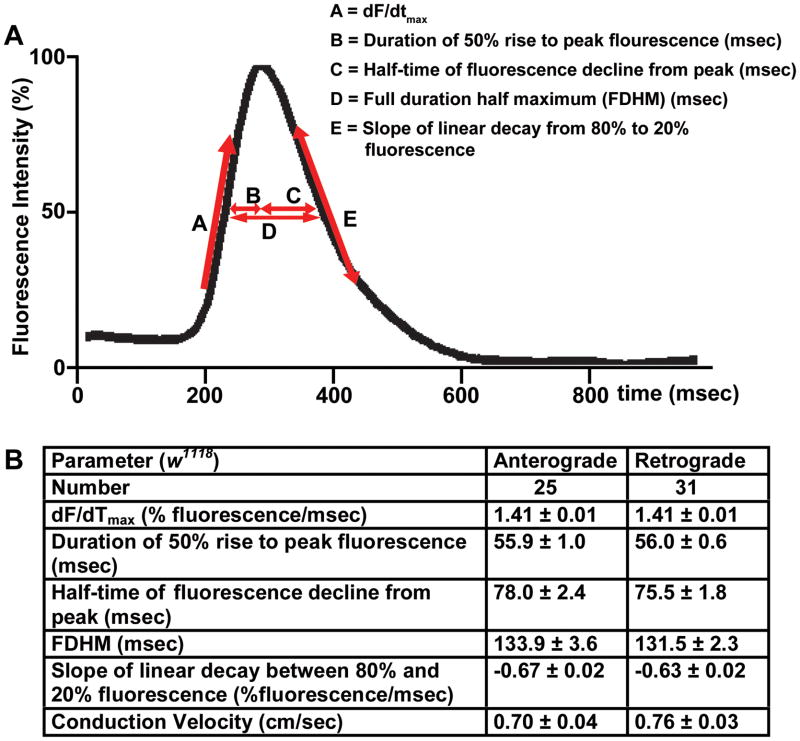
To characterize myocardial Ca2+ handling in w1118 flies we measured parameters associated with the generated Ca2+ transients reflecting changes in the intracellular Ca2+ concentration. These parameters included the maximal slope of increasing fluorescence intensity (dF/dtmax), the duration from the 50% rise in intensity to peak intensity, the half-time of fluorescence decline from peak, the full duration at half maximal intensity (FDHM), and the linear slope from 80% to 20% intensity decay (Figure 3A). Additionally, conduction velocities for propagating Ca2+ transients along the heart tube were determined. Since the majority of generated Ca2+ transients occurred in the anterograde and retrograde directions, the properties of the Ca2+-induced fluorescence intensities and conduction velocities in both groups were compared. All parameters including conduction velocities were similar whether propagation was anterograde or retrograde (p=NS) (Figure 3B). Additionally, the effects of motion on the generated Ca2+ transient parameters were determined by comparing fly hearts treated with cytochalasin-D vs. vehicle alone. The beating fly heart preparations had significant delays the generated Ca2+ transient parameters suggesting that contraction artifact can alter interpretation of fluorescence measurements (Online Figure I).
Figure 3. Comparison of anterograde and retrograde directed Ca2+ transients in adult hearts from w1118; tinC-GCaMP2.
A. Representative trace of averaged pixel intensities from a w1118; tinC-GCaMP2 heart. Definitions of dF/dtmax (A), the duration of the 50% rise to peak fluorescence intensity (B), the duration of the peak to 50% decay in fluorescence intensity (C), FDMH (D), and the slope of linear decay from 80% to 20% fluorescence intensity (E) are shown. B. Comparison between parameters of pixel intensity traces and conduction velocity for anterograde (i.e.; caudal to rostral) (n=25) and retrograde (i.e.; rostral to caudal) (n=31) Ca2+ transients in w1118; tinC-GCaMP2 hearts. There were no statistically significant differences in the parameters measured between anterograde and retrograde Ca2+ wave propagations.
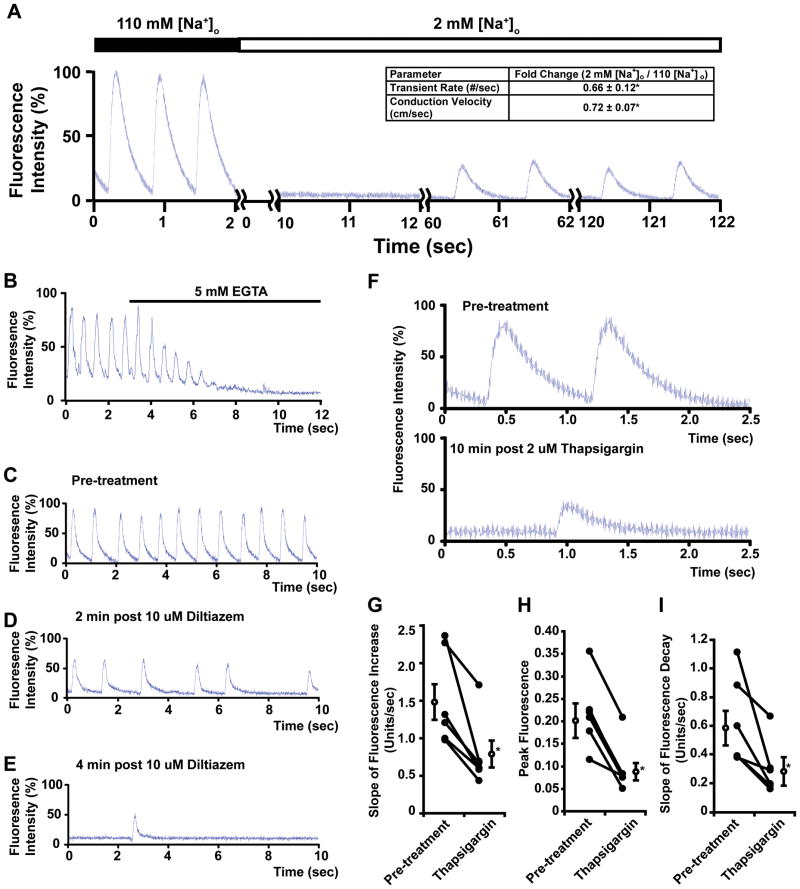
Next, we addressed the ability to manipulate fly myocardial Ca2+ handling using pharmacological interventions to examine the relevance of our model to the mammalian heart. To examine the contribution of voltage-dependent Na+ channels to the generated Ca2+ transients we examined the effects of low [Na+]o buffer, TTX, or cadmium (Cd2+). Low [Na+]o buffer (2mM) caused a complete block of Ca2+ transients followed by a resumed Ca2+ transients suggestive of Ca2+ waves (SR overload-induced spontaneous Ca2+ release) or Na+-independent excitability under experimental conditions (Figure 4A). The resumed propagated Ca2+ transients had slower conduction velocities (0.71 ± 0.05 cm/sec for 110mM [Na+]o buffer vs. 0.49 ± 0.04 cm/sec in 2 mM [Na+]o buffer, p<0.05, n=9). The administration of TTX (100 uM) did not block the propagating Ca2+ transients (data not shown).
Figure 4. Ca2+ transients in adult hearts from w1118; tinC-GCaMP2 are dependent on extracellular Na+, extracellular Ca2+ and intracellular Ca2+ stores.
A. Representative trace of the generated Ca2+ transients in w1118; tinC-GCaMP2 in buffer containing 110 mM [Na+]o and after exposure to 2 mM [Na+]o buffer. Two second segments of the time course are shown. Inset table shows fold changes in transient rate and conduction velocity after exposure to 2 mM [Na+]o. (*p<0.05 by paired t-test, n=9). B. Representative trace of generated Ca2+ transients in w1118; tinC-GCaMP2 before and after the administration of 5 mM EGTA. C–E. Representative serial traces of the generated Ca2+ transients from w1118; tinC-GCaMP2 before and after the administration of 10 uM diltiazem. F. Representative traces of the generated Ca2+ transients from w1118; tinC-GCaMP2 before and after the administration of 2 uM thapsigargin. G–I. Summary data for the effects of thapsigargin on generated Ca2+ transients. The changes in the slopes of fluorescence increases (G), peak fluorescence (H), and slopes of fluorescence decreases (I) are shown. *p<0.05 by paired t-tests for changes in parameters before and after thapsigargin treatment (n=6).
To examine the contributions of extracellular Ca2+ and L-type Ca2+ channels, we performed experiments in the presence of EGTA or diltiazem. The chelation of free extracellular Ca2+ resulted in the complete inhibition of generated Ca2+ transients (Figure 4B). Next, we used diltiazem, an inhibitor of L-type Ca2+ channels.49 The administration of diltiazem resulted in attenuation and subsequent cessation of generated Ca2+ transients (Figure 4C–D). Additionally, the generated Ca2+ transients were completely blocked by 200 uM Cd2+ (data not shown).
We examined the effects of intracellular Ca2+ stores depletion on generated Ca2+ transients by using thapsigargin, an inhibitor of SERCA.50, 51 Fly hearts that were treated with the thapsigargin had reductions in generated Ca2+ transients with decreases in peak fluorescence and the slopes of fluorescence increase and decrease (Figure 4F–I). Lastly, we examined the effects of octopamine, a G-protein coupled receptor (GPCR) agonist, and observed a decrease in the FDHM and a more steep linear slope of intensity decay suggesting that the model responds to agonist stimulation (Online Table I).
Since dilated cardiomyopathies and heart failure have been associated with alterations in myocardial Ca2+ handling, we next tested whether a dilated heart in the fly would recapitulate the known abnormalities in generated Ca2+ transients in mammalian hearts. Previously, we identified a mutant, hdp2 that has a point mutation in Troponin I conserved among multiple species including Drosophila and humans, and has a dilated heart phenotype.17 hdp2; tinC-GCaMP2 flies were generated by genetic crosses. The cardiac-specific expression of GCaMP2 did not significantly affect cardiac function in w1118 or hdp2 as determined by OCT measurements (Online Figure II).
We measured generated Ca2+ transients in hearts from adult, age-matched hdp2; tinC-GCaMP2 and w1118; tinC-GCaMP2 (Figure 5A). Compared to w1118, hdp2 mutant hearts had similar dF/dtmax but significant differences in the duration of the 50% rise in intensity to peak intensity, the half-time of fluorescence decline from peak, FDHM, and the linear slope of decay from 80% to 20% intensity decay (Figure 5B–F). The conduction velocities were not statistically different between w1118 and hdp2 hearts (0.76 ± 0.04 vs. 0.65 ± 0.04 cm/sec, p=NS for w1118 vs. hdp2, respectively). Additionally, the duration of the fluorescent Ca2+ transient was significantly different between w1118 and hdp2 hearts over a range of defined intensities (Online Figure III).
Figure 5. The hdp2; tinC-GCaMP2 mutants have altered myocardial Ca2+ handling compared to w1118; tinC-GCaMP2.
A. Representative average pixel fluorescence intensity traces for w1118; tinC-GCaMP2 (black) and hdp2; tinC-GCaMP2 (red) hearts. B–F. Measurements of dF/dtmax (panel B), the duration of the peak to 50% decay in fluorescence intensity (panel C), the duration of the 50% rise to peak in fluorescence intensity (panel D), FDHM (panel E), and the slope of linear decay from 80% to 20% fluorescence intensity (panel F) in w1118; tinC-GCaMP2 (n=55 recordings from 19 individual preparations) and hdp2; tinC-GCaMP2 (n=81 recordings from 19 individual preparations) hearts. Individual measurements (black circles) and the mean (open circles) with SEM are shown. *p<0.05 for w1118; tinC-GCaMP2 vs. hdp2; tinC-GCaMP2 for the indicated parameter.
Abnormalities in mRNA transcript levels in key components of Ca2+ cycling have been associated with myocardial dysfunction. Therefore, we performed qPCR analyses of several well recognized components involved in intracellular Ca2+ cycling in hearts from w1118 and hdp2 mutants (Figure 6A). Interestingly, hdp2 mutants had significant reductions in ryanodine receptor transcripts compared to w1118. There were no significant differences in SERCA, L-type Ca2+ channel, IP3 Receptor, or sodium-Ca2+ exchanger transcripts between hdp2 and w1118. Additionally, hdp2 hearts had reductions in caffeine-augmented generated Ca2+ transients (Figure 7B). These findings are consistent with the known changes in a variety of vertebrate animal models and humans with dilated cardiomyopathy.6
Figure 6. Hearts from hdp2 mutants have reductions in ryanodine receptor receptor transcript levels compared to w1118.
A. qPCR measurements of transcript expression levels in hearts from hdp2 (open bars) relative to w1118 (closed bars) for L-type Ca2+ channel (LTCC), ryanodine receptor(RYR) sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), Sodium calcium exchanger (NCX) and inositol-3-phosphate receptor(IP3R). n=5 independent experiments with 60 fly hearts per group per experiment. *p<0.05 for hdp2 vs. w1118 by t-test. B. Representative traces of generated Ca2+ transients before and after the administration of 2 mM caffeine in w1118; tinC-GCaMP2 hearts. C. Summary data for the fold changes in the area under the peaks of fluorescence after caffeine administration to w1118; tinC-GCaMP2 vs. hdp2; tinC-GCaMP2 hearts.
Discussion
We developed a new method based on the cardiac expression of GCaMP2, a genetically encoded Ca2+ indicator, to measure myocardial Ca2+ cycling in adult Drosophila. Genetically encoded Ca2+ indicators have been used to examine cardiac Ca2+ handling in other models including the mouse and zebrafish.52–56 However, the genetic resources to map and rapidly identify mutants that affect myocardial Ca2+ handling in these genetic models can be quite laborious. The method that we describe has a number of significant advantages. First and foremost, Drosophila genetics and genomics offers unique resources for gene discovery that are not available in other model systems. These genetic resources facilitate the mapping and identification of gene mutations and have been used to identify a variety of genes that affect different aspects of Drosophila cardiac contractile function.13, 19 Flies that have molecularly-defined genomic deficiencies, P-element insertions, or harbor RNAi for specific gene knockdown can be introduced into the tinC-GCaMP2 background to investigate how individual genes potentially affect myocardial Ca2+ handling. Alternative approaches that rely on applying Ca2+ indicator dyes can lead to non-specific, extra-cardiac tissue distribution. For example, dye incorporation into the non-tinC expressing striated ventral longitudinal muscle can prevent adequate labeling of the fly heart and lead to diminished specificity during cardiac Ca2+ measurements. Our method has the advantage of genetically targeted cardiac-specific Ca2+ measurements that circumvent this potential problem.
The circulatory system in flies is simple compared to mammals, however the generated Ca2+ transients in adult Drosophila hearts share several characteristics with mammalian myocardial Ca2+ handling. First, the Ca2+-dependent fluorescence wave in tinC-GCaMP2 hearts moves along the heart tube in an organized manner. However, unlike mammalian hearts, the fly heart has two pacemakers that control anterograde and retrograde contraction.31, 47, 48 Second, the generated Ca2+ transients in fly hearts are dependent on extracellular Ca2+ via L-type Ca2+ channels and intracellular calcium stores. Fourth, the generated Ca2+ transients appear to require sodium channels since low [Na+]o causes a transient cessation of Ca2+ transients. Fifth, Ca2+ transient parameters undergo changes after stimulation with octopamine, an agonist of the octopamine GPCR. Sixth, the fly heart appears to have altered Ca2+ handling in the context of the hdp2 mutant. The hdp2 mutant has a well characterized deterioration of the indirect flight muscles, progressive myopathy of other muscle groups, and a dilated cardiomyopathic phenotype.17, 57, 58 Compared to w1118, hearts from mutant hdp2 have a prolonged duration of 50% rise to peak intensity, but no significant changes in dF/dtmax. This suggests that while the early rise in cytosolic Ca2+ concentration is similar, the late rise in cytosolic Ca2+ is delayed. Furthermore, in hdp2 hearts, the half-time of fluorescence decline from peak is prolonged and the linear slope from 80% to 20% of fluorescence decay is decreased. These findings suggest that Ca2+-reuptake into the SR and/or Ca2+ extrusion into the extracellular space are impaired in the hearts from hdp2 compared to w1118.
The mechanisms that are responsible for the observed changes in cytosolic Ca2+ cycling and contractile dysfunction in hdp2 hearts remain to be identified. Hearts from hdp2 mutants had reductions in RyR and IP3R transcript levels consistent with the prolonged duration of 50% rise to peak intensity that we observed in the GCaMP2 flies. Additionally, hdp2 hearts had reductions in caffeine- augmented increases in the generated Ca2+ transients indicating a reduced SR Ca2+ load. These results are consistent with some previous studies in mammalian models of heart failure.6 However, the transcript levels for SERCA were not significantly different between w1118 and hdp2 although the duration of peak intensity to 50 % decay is prolonged and the linear slope from 80% to 20% of fluorescence decay is decreased in hdp2 hearts. A possible explanation for these findings is that alterations of Ca2+-reuptake in hdp2 hearts may result from changes in the post-transcriptional level of SERCA or alterations in proteins that control SERCA function.
Alternatively, the troponin I mutation in hdp2 flies may change the structure and Ca2+ binding properties of contractile protein complexes thereby altering the buffering capacity of these complexes for cytosolic Ca2+. Recent studies using preparations of indirect flight muscle from Drosophila have demonstrated that the site of the hdp2 troponin I mutation decreases the concentration of Ca2+ necessary for thin filament activation.57–59 Furthermore, based on comparisons of the conserved site of the mutation in troponin I in hdp2 flies and mammals, studies suggest that the change in Ca2+ binding to the troponin I/troponin C complex is an indirect effect of the hdp2 mutation.58 Thus, changes in the interaction between contractile proteins and Ca2+ may also explain some of the changes in Ca2+ transients in hdp2 mutants.
There are limitations to our approach to measuring myocardial Ca2+ handling in adult flies. First, GCaMP2 is a nonratiometric Ca2+ indicator, similar to several nonratiometric Ca2+ indicator dyes.55, 60, 61 Additionally, the amount of GCaMP2 is variable in each fly heart. Therefore, the absolute changes in cytosolic Ca2+ concentration cannot be determined and relative changes must be used for comparison among experiments. Second, the fluorescence intensity of GCaMP2 is lower than fluorescence dyes since the intensities of the fluorescence dyes depend on the loading conditions.45, 55, 56 Thus, GCaMP2 expression in the fly heart is not sufficient enough to permit imaging through the cuticle of the live insect. Therefore, the tissue surrounding the heart must be partially dissected away prior to imaging. The generation of newer genetic indicators with improved signal-to-noise may circumvent the inability to directly measure Ca2+-dependent fluorescence intensity through the cuticle. Third, although GCaMP2 and Ca2+ indicator dyes are nonratiometric, GCaMP2 has different affinities and kinetics regarding Ca2+ binding and unbinding compared to chemical dyes.45, 55, 56 The response of GCaMP2 to Ca2+ binding and unbinding is slower than chemical dyes and therefore will tend to prolong the Ca2+ duration. Fourth, the fly heart must be electromechanically dissociated prior to imaging to minimize changing optical properties in the tissue that occurs during contraction. In fact, in the beating fly heart approximately 50% of the change in fluorescence intensity is the result of motion. Additionally, the parameters associated with the generated Ca2+ transients from fly hearts that were electromechanically dissociated are significantly different from hearts that were contracting. Therefore, measurements of myocardial Ca2+ in our fly model require pre-treatment with cytochalasin-D to uncouple true Ca2+-dependent fluorescence from motion artifact. Unfortunately, blebbistatin did not stop fly heart contractions and therefore the use of cytochalasin-D introduces another potential limitation since cytochalasin-D is known to affect intracellular Ca2+.62
Lastly, our studies suggest that the propagating Ca2+ transient is mediated by electrical action potential propagation. The generated Ca2+ transients in fly hearts are transiently blocked by low extracellular Na+ buffer suggesting that sodium-dependent actions potentials are necessary for the observed Ca2+ transients. The generated Ca2+ transients are also sensitive to Cd2+ consistent with a dependence on voltage-sensitive Na+ channels similar to that observed in mammalian hearts.63–65 However, the dose of Cd2+ required to block fly heart Ca2+ transients is in a range that can also inhibit L-type Ca2+ channels. The effects of TTX were modest and suggest that the fly heart may not be sensitive to TTX. Therefore, more definitive studies employing voltage-sensitive dyes will be necessary to understand the contribution of the electrical action potential to the Ca2+ transient in the fly heart.
Overall, our results demonstrate the potential utility of Drosophila as a model of myocardial Ca2+ handling. Future applications including an examination of fly mutants using this methodology have the potential to identify and characterize new genes that regulate intracardiac Ca2+ signaling and may lead to new insights into human cardiovascular diseases.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is known?
The fruit fly, Drosophila melanogaster, is recognized as a model of mammalian cardiovascular disease and can recapitulate aspects of mammalian cardiomyopathies.
Abnormalities in myocardial calcium handling are observed in mammalian models of heart failure; however, the myocardial calcium handling in Drosophila has not been well-characterized.
What new information does this article contribute?
A new transgenic fruit fly harboring a cardiac-specific, reversible calcium-dependent fluorescent protein reporter, GCaMP2, was engineered and myocardial calcium transients were investigated using high speed fluorescence imaging.
The propagating calcium transients in adult fly hearts originate from two different pacemakers and are dependent on extracellular calcium via L-type-calcium channels and intracellular calcium stores.
The study results suggest that components that underlie myocardial calcium transients are evolutionarily conserved between Drosophila and mammals and the fruit fly may provide new insights into genes that potentially regulate calcium handing in mammalian hearts.
The study describes a new method to measure calcium transients in the adult fruit fly heart using flies that express a cardiac-specific, reversible calcium-dependent fluorescent protein reporter and a high-speed imaging system. By applying this technique, the propagating calcium transients were measured in adult fly hearts from controls and mutants that have dilated cardiomyopathic phenotypes. Drosophila myocardial calcium transients propagate in two directions and are similar to mammalian myocardial calcium transients in that they require extracellular sodium, extracellular calcium via L-type calcium channels, and intracellular calcium stores. These findings further support the fruit fly as a model of cardiovascular disease. Furthermore, this method suggests that the fly can serve as a genetically tractable system for identifying genes that affect myocardial calcium handling in mammals.
Acknowledgments
Sources of Funding: This work was supported by the NIH through a K08HL085072 to M.J.W. and HL-083065 to H.A.R.; the American Heart Association through a NCRP Innovative Research Grant (0970391N) to M.J.W., and a Robert J. Lefkowitz Innovation Research Award to M.J.W.
Non-standard abbreviations and acronyms
- CV
conduction velocity
- dF/dTmax
slope of maximum fluorescence intensity increase
- EDD
end-diastolic dimension
- EMCCD
electron-multiplying charge-coupled device
- ESD
end-systolic dimension
- FDHM
full duration half maximum
- FS
fractional shortening
- GCaMP2
circularly permutated enhanced green fluorescent protein fused with M13 helix of myosin light chain at the N-terminus and calmodulin at the C-terminus
- GPCR
G-protein coupled receptor
- hdp2
held-up2 mutant fly
- IP3
inositol-3-phosphate
- LTCC
L-type calcium channel
- NCX
sodium-calcium exchanger
- OCT
optical coherence tomography
- RyR
Ryanodine receptor
- SERCA
sarcoendoplasmic reticulum calcium ATPase
- SR
sarcoplasmic reticulum
- tinC
DNA regulatory region of the Drosophila tin gene
- tinC-GCaMP2
transgenic fly harboring GCaMP2 under the control of the tinC DNA element
- TTX
tetrodotoxin
- W1118
laboratory stock fly
Footnotes
Disclosures: None
References
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann N Y Acad Sci. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Altered cardiac myocyte ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 4.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 5.Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell Biochem. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- 6.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 7.Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, Brown JH, Molkentin JD, Kranias EG, Dorn GW., 2nd Endoplasmic reticulum-mitochondria crosstalk in nix-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitsis RN, Molkentin JD. Apoptotic cell death “nixed” by an er-mitochondrial necrotic pathway. Proc Natl Acad Sci U S A. 2010;107:9031–9032. doi: 10.1073/pnas.1003827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: Mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 10.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 11.St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet. 2002;3:176–188. doi: 10.1038/nrg751. [DOI] [PubMed] [Google Scholar]
- 12.Houser SR, Piacentino V, 3rd, Mattiello J, Weisser J, Gaughan JP. Functional properties of failing human ventricular myocytes. Trends Cardiovasc Med. 2000;10:101–107. doi: 10.1016/s1050-1738(00)00057-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim IM, Wolf MJ, Rockman HA. Gene deletion screen for cardiomyopathy in adult drosophila identifies a new notch ligand. Circ Res. 2010;106:1233–1243. doi: 10.1161/CIRCRESAHA.109.213785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW, Bodmer R. Kcnq potassium channel mutations cause cardiac arrhythmias in drosophila that mimic the effects of aging. Proc Natl Acad Sci U S A. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taghli-Lamallem O, Akasaka T, Hogg G, Nudel U, Yaffe D, Chamberlain JS, Ocorr K, Bodmer R. Dystrophin deficiency in drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell. 2008;7:237–249. doi: 10.1111/j.1474-9726.2008.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 17.Wolf MJ, Amrein H, Izatt JA, Choma MA, Reedy MC, Rockman HA. Drosophila as a model for the identification of genes causing adult human heart disease. Proc Natl Acad Sci U S A. 2006;103:1394–1399. doi: 10.1073/pnas.0507359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf MJ, Rockman HA. Drosophila melanogaster as a model system for genetics of postnatal cardiac function. Drug Discov Today Dis Models. 2008;5:117–123. doi: 10.1016/j.ddmod.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L, Lee T, Lin N, Wolf MJ. Affecting rhomboid-3 function causes a dilated heart in adult drosophila. PLoS Genet. 2010;6:e1000969. doi: 10.1371/journal.pgen.1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. doi: 10.1016/j.gene.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Mann T, Bodmer R, Pandur P. The drosophila homolog of vertebrate islet1 is a key component in early cardiogenesis. Development. 2009;136:317–326. doi: 10.1242/dev.022533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neely GG, Kuba K, Cammarato A, Isobe K, Amann S, Zhang L, Murata M, Elmen L, Gupta V, Arora S, Sarangi R, Dan D, Fujisawa S, Usami T, Xia CP, Keene AC, Alayari NN, Yamakawa H, Elling U, Berger C, Novatchkova M, Koglgruber R, Fukuda K, Nishina H, Isobe M, Pospisilik JA, Imai Y, Pfeufer A, Hicks AA, Pramstaller PP, Subramaniam S, Kimura A, Ocorr K, Bodmer R, Penninger JM. A global in vivo drosophila rnai screen identifies not3 as a conserved regulator of heart function. Cell. 2010;141:142–153. doi: 10.1016/j.cell.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles W, Ho SY, Benson DW, Silberbach M, Shou W, Chien KR. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 24.Perrin L, Monier B, Ponzielli R, Astier M, Semeriva M. Drosophila cardiac tube organogenesis requires multiple phases of hox activity. Dev Biol. 2004;272:419–431. doi: 10.1016/j.ydbio.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Zeitouni B, Senatore S, Severac D, Aknin C, Semeriva M, Perrin L. Signalling pathways involved in adult heart formation revealed by gene expression profiling in drosophila. PLoS Genet. 2007;3:1907–1921. doi: 10.1371/journal.pgen.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian L, Bodmer R. Partial loss of gata factor pannier impairs adult heart function in drosophila. Hum Mol Genet. 2009;18:3153–3163. doi: 10.1093/hmg/ddp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian L, Liu J, Bodmer R. Neuromancer tbx20-related genes (h15/midline) promote cell fate specification and morphogenesis of the drosophila heart. Dev Biol. 2005;279:509–524. doi: 10.1016/j.ydbio.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Zaffran S, Reim I, Qian L, Lo PC, Bodmer R, Frasch M. Cardioblast-intrinsic tinman activity controls proper diversification and differentiation of myocardial cells in drosophila. Development. 2006;133:4073–4083. doi: 10.1242/dev.02586. [DOI] [PubMed] [Google Scholar]
- 29.Bodmer R, Venkatesh TV. Heart development in drosophila and vertebrates: Conservation of molecular mechanisms. Dev Genet. 1998;22:181–186. doi: 10.1002/(SICI)1520-6408(1998)22:3<181::AID-DVG1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Monier B, Astier M, Semeriva M, Perrin L. Steroid-dependent modification of hox function drives myocyte reprogramming in the drosophila heart. Development. 2005;132:5283–5293. doi: 10.1242/dev.02091. [DOI] [PubMed] [Google Scholar]
- 31.Wasserthal LT. Drosophila flies combine periodic heartbeat reversal with a circulation in the anterior body mediated by a newly discovered anterior pair of ostial valves and ‘venous’ channels. J Exp Biol. 2007;210:3707–3719. doi: 10.1242/jeb.007864. [DOI] [PubMed] [Google Scholar]
- 32.Curtis NJ, Ringo JM, Dowse HB. Morphology of the pupal heart, adult heart, and associated tissues in the fruit fly, drosophila melanogaster. J Morphol. 1999;240:225–235. doi: 10.1002/(SICI)1097-4687(199906)240:3<225::AID-JMOR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Kim IM, Wolf MJ. Serial examination of an inducible and reversible dilated cardiomyopathy in individual adult drosophila. PLoS One. 2009;4:e7132. doi: 10.1371/journal.pone.0007132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal SM, Qian L, Lacin H, Bodmer R, Skeath JB. Neuromancer1 and neuromancer2 regulate cell fate specification in the developing embryonic cns of drosophila melanogaster. Dev Biol. 2009;325:138–150. doi: 10.1016/j.ydbio.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Qian L, Wessells RJ, Bidet Y, Jagla K, Bodmer R. Hedgehog and ras pathways cooperate in the anterior-posterior specification and positioning of cardiac progenitor cells. Dev Biol. 2006;290:373–385. doi: 10.1016/j.ydbio.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Mery A, Taghli-Lamallem O, Clark KA, Beckerle MC, Wu X, Ocorr K, Bodmer R. The drosophila muscle lim protein, mlp84b, is essential for cardiac function. J Exp Biol. 2008;211:15–23. doi: 10.1242/jeb.012435. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen HT, Bodmer R, Abmayr SM, McDermott JC, Spoerel NA. D-mef2: A drosophila mesoderm-specific mads box-containing gene with a biphasic expression profile during embryogenesis. Proc Natl Acad Sci U S A. 1994;91:7520–7524. doi: 10.1073/pnas.91.16.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian L, Mohapatra B, Akasaka T, Liu J, Ocorr K, Towbin JA, Bodmer R. Transcription factor neuromancer/tbx20 is required for cardiac function in drosophila with implications for human heart disease. Proc Natl Acad Sci U S A. 2008;105:19833–19838. doi: 10.1073/pnas.0808705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin Z, Frasch M. Regulation and function of tinman during dorsal mesoderm induction and heart specification in drosophila. Dev Genet. 1998;22:187–200. doi: 10.1002/(SICI)1520-6408(1998)22:3<187::AID-DVG2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Yin Z, Xu XL, Frasch M. Regulation of the twist target gene tinman by modular cis-regulatory elements during early mesoderm development. Development. 1997;124:4971–4982. doi: 10.1242/dev.124.24.4971. [DOI] [PubMed] [Google Scholar]
- 41.Alayari NN, Vogler G, Taghli-Lamallem O, Ocorr K, Bodmer R, Cammarato A. Fluorescent labeling of drosophila heart structures. J Vis Exp. 2009 doi: 10.3791/1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ocorr K, Fink M, Cammarato A, Bernstein S, Bodmer R. Semi-automated optical heartbeat analysis of small hearts. J Vis Exp. 2009 doi: 10.3791/1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akerboom J, Rivera JD, Guilbe MM, Malave EC, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER. Crystal structures of the gcamp calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009;284:6455–6464. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakai J, Ohkura M. Probing calcium ions with biosensors. Biotechnol Genet Eng Rev. 2003;20:3–21. doi: 10.1080/02648725.2003.10648035. [DOI] [PubMed] [Google Scholar]
- 45.Nakai J, Ohkura M, Imoto K. A high signal-to-noise ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 46.Molina MR, Cripps RM. Ostia, the inflow tracts of the drosophila heart, develop from a genetically distinct subset of cardial cells. Mech Dev. 2001;109:51–59. doi: 10.1016/s0925-4773(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 47.Dulcis D, Levine RB. Innervation of the heart of the adult fruit fly, drosophila melanogaster. J Comp Neurol. 2003;465:560–578. doi: 10.1002/cne.10869. [DOI] [PubMed] [Google Scholar]
- 48.Dulcis D, Levine RB. Glutamatergic innervation of the heart initiates retrograde contractions in adult drosophila melanogaster. J Neurosci. 2005;25:271–280. doi: 10.1523/JNEUROSCI.2906-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KS, Tsien RW. Mechanism of calcium channel blockade by verapamil, d600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983;302:790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- 50.Kirby MS, Sagara Y, Gaa S, Inesi G, Lederer WJ, Rogers TB. Thapsigargin inhibits contraction and ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum ca2+ pump. J Biol Chem. 1992;267:12545–12551. [PubMed] [Google Scholar]
- 51.Rogers TB, Inesi G, Wade R, Lederer WJ. Use of thapsigargin to study ca2+ homeostasis in cardiac cells. Biosci Rep. 1995;15:341–349. doi: 10.1007/BF01788366. [DOI] [PubMed] [Google Scholar]
- 52.Adam SA, Adam EJ, Chi NC, Visser GD. Cytoplasmic factors in nls-mediated targeting to the nuclear pore complex. Cold Spring Harb Symp Quant Biol. 1995;60:687–694. doi: 10.1101/sqb.1995.060.01.074. [DOI] [PubMed] [Google Scholar]
- 53.Chi NC, Bussen M, Brand-Arzamendi K, Ding C, Olgin JE, Shaw RM, Martin GR, Stainier DY. Cardiac conduction is required to preserve cardiac chamber morphology. Proc Natl Acad Sci U S A. 2010;107:14662–14667. doi: 10.1073/pnas.0909432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008;6:e109. doi: 10.1371/journal.pbio.0060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotlikoff MI. Genetically encoded ca2+ indicators: Using genetics and molecular design to understand complex physiology. J Physiol. 2007;578:55–67. doi: 10.1113/jphysiol.2006.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal ca2+ indicator gcamp2. Proc Natl Acad Sci U S A. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nongthomba U, Clark S, Cummins M, Ansari M, Stark M, Sparrow JC. Troponin i is required for myofibrillogenesis and sarcomere formation in drosophila flight muscle. J Cell Sci. 2004;117:1795–1805. doi: 10.1242/jcs.01024. [DOI] [PubMed] [Google Scholar]
- 58.Vikhorev PG, Vikhoreva NN, Cammarato A, Sparrow JC. In vitro motility of native thin filaments from drosophila indirect flight muscles reveals that the held-up 2 tni mutation affects calcium activation. J Muscle Res Cell Motil. 2010;31:171–179. doi: 10.1007/s10974-010-9221-x. [DOI] [PubMed] [Google Scholar]
- 59.Cammarato A, Hatch V, Saide J, Craig R, Sparrow JC, Tobacman LS, Lehman W. Drosophila muscle regulation characterized by electron microscopy and three-dimensional reconstruction of thin filament mutants. Biophys J. 2004;86:1618–1624. doi: 10.1016/S0006-3495(04)74229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grynkiewicz G, Poenie M, Tsien RY. A new generation of ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 61.Robinson JA, Jenkins NS, Holman NA, Roberts-Thomson SJ, Monteith GR. Ratiometric and nonratiometric ca2+ indicators for the assessment of intracellular free ca2+ in a breast cancer cell line using a fluorescence microplate reader. J Biochem Biophys Methods. 2004;58:227–237. doi: 10.1016/j.jbbm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Baeker TR, Simons ER, Rothstein TL. Cytochalasin induces an increase in cytosolic free calcium in murine b lymphocytes. J Immunol. 1987;138:2691–2697. [PubMed] [Google Scholar]
- 63.Backx PH, Yue DT, Lawrence JH, Marban E, Tomaselli GF. Molecular localization of an ion-binding site within the pore of mammalian sodium channels. Science. 1992;257:248–251. doi: 10.1126/science.1321496. [DOI] [PubMed] [Google Scholar]
- 64.Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 65.Satin J, Kyle JW, Chen M, Bell P, Cribbs LL, Fozzard HA, Rogart RB. A mutant of ttx-resistant cardiac sodium channels with ttx-sensitive properties. Science. 1992;256:1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.