Summary
Subliminal visual stimuli affect motor planning [1] but the size of such effects differs greatly between individuals [2, 3]. Here we investigated whether such variation may be related to neurochemical differences between people. Cortical responsiveness is expected to be lower under the influence of more of the main inhibitory neurotransmitter, GABA [4]. Thus we hypothesized that if an individual has more GABA in the supplementary motor area (SMA) – a region previously associated with automatic motor control [5] – this would result in smaller subliminal effects. We measured the reversed masked prime – or negative compatibility – effect, and found that it correlated strongly with GABA concentration, measured with magnetic resonance spectroscopy. This occurred specifically in the SMA region and not in other regions from which spectroscopy measurements were taken. We replicated these results in an independent cohort: more GABA in the SMA region is reliably associated with smaller effect size. These findings suggest that, across individuals, the responsiveness of subconscious motor mechanisms is related to GABA concentration in the SMA.
Results and discussion
Differences between people have long been studied at the level of personality or intelligence, but people also differ in much more basic neural processes, even ones that are subconscious and automatic [e.g. 2, Fig. 4, 3, supplementary Fig. S3]. In the study of such low-level phenomena, behaviour across a group of individuals is usually averaged and differences are treated as unwanted noise (random variation). However, this approach overlooks the inescapable fact that a component of the measured differences may reflect stable individual traits, no matter how low-level the mechanism (supplementary Fig. S1). Such basic traits may also hold essential clues into how individual differences translate into mental disorders. To our knowledge, no explanation has ever been provided for individual differences in automatic mechanisms operating at the threshold of conscious awareness, even though they potentially offer a cleaner index than measures of conscious behaviour. Recent advances in magnetic resonance spectroscopy (MRS) allow us to ask whether these traits might be predicted by differences in neurotransmitter concentration in specific brain regions [6-13].
We studied reversed masked priming using a standard paradigm [14, 15]. Participants must respond to arrows pointing left or right by pressing different buttons, and each target arrow is preceded by a very brief and backward-masked prime arrow (Fig. 1a). Prior to the main priming procedure, the primes were set to be below the threshold of conscious perception determined for each participant using a staircase discrimination task. Nevertheless, these prime stimuli influenced responses to the target arrows.
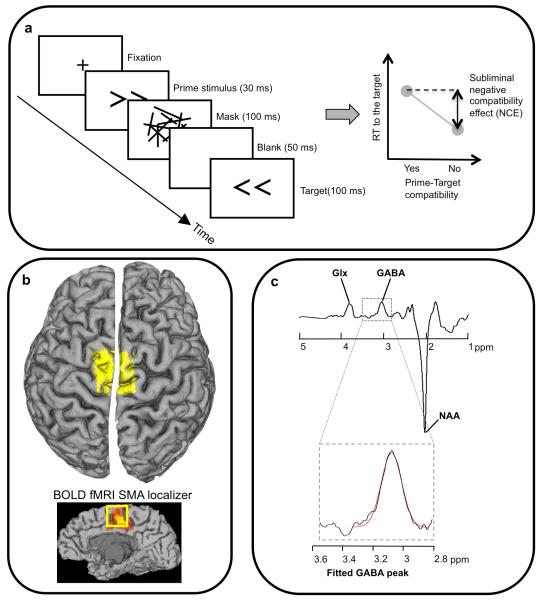
Figure 1. Methodology for masked priming and GABA spectroscopy.
a, target arrows were preceded by masked primes presented below the threshold for conscious discrimination. For the stimulus timing illustrated, responses tend to be slower when prime and target are the same (compatible) than when they are not (right hand illustration). This is the measure of subliminal suppression, and the magnitude differs between individuals. b, the MRS voxel (yellow, (3 cm)3 voxel) was placed over the anatomical location of SMA. As a check on voxel placement, for two participants we acquired a functional localiser for the SMA using fMRI (see Methods and bottom sagital view). Edited MR spectra (c) allow the quantification of GABA concentration by extracting the area under the GABA peak [6, 8, 9, 48] (glutamine/ glutamate, Glx, and N-acetyl-aspartate, NAA, peaks are also marked). The peak will also contain co-edited macromolecules. See Methods and supplementary fig S2 for more details and individual spectra.
When the time between prime and target was very short, responses were facilitated by primes pointing in the same direction as targets (mean 21 ms; p<.017). This is known as the positive compatibility effect (PCE), and is taken as an index of subliminal activation by the prime [1]. However, when the target was presented slightly later, responses were relatively delayed for targets pointing in the same direction as primes (mean 26 ms; p<.008). This ‘reversed’ masked prime effect is known as the negative compatibility effect (NCE) and is taken as an index of an automatic inhibitory mechanism that is triggered to suppress the initial subliminal motor activation evoked by the primes [16-19]. Importantly for our study, this mechanism has been linked with a specific brain area, the supplementary motor area (SMA): the NCE has been reported absent in patients with very specific lesions in this region [5], and in healthy participants, the behavioural NCE is accompanied by fMRI modulation in the SMA [20].
Participants varied considerably in the size of their NCE (range −45 to −8 ms), and this is a robust trait: we found a high correlation between measurements taken in the same person several weeks apart (supplementary Fig. S1). We tested whether this individual variation is correlated with subtle differences in resting brain chemistry in the SMA. Neuronal activity in all cortical regions reflects a complex interplay between excitatory and inhibitory synapses. The latter mainly employ the neurotransmitter GABA (gamma-aminobutyric acid), and we predicted therefore that the size of the NCE might be related to the level of GABA concentration in the SMA, given that the SMA is thought to play an important role in the NCE [5, 20].
Further, opposing predictions can be made depending on the exact role of the SMA: If the main role of the SMA in reversed priming is to be the site of inhibition, then we would predict that more GABA would be associated with more inhibition, and thus a larger NCE. If on the other hand, the SMA is more involved upstream, with eliciting the inhibition process, we would predict smaller NCEs in those participants with higher GABA concentration in the SMA, because more GABAergic inhibition within a region would make that region less responsive [4] (for example, the GABA agonist muscimol is commonly used to temporarily deactivate a region in animal research e.g. [21]).
Using edited MR spectroscopy [6, 8], we measured GABA concentration from a (3cm)3 region of dorsal medial frontal cortex including, but not limited to, both right and left SMA (we refer to this as the SMA region, Fig. 1b,c, see supplementary Fig. S2 for individual GABA peaks within the spectra). This correlated well with the magnitude of the NCE, such that participants with higher GABA concentration in this region had smaller NCEs (r=0.77, p<.005 uncorrected, n=12, bootstrap 95% confidence interval, r = 0.51 to 0.93; Fig. 2a, left). We replicated this result in a separate cohort of 13 subjects (r=0.62, p=.025, bootstrap 95% confidence interval, r= 0.11 to 0.85; Fig. 2a, right). In both groups, therefore, the magnitude of the NCE was inversely related to GABA concentration in SMA.
Figure 2. Subliminal suppression correlates with GABA in the SMA region.
Higher GABA concentration in the region around human SMA predicts smaller negative compatibility effect (NCE) across individuals (a). This result was replicated in a second cohort. b, There was no correlation between the NCE and GABA concentration in dorsolateral prefrontal cortex (DLPFC), parietal cortex, anterior cingulated cortex (ACC) or inferior frontal gyrus (IFG). Positioning of these MRS voxels is shown for one participant (yellow rectangles on inset brains). Note that although the word cortex is included in some labels (to follow standard abbreviations for these regions) all voxels necessarily included both grey and white matter. Filled symbols and bold R-values reflect measurements from the second cohort. GABA concentration measurements are stated in institutional units (i.u.). All p-values are given 2-tailed but uncorrected for multiple comparisons; the main relationship of interest between the NCE and the SMA was specified a priori [5], but even if it had not been (and the first p-value is corrected), the replication demonstrates that the relationship is robust.
In the same participants, we also measured GABA concentration in other regions that have been associated with controlling the interaction between visual stimuli and action plans: dorsolateral prefrontal cortex, parietal cortex (both cohorts), anterior cingulate and inferior frontal gyrus (first cohort) [22-25]. In these regions GABA concentration did not correlate with the NCE (Fig. 2b; see also supplementary Fig. S3). Thus our data show regional specificity for the relationship between GABA and subconscious motor suppression.
We also find that the NCE correlates behaviorally with positive priming (the PCE, 1st cohort, r = −0.8, p< .005, 2nd cohort, r = −0.5, p< .05 1-tailed, Fig. 3a, b). People with smaller NCEs tend also to have smaller PCEs, which rules out the possibility that smaller NCEs simply reflected difficulty in overturning large initial positive priming phases. Rather it suggests that in some people both mechanisms are less responsive. However, GABA in the SMA region does not significantly correlate with the PCE (Fig. 3c, d), and thus the association between PCE and NCE must arise elsewhere. This is consistent with previous research, in which lesions in the locality of the SMA disrupted the NCE, but a PCE was present [5].
Fig. 3. Correlations of the PCE with the NCE and GABA in the SMA.
a,b, higher positive compatibility effect (PCE, subliminal activation) predicts higher (more negative) NCE (subliminal suppression). Further analyses of previously published data [3] also revealed strong correlations between NCE and PCE (experiment 1: r = −0.69, p< .013; experiment 2: r = −0.72, p< 0.008 & experiment 3: r = −0.63, p< .03). Thus it seems a general and robust phenomenon that the magnitudes of the PCE and NCE are correlated across individuals. However, although there is weak correlation in both cohorts between the PCE and GABA concentration in the SMA region (c, d), this was not significant (even across cohorts) and is presumably just mediated by the correlations between NCE and GABA and between NCE and PCE. Thus it appears that the common factor between NCE and PCE does not lie with GABA in the SMA.
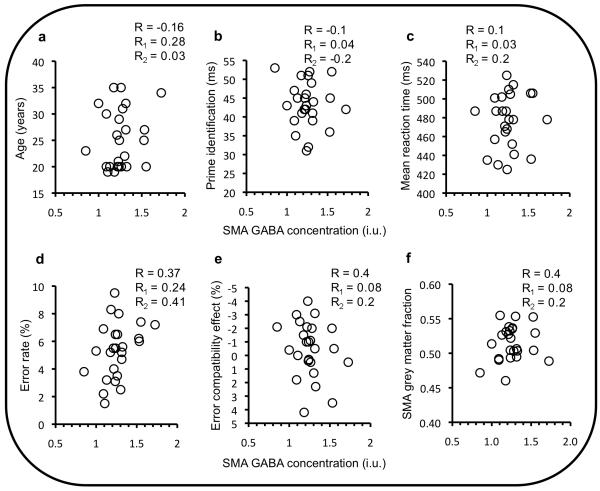
We tested whether the correlation between the NCE and GABA in the SMA region might arise due to other individual factors: age; prime visibility; overall speed of responding; error rate; grey matter volume. It did not; there were no significant correlations of either the NCE or GABA in SMA with any of these factors, and when controlling for them, the correlation between SMA GABA and the NCE remained (Fig. 4).
Fig. 4. GABA in SMA region does not correlate with other potential mediating factors.
(a: age, b: prime identification, c: mean reaction time, d: error rate, e: error compatibility effect, and f: fraction of grey matter in SMA MRS voxel) R-values are the correlation coefficient obtained putting both cohorts together; R1- and R2-values are the coefficient obtained for cohort 1 and 2 separately. There was also no significant correlation of any of these factors with the NCE (all |R|, |R1| or |R2| < 0.44, p> .16). Most importantly, when these factors were controlled for, the (partial) correlation (Rp) between the NCE and GABA in the SMA region remained. When controlling for the amount of grey matter, R1p = 0.8, p< .003, R2p = .53, p< .04, one-tailed. Similarly when controlling for age (R1p = 0.77, p< .005, R2p = .62, p< .035), average speed (R1p = 0.84, p< .001, R2p = .61, p< .03), prime visibility (R1p = 0.8, p< .003, R2p = .55, p< .03, one-tailed) and error rate (R1p = 0.75, p< .008, R2p = .51, p< .045, one-tailed). Note that as a neurotransmitter, the concentration of GABA is expected to higher in grey matter (GM) than in white matter, so one might predict a correlation between GM volume and GABA. However, the GM proportion in the voxel was very similar across participants (i.e. it was well controlled for), so there was little opportunity for a correlation to be revealed. GM proportion ranged from 49% to 54% in cohort 1 and from 46% to 55% in cohort 2. The essential point is not whether GM correlates with GABA, but that this relationship does not account for the correlation of GABA with the NCE.
What then is the cause of the relationship between a person’s NCE and GABA in their SMA region? The direction of the relationship implies that SMA is involved in the production of suppression rather than being the site where it occurs. The intuitive expectation might be that more GABA is associated with more inhibition, and thus a larger NCE. This relationship would be consistent with suppression within the SMA. However, directional expectations are complicated because the SMA is part of a network with other regions. If it is involved upstream, in the eliciting of inhibition rather than the implementation of inhibition, then lowering the responsiveness of this region with more baseline GABA would be predicted to lessen its functional effect, reducing the NCE.
The latter prediction is consistent with the deactivating effect of GABA agonist muscimol, and also with the absence of the NCE in patients in which the area is deactivated by actual lesions [5]. It is also consistent with two MRS studies measuring GABA in primary motor cortex: lowered cortical excitability following theta burst stimulation was associated with increased GABA concentration [4], and motor learning, which is thought to increase cortical excitability, was associated with lower GABA levels [26]. Thus the direction of correlation we found suggests that GABA levels in the SMA have a greater influence on the production of the suppression process creating the NCE than its implementation. The site of inhibition may be basal ganglia [27].
The relationship does not reflect general differences in caution or arousal, because neither the NCE or SMA GABA correlated with overall speed or accuracy (Fig. 4). Nor is it explained by any factor common to all sensorimotor tasks containing elements of response compatibility, conflict or inhibition, because neither SMA GABA or the NCE correlated with other tasks we have measured, including the Simon task [28], the Eriksen flanker task [29] and the STOP task [30] (supplementary Fig. S4).
In the Simon task [28], participants made left or right responses to the identity of letters appearing on the left or right of fixation. The location of the letter is irrelevant, but there is an automatic spatial compatibility effect such that responses tend to be faster when the stimulus appears on the same side as the required response. The fact that this “Simon effect” does not correlate behaviourally with the NCE (or PCE), or with GABA in the SMA region, indicates that individual differences in these two phenomena reflect dissociable traits. Similarly, in the Eriksen flanker task [29], participants respond to a central arrow, which is flanked by irrelevant arrows that also cause a compatibility effect: responses tend to be faster when the flankers point in the same direction as the target. Although recent evidence suggests some shared mechanism between subliminal suppression and the control of flanker interference [31], individual differences in the flanker effect appear dissociable from individual differences in the NCE, and do not reflect SMA GABA concentration.
In the STOP task [30], participants made speeded button presses to a shape cue, but on a subset of trials a second stimulus was presented that instructed them to withhold their response. The interval between go and stop signals is modulated to find the interval at which participants successfully stop on 50% of the stop trials. This ‘stop signal reaction time’ varied between participants, but importantly did not correlate with the NCE or with GABA in the SMA region.
Thus we find some specificity in the relationship between SMA GABA and functional inhibitory mechanisms, but this is not to say that SMA GABA only influences the NCE. In general we argue that control of specific functions will be subject to influence by the GABA level in areas of the brain that are causally involved in that function. Thus GABA concentration in SMA presumably affects other functions for which SMA plays a critical role.
It is not yet known why natural differences in baseline GABA concentration occur, and what factors create their regional specificity (supplementary Fig. S3; see also [32]). The differences in MRS signal we measure probably reflect different densities of GABA interneurons or synapses. Abnormalities in GABAergic inhibition have been associated with a number of clinical conditions, including schizophrenia, epilepsy, attention deficit/hyperactivity disorder, obsessive compulsive disorder, depression and bipolar disorder [33-39], but the relationship between symptoms and pathophysiology remains little understood.
The ability to relate specific and even subconscious traits to GABA in specific brain regions in healthy individuals promises to inform the study of such disorders, where there is no clear division between healthy and clinical populations. Moreover, our finding that differences in GABA concentration are regionally specific (as opposed to globally correlated) underlines the importance of targeting specific brain regions in clinical GABA MRS studies [40, 41], rather than inferring global changes from measurements of one region [42, 43].
In sum, we have found that individual variation in an automatic motor mechanism operating at the threshold of conscious awareness is reliably correlated with GABA concentration specifically in a region of medial frontal cortex, but not in other frontal regions or parietal cortex. This result promises that we can begin to understand differences in people’s basic behaviour in terms of the neurochemistry of specific brain regions.
Experimental Procedures
Overview
In the first experiment, we acquired (over two MR sessions per participant) MRS measurements from a (3 cm)3 voxel around the supplementary motor area (SMA), as well as an anatomical MRI scan and further MRS measurements from voxels in the parietal lobe, dorso-lateral pre-frontal cortex (DLPFC), inferior frontal gyrus (IFG), and the anterior cingulate cortex (ACC). Note that in MRS we acquire an average spectrum from a single predefined volume (it is not an imaging-like technique), and thus measurements for each volume was taken separately (12 mins each). On a separate occasion (not in the scanner), each participant was tested in the masked priming tasks, Simon task, Eriksen flanker task and STOP task.
The aim of the second experiment was simply to test in a second, independent, cohort the robustness of the relationship found between GABA and behaviour in the first experiment. There was one MR session per participant consisting of an anatomical MRI scan followed by three MRS measurements from voxels in the SMA region, DLPFC and parietal cortex. On a separate occasion, each participant was tested in the masked priming tasks and Simon task.
Participants
For the first experiment, twelve volunteers (all male, aged 21-32) were recruited within the School of Psychology, Cardiff University. For the second experiment, thirteen volunteers were similarly recruited (all male, aged 19-35). All had normal or corrected-to-normal vision, no neurological history and received payment for their time. All were naïve to the purpose of the study. The local Ethics Committee approved all procedures.
Anatomical MRI
A 1 mm3 isotropic resolution, T1-weighted anatomical MRI scan (FSPGR) was carried out to allow MRS voxel placement, and subsequent reconstruction of the cortical surface and segmentation of the MRS voxel. To segment the volume we used both FAST (http://www.fmrib.ox.ac.uk/fsl/) and FreeSurfer (http://surfer.nmr.mgh.harvard.edu/), and these methods showed a high degree of correlation for grey matter volume (r>0.95). In the reported results, grey matter estimates came from FreeSurfer.
MRS
In both experiments, GABA-edited MEGA-PRESS spectra [6, 8] were acquired from voxels positioned according to anatomical landmarks. The SMA voxel was placed symmetrically over the midline with its backward face anterior to the central sulci. All voxels, except in the ACC, were (3 cm)3 with one face of the cube aligned with the cortical surface. The ACC voxel was 2×3×4 cm3 in order to restrict it mainly to the appropriate region. The order of MRS voxels was counterbalanced across participants. Note that the MRS voxel is chosen a priori, and must be large enough to ensure sufficient signal quality to investigate individual differences in GABA concentration. Each MRS measurement was preceded by several brief anatomical imaging acquisitions in different orientations to allow accurate voxel placement.
The field strength was 3T and the following experimental parameters were used: TE 68 ms; TR 1800 ms; 400 transients of 4096 data points were acquired in 12 min; 16 ms Gaussian editing pulses were applied either to the GABA spins at 1.9 ppm, or at 7.5 ppm in an interleaved manner. Phased-array coil data were combined (using the first point of the unsuppressed water free induction decay signal) and spectra were processed by locally written software. Three-Hertz exponential line broadening and a high-pass water filter were applied, and the MEGA-PRESS difference spectrum was produced. The edited GABA signal at 3 ppm and the unsuppressed PRESS water signal were integrated: the integral of the GABA peak was calculated automatically using a linear fit of the baseline and a Gaussian fit to the peak itself (supp. Fig. S2); the water signal was fitted using a Lorentzian-Gaussian lineshape [44]. The GABA fitted amplitude was scaled to account for the fraction of cerebrospinal fluid (CSF) within the voxel, and the water amplitude was scaled to account for the different water content in CSF, grey and white matter [45]. A concentration measurement in institutional units was derived from the ratio of the GABA and water signals by using a single scalar to adjust for the editing efficiency and the T1 and T2 relaxation times of water and GABA. The GABA peak will also contain signal from co-edited macromolecules (e.g. cytosol), and this may contribute 30-40% of the integrated area [46]. However, we have no reason to expect that these would differ between individuals or have an influence on sensorimotor behaviour. Confidence that individual differences in our measure of GABA concentration reflect actual GABA differences can be drawn from recently reported association of this measurement with gamma frequency, BOLD signal, TMS and sensory tuning, all of which are well modelled by variation in GABA [4, 10-12, 47].
Functional localizer
As a check on the placement of our main voxel of interest, for two participants we acquired a functional localiser for the SMA using fMRI. A standard boxcar protocol was used with 15 seconds of sequential finger movements and 15 seconds of rest. We used a gradient echo EPI sequence taking 26 oblique-axial slices at 3 mm isotropic voxel resolution; 265 T2*-weighted volumes (TR = 1500 ms, TE = 35 ms, 90° flip angle, acquisition matrix = 64 × 64). Due to time-constraints this could not be done for all participants in the MRS sessions.
Masked priming
Stimulus presentation was performed by a PC-controlled Cambridge Research Systems (CRS) Visage® connected to a 21 in. Sony GDM-F520 Trinitron monitor. Stimulus presentation was synchronized with the screen refresh rate of 100 Hz, and timings were controlled and measured by the CRS clock and thus not subject to the errors produced by normal PC operating systems. Manual responses were collected using a CRS CB6 button box.
Participants had to make speeded responses with a left- or right-hand key press to left/right arrows (1° × 1°), which occurred in random order and within 4° of fixation. A fixation cross was visible at the centre of the screen at the beginning of each trial. The primes were identical to either one or the other targets, but presented for a briefer duration determined by prior adaptive staircase procedure (described below) and appeared within 0.3° of fixation. In all conditions, the prime was immediately followed by a mask of 2° × 2°, presented for 100 ms and constructed of 35 randomly orientated lines, excluding any orientation closer than 5° to the orientations in the arrow stimuli. A new mask was constructed on each trial.
To be sure that the masked-prime stimuli were subliminal at the start of the priming blocks, we used a psychophysical adaptive staircase procedure to determine the presentation duration for which an individual could consciously report the direction of the prime [for similar method, see 19]. Note that although we did not measure prime visibility again after the main task, the settings we used we similar to studies in which we have measured visibility afterwards, and in our experience prime discriminability does not grow during priming blocks (in which the participant is ignoring the prime). To measure the NCE, the delay between prime offset and target onset was set to 150 ms. To measure the PCE, this delay was 40 ms. A control experiment, as well as previous work, found these timings to provide robust and approximately maximal PCE and NCE effects [3]. There were 400 trials in each block (PCE and NCE).
Several weeks (2-8) after the first measurement, we assessed the repeatability of the NCE measurement by submitting participants to a further 200 trials of the same masked-prime paradigm. Details of the other tasks we measured can be found in supplementary information.
Highlights.
The magnitude of reversed masked priming robustly correlates with the concentration of GABA measured by magnetic resonance spectroscopy in the supplementary motor area (SMA).
This relationship appears specific to the SMA region – GABA measured in other regions associated with sensorimotor control does not correlate with priming.
Supplementary Material
Acknowledgements
This work was supported by the Wellcome Trust, the Wales Institute of Cognitive Neuroscience (WICN) and the NIHR CBRC at UCL/UCLH. CUBRIC was established with support from the UK Department of Trade and Industry, Cardiff University and the Welsh Assembly government. We thank also Tracy Herlihey, Sian Griffiths and Aline Bompas for comments on the manuscript.
References
- 1.Neumann O, Klotz W. Motor-Responses to Nonreportable, Masked Stimuli - Where Is the Limit of Direct Parameter Specification. In: Umiltà C, Moscovitch M, editors. Attention and Performance XV: Conscious and nonconscious information processing. MIT Press; Cambridge, MA: 1994. pp. 123–150. [Google Scholar]
- 2.Hermens F, Sumner P, Walker R. Inhibition of masked primes as revealed by saccade curvature. Vision Res. 50:46–56. doi: 10.1016/j.visres.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Boy F, Sumner P. Tight coupling between positive and reversed priming in the masked prime paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2010;36(4):892–905. doi: 10.1037/a0017173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J. Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumner P, Nachev P, Morris P, Jackson SR, Kennard C, Husain M. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron. 2007;54:697–711. doi: 10.1016/j.neuron.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 8.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn. Reson. Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21:22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- 10.Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. USA. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J. Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nature Neuroscience. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 14.Eimer M, Schlaghecken F. Response facilitation and inhibition in subliminal priming. Biol. Psychol. 2003;64:7–26. doi: 10.1016/s0301-0511(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 15.Sumner P. Negative and positive masked-priming – implications for motor inhibition. Advances in Cognitive Psychology. 2007;3:317–326. doi: 10.2478/v10053-008-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaghecken F, Eimer M. Active masks and active inhibition: A comment on Lleras and Enns (2004) and on Verleger, Jaskowski, Aydemir, van der Lubbe, and Groen (2004) Journal of Experimental Psychology-General. 2006;135:484–494. doi: 10.1037/0096-3445.135.3.484. [DOI] [PubMed] [Google Scholar]
- 17.Sumner P. Mask-induced priming and the negative compatibility effect. Experimental Psychology. 2008;55:133–141. doi: 10.1027/1618-3169.55.2.133. [DOI] [PubMed] [Google Scholar]
- 18.Jaskowski P. Negative compatibility effect: the object-updating hypothesis revisited. Exp. Brain Res. 2009;193:157–160. doi: 10.1007/s00221-008-1700-6. [DOI] [PubMed] [Google Scholar]
- 19.Boy F, Clarke K, Sumner P. Mask stimulus triggers inhibition in subliminal visuomotor priming. Exp. Brain Res. 2008;190:111–116. doi: 10.1007/s00221-008-1515-5. [DOI] [PubMed] [Google Scholar]
- 20.Boy F, Husain M, Singh K, Sumner P. Supplementary Motor Area Activations in Unconscious Inhibition of Voluntary Action. Exp. Brain Res. doi: 10.1007/s00221-010-2417-x. in press. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Sakai K, Hikosaka O. Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J. Neurophysiol. 1999;82:1063–1068. doi: 10.1152/jn.1999.82.2.1063. [DOI] [PubMed] [Google Scholar]
- 22.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- 23.Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. Neuroimage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who’s in control? Nature Neuroscience. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- 25.Buneo CA, Andersen RA. The posterior parietal cortex: Sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 27.Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: Evidence from functional MRI and Huntington’s disease. Brain. 2003;126:713–723. doi: 10.1093/brain/awg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon JR, Wolf JD. Choice Reaction-Time as a Function of Angular Stimulus-Response Correspondence and Age. Ergonomics. 1963;6:99–105. [Google Scholar]
- 29.Eriksen BA, Eriksen CW. Effects of Noise Letters Upon Identification of a Target Letter in a Nonsearch Task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- 30.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 31.Boy F, Husain M, Sumner P. Unconscious inhibition separates two forms of cognitive control. Proc Natl Acad Sci U S A. 2010;107:11134–11139. doi: 10.1073/pnas.1001925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grachev ID, Apkarian AV. Aging alters regional multichemical profile of the human brain: an in vivo 1H-MRS study of young versus middle-aged subjects. J. Neurochem. 2001;76:582–593. doi: 10.1046/j.1471-4159.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 33.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 34.Petty F, Kramer GL, Dunnam D, Rush AJ. Plasma GABA in mood disorders. Psychopharmacol. Bull. 1990;26:157–161. [PubMed] [Google Scholar]
- 35.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 36.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress. Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- 37.Zai G, Arnold P, Burroughs E, Barr CL, Richter MA, Kennedy JL. Evidence for the gamma-amino-butyric acid type B receptor 1 (GABBR1) gene as a susceptibility factor in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:25–29. doi: 10.1002/ajmg.b.30152. [DOI] [PubMed] [Google Scholar]
- 38.Pouget P, Wattiez N, Rivaud-Pechoux S, Gaymard B. A fragile balance: perturbation of GABA mediated circuit in prefrontal cortex generates high intraindividual performance variability. PloS one. 2009;4:e5208. doi: 10.1371/journal.pone.0005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin. Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 40.Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J, Drevets WC, Charney DS. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol. Psychiatry. 2005;58:969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, P MM, P JC. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 42.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol. Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 43.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 44.Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, Seckl J. Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magn. Reson. Med. 2000;44:646–649. doi: 10.1002/1522-2594(200010)44:4<646::aid-mrm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 45.Ernst T, Kreis R, Ross BD. Absolute Quantitation of Water and Metabolites in the Human Brain .1. Compartments and Water. Journal of Magnetic Resonance Series B. 1993;102:1–8. [Google Scholar]
- 46.Choi C, Bhardwaj PP, Kalra S, Casault CA, Yasmin US, Allen PS, Coupland NJ. Measurement of GABA and contaminants in gray and white matter in human brain in vivo. Magn. Reson. Med. 2007;58:27–33. doi: 10.1002/mrm.21275. [DOI] [PubMed] [Google Scholar]
- 47.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.07.017. DOI: 10.1016/j.neuroimage.2010.1007.1017. [DOI] [PubMed] [Google Scholar]
- 48.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J. Magn. Reson. Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 49.Verbruggen F, Logan GD, Stevens MA. STOP IT: Windows executable software for the stop-signal paradigm. Behavior Research Methods. 2008;40:479–483. doi: 10.3758/brm.40.2.479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.