Abstract
Background
Cardiac magnetic resonance imaging (CMR) can accurately determine infarct size. Prior studies using indirect methods and CMR, to assess infarct size have shown that patients with larger myocardial infarctions have worse prognoses. Implantable cardioverter defibrillators (ICD) have shown to improve survival among patients with severe left ventricular (LV) dysfunction. However, the majority of cardiac arrests occur in patients with higher ejection fractions.
Methods
The Defibrillators To Reduce Risk By Magnetic Resonance Imaging Evaluation study (DETERMINE) is a prospective, multi-center, randomized, clinical trial in patients with coronary artery disease (CAD) and mild to moderate LV dysfunction. The purpose of this trial is to test the hypothesis that patients with an infarct size ≥10% of LV mass, randomized to ICD plus appropriate medical therapy will have reduced mortality as compared to patients randomized to medical therapy. Cine and myocardial delayed contrast CMR will be performed in patients with CAD. The primary endpoint is death from any cause. At least 10,000 patients with CAD will undergo CMR. The target enrollment is 1,550 patients with an estimated 36-month enrollment period. Patients will be followed for 24 months after the last patient randomization. 330 deaths are estimated to occur during the follow-up period. This study is powered to detect a 28% reduction in mortality by ICD therapy.
Conclusion
The DETERMINE trial will assess the efficacy of ICD therapy to improve survival among patients with CAD, mild to moderate LV dysfunction and infarct size ≥10% of LV mass as measured by CMR.
Keywords: implantable cardioverter defibrillators, cardiac magnetic resonance imaging, sudden cardiac death, coronary artery disease, LV dysfunction
Introduction
American Heart Association (AHA) statistics suggest that up to 400,000 people die suddenly every year presumably due to cardiac arrhythmias.[1] Prior myocardial infarction (MI) is the most common substrate for cardiac arrhythmias in patients with coronary artery disease (CAD).[2–5] Most patients who suffer an out-of-hospital cardiac arrest do not survive. [6,7] Thus, using a prophylactic implantable cardioverter defibrillator (ICD) in patients at risk for sudden cardiac death (SCD) is a conceptually attractive option. Several clinical trials have previously shown that the ICD reduces mortality in patients with CAD and low left ventricular (LV) ejection fraction who had not yet suffered a life threatening arrhythmia. [8,9] However, the majority of out-of-hospital cardiac arrest occur in patients with ejection fractions >35%. However, the relative risk of sudden death in such patients is lower than for patients with LV ejection fraction ≤35%.[1] Thus, risk stratification is needed to define a subgroup of patients with better preserved LV ejection fraction who are at risk for SCD. A number of non-invasive tests have been developed to help predict SCD. In randomized trials, only LV ejection fraction, the presence of heart failure, and the presence of inducible ventricular tachycardia (VT) during programmed electrical stimulation (EPS) have been shown to identify patient who benefit from ICD therapy.[8,10]
In recent years, the development of cardiac magnetic resonance imaging (CMR) techniques using late delayed contrast enhancement have made it possible to delineate infarcted regions of the myocardium with high accuracy and spatial resolution.[11] Animal studies have demonstrated that inversion recovery CMR delineates infarct morphology with an excellent degree of correlation to infarct morphology by pathologic analysis.[12,13] Infarct size predicts subsequent LV remodeling after MI [14] and the presence of silent MI has been associated with higher mortality.[15–20] Infarct size assessed with contrast-enhanced CMR can help predict mortality in patients with healed myocardial infarction. Roes et al. performed CMR in 231 patients. The spatial extent of infarct size and the total score of the scar were stronger predictors of total mortality than LV ejection fraction or LV volumes.[21] A prospective study that has been presented in preliminary form demonstrated that both infarct mass and LV ejection fraction were independent predictors of survival in a population of patients with CAD.[16] Kwong et al examined the relationship between hyperenhancement and total mortality in patients with CAD. They found that the presence and extent of hyperenhancement predicted cardiac mortality.[22] Based on these results and since infarct mass has been found to be a better predictor of inducibility of VT during EPS than LV ejection fraction, we hypothesized that infarct size as measured by CMR may be a better predictor of arrhythmic risk and survival then LV ejection fraction.[15] While there is a consensus that myocardial delayed hyperenhancement predicts outcome, other investigators have each used slightly different criteria in defining the extent of myocardial infarction. For those studies that have characterized infarct size, a lower limit of 10% of the LV mass appears to provide reasonable sensitivity and specificity for classifying those patients at risk for overall mortality. This criterion is similar to that found to predict inducibility of VT in other studies [16] and thus the infarct mass cut-off of 10% or greater was utilized in the DETERMINE study to define those patients who would be at risk for mortality and be eligible for randomization to ICD or standard therapy.
Methods
Study Objectives, study population, and inclusion and exclusion criteria
To test the hypothesis that therapy with an ICD combined with appropriate medical therapy improves long-term survival compared to aggressive medical therapy alone in patients with CAD, infarct mass measuring ≥10% of the LV mass and LV systolic dysfunction will be randomized. Patients must have an ejection fraction >35%, or have an ejection fraction of 30–35% without inducible ventricular tachycardia and without New York Heart Association (NYHA) functional class II or greater heart failure. Details of the inclusion and exclusion criteria are shown in tables 1 and 2.
Table 1.
Eligibility
Inclusion Criteria: (Randomized Trial)
|
CAD will be confirmed by evidence of one of the following three (3) criteria 1) Prior myocardial infarction, 2) Significant stenosis of a major epicardial vessel (>50% proximal or 70% distal) by coronary angiography, 3) Prior revascularization (percutaneous coronary intervention or coronary artery bypass surgery. Patients may not be randomized until 90 days after revascularization.
MI should be documented by the presence of two (2) of the following three (3) criteria: 1) Symptoms consistent with myocardial infarction (i.e. chest pain, shortness of breath), 2) Q-waves on electrocardiogram and 3) Elevated cardiac enzymes (CPK elevation > two times or troponin elevation > three times the upper limit of normal for the lab). Patients may not be randomized until 40 days after myocardial infarction.
Table 2.
Exclusions
Exclusion Criteria
|
Justification of Patient Selection Criteria
Several large scale primary prevention trials have identified patients with clinical characteristics who appear to benefit from prophylactic ICD implantation.[23] These characteristics include symptoms of heart failure and LV dysfunction.[8,9,23–25] However, a meta-analysis by Al Khatib et al [26] demonstrated that the benefit of ICD implantation may be more modest in patients with LV ejection fractions between 30 and 35%. In this group of patients, heart failure class may provide additional risk stratification information.[26] We thus carefully considered the appropriate ejection fraction cut-off for inclusion in the present trial and set the ejection fraction cut-off at 35% with NYHA functional class II and class III heart failure and 30% for those with class I CHF.
Data regarding the relationship of infarct mass to mortality and SCD risk in patients with CAD are described in the background section. This information suggests that a cut off of 10% of the LV mass provides reasonable sensitivity and specificity for defining the risk of death from arrhythmia. We considered using other parameters such as infarct surface area, peri-infarct area, or infarct transmurality as part of the inclusion criteria. However, preliminary data cited above demonstrate that not enough information is available to fully implement these techniques on a broad basis and to reliably use these parameters as inclusion criteria in a clinical trial. Thus we have chosen infarct mass, represented as the percent of the current LV mass as the primary endpoint.
Infarct resorption and LV remodeling can occur weeks to months following MI. Yan, et al showed that after the acute phase of an MI, the extent of scar defined by delayed contrast enhanced CMR is an independent predictor of post-MI all-cause (P=0.005) and cardiovascular mortality (P=0.01).[27] Infarct size has also been shown to decrease over time. Several studies have looked at the change in infarct size by measuring infarct mass during the immediate post-MI period and then again during chronic follow-up. In a study by Wu et al, the late measure of infarct mass decreased by 22% of the initial size with an absolute decrease of 4.3 ± 5.5%.[14] Ingkanisorn, et al showed that the percent of infarcted myocardium decreased from 16 ± 12% to 11 ± 9%.[28] Therefore, for DETERMINE in the instance where infarct mass is measured within 40 days of a myocardial infarction, the infarct mass required for randomization will be ≥15% of LV mass.
Study Organization
The study organization includes the Northwestern University clinical coordinating center and the data coordinating center, a data safety and monitoring board (DSMB), an executive steering committee, an electrophysiology steering committee, a CMR steering committee, the CMR core laboratory at the University of California, Los Angeles, the genetics and biomarker and registry endpoint core laboratories at Brigham and Women’s Hospital in Boston, and the investigational sites. The study is funded by St. Jude Medical Cardiac Rhythm Management Division, which holds the IDE for the trial. The DSMB, the endpoints committee and the principal investigators have disclosed potential conflict of interests based on the AHA and the American College of Cardiology (ACC-AHA) guidelines.
Study Design
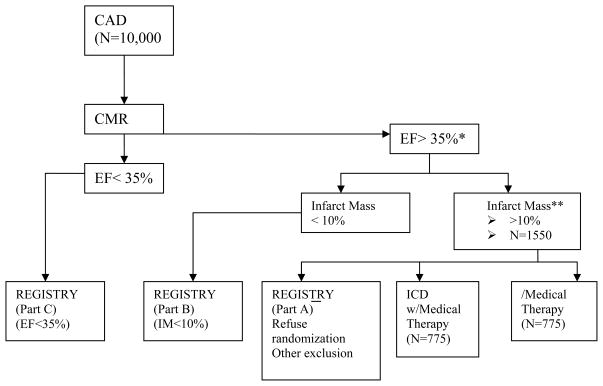
DETERMINE is a prospective, randomized, multi-center study (figure 1). At baseline, evaluation of entry criteria will be verified, and patients who have a history of CAD with documentation of either MI or LV dysfunction, may be screened for eligibility for this trial.
Figure 1.
DETERMINE. Study Overview
* Eligibility for patients with an ejection fraction of 30–35% will be determined based upon their history of heart failure, ventricular tachyarrhythmias or inducibility during electrophysiologic testing.
** Infarct mass must be >15% if measured < 40 days after myocardial infarction
Approximately 10,000 patients with CAD who underwent CMR within 12 months for routine clinical evaluation will be enrolled in the DETERMINE non-investigational registry. Of these 10,000 patients, those with an ejection fraction >35% or 30–35% if they do not currently have an indication for an ICD, will be eligible for enrollment in the randomized portion of the trial. The first 1,550 patients will be randomized to either medical therapy (Control Group) or ICD therapy in combination with medical therapy (Treatment Group) in a 1:1 fashion.
Non-investigational Registry
The DETERMINE registry will enroll approximately 10,000 patients with a diagnosis of CAD who have undergone CMR. Patients enrolled in the registry who do not undergo randomization will be asked to consent to long-term follow-up as part of the NIH/NHLBI and St. Jude Medical sponsored observational cohort study (Pre-DETERMINE). SCD and other causes of death will be documented over a 3–5 year follow-up period. One of the primary objectives of this cohort study will be to test the hypothesis that infarct mass as measured by contrast enhanced CMR is a better predictor for SCD than LV ejection fraction.
Patients who are screened but not randomized will fall into one of three categories that will be used for sub-cohort analysis:
Subjects may meet ejection fraction and infarct mass criteria but are not randomized because of exclusion criteria or because of refusal to provide informed consent.
Subjects may not qualify for randomization because their infarct mass does not meet eligibility criteria for randomization
Subjects may not qualify for randomization because their LV ejection fraction is determined to be less than 35% (or 30% if NYHA class I heart failure is present).
Randomization
Randomizations will be stratified by enrolling center, infarct mass of 10% or 15% and presence of chronic atrial fibrillation at the time of enrollment. Randomization will be considered Time 0 for both groups for the purpose of the primary endpoint.
Crossover and loss to follow-up
Patients will be included in the analysis on an intention-to-treat basis. If there is a decision to use anti-arrhythmic therapy and not to implant an ICD in the ICD arm or if the ICD is explanted or de-activated or if there is failure to implant the ICD system, patients will be considered “crossed over”. In addition, an attempt will be made to verify the vital status of subjects lost to follow-up through means including but not limited to, the National Death Index/Social Security Index.
Baseline evaluation
After the patient signs informed consent, a baseline examination will be performed that will include a clinical history and physical examination, 12-lead ECG, and collection of blood sample for biomarker and genetic analysis. In addition, the patient will be asked to complete a 6-minute walk test and quality of life (QOL) questionnaires.
Follow-up
Enrollment is expected to occur over three years. Study follow-up will occur every three months and will include clinic visits at six-month intervals alternating with telephone contact. It is anticipated that the average length of follow-up will be 3.5 years per patient. All patients will be followed until the last patient randomized completes 24 months of follow-up. All patients will be followed for evaluation of clinical status, vital status and QOL. In patients randomized to ICD, follow-up will also include ICD interrogation. Additionally, a 6-minute walk test and a standard ECG will be performed annually. (Table 3)
Table 3.
DETERMINE TRIAL: Follow up
| Screening Baseline | Post Randomization (Months) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | 36 | 39 | 42 | 45 | 48 | ||
| Medical History | X | ||||||||||||||||
| Physical Exam | X | X | X | X | X | X | X | X | X | ||||||||
| MRI data | X | ||||||||||||||||
| ICD Interrogation | X | X | X | X | X | X | X | X | |||||||||
| Medications | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 12-Lead ECG | X | X | X | X | X | ||||||||||||
| 6 Minute Walk Test | X | X | X | X | X | ||||||||||||
| QOL Questionnaire | X | X | X | X | X | X | X | X | X | ||||||||
| Genetic Sample | X | ||||||||||||||||
| Adverse Events | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Telephone Contact (vital status) | X | X | X | X | X | X | X | X | |||||||||
All patients will continue the follow-up schedule (every 6 months clinic visit alternating with telephone follow-up) until the last randomized patient has been followed for 24 months.
Device selection and ICD implantation
The devices used in this clinical investigation include commercially available St. Jude Medical single and dual chamber ICDs and lead systems. Dual chamber devices may be implanted for sinus node dysfunction, atrioventricular block or discrimination of atrial tachyarrhythmias. Biventricular pacing for cardiac resynchronization therapy is not currently indicated in this patient population and will not be used in this trial unless guidelines change. Defibrillation testing will consist of a standard clinically applicable approach. A single defibrillation success with a 15J safety margin or 2 consecutive successes with at least 7J safety margin will be considered adequate for implantation. Non-invasive programmed stimulation will be performed to optimize device programming either during ICD implantation or prior to hospital discharge.
The following ICD programming parameters will be used:
-
ICD should be programmed to minimize ventricular pacing and inappropriate shocks
Single ventricular fibrillation zone greater than or equal to 180 BPM
Bradycardia at 40 BPM with hysteresis
Single or dual chamber ICD as appropriate
ATP can be programmed if monomorphic ventricular tachycardia is induced
Medical therapy
It is recommended that all patients, regardless of randomization group, receive appropriate pharmacologic therapy as described in the ACC-AHA guidelines for patients after MI [29] including antiplatelet agents, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and beta blockers. In addition, all patients should be administered pharmacologic therapy for lipid lowering as described in guidelines for patients post MI.[30] Anti-arrhythmic therapy for atrial fibrillation is allowed in both groups. Anti-arrhythmic drug therapy for ventricular arrhythmias will not be allowed except when clinically required for symptomatic arrhythmias.
CMR protocol
CMR will have been performed for clinical indications for evaluation of LV function. In order to ensure consistent infarct mass assessments, the cardiac CMR study submitted to assess eligibility for randomization must meet the following criteria:
The contrast agent must be an FDA approved, gadolinium-based extracellular agent.
CMR images must be of sufficient quality to enable confident analysis of LV function and LV myocardial mass.
CMR images must be of sufficient quality to enable confident discrimination between scarred and viable myocardium (on delayed contrast hyper-enhancement images) throughout the entirety of the LV.
The CMR must be available for electronic submission to the CMR Core lab for analysis
For the images to be accepted by the core lab, two set of images will be required. The first set involves steady-state free precession cine images using a phased-array cardiac coil during repeated breath holds.[31–34]
Images will be acquired in multiple short-axis (at 1 cm intervals throughout the entire left ventricle) and 3 long-axis planes. A second set of contrast enhanced images following administration of gadolinium will employ a segmented inversion-recovery sequence in the identical planes to the cine images. The cine and gadolinium-enhanced images will be evaluated separately by the consensus of 2 observers unaware of any patient information. Hyperenhanced myocardium on the delayed gadolinium-enhanced images will be assumed to represent scar. The myocardial borders will be planimetered on all of the short-axis cine images to determine LV volumes, ejection fraction, and mass (assuming density = 1.05 g/cm3). Regional parameters will be assessed using a standard 17-segment model.[35] Segmental gadolinium enhancement with respect to the extent of transmural MI as well as the total volume of hyperenhancement will be divided by the total volume of LV myocardial tissue to obtain infarct mass as a percentage of LV mass.
Study Endpoints
Primary endpoint
The primary endpoint of the study is all-cause mortality.
Secondary endpoint
The secondary endpoint of the study will be arrhythmic mortality. Cause-specific mortality will be determined by an independent events committee.[36,37]
Additional secondary objectives
Additional analyses will look at appropriate ICD shocks and QOL. This study will include a careful assessment of the effects of ICD implantation and presence on continued QOL and will be performed using two assessment tools. Changes in QOL throughout the trial will be assessed using one global [Short form 36 (SF-36)] and one disease-specific [Seattle Angina Questionnaire (SAQ)] instrument. This set of variables will measure psychological profiles that may determine the susceptibility to the placebo effect with ICD. The QOL instruments will be obtained at the enrolling center for all enrolled subjects at baseline and then every 6 months. In the event that the patient receives a shock from the ICD, the SF-36 and SAQ questionnaires will also be administered. In addition, two ICD specific QOL tools, the Florida Patient Acceptance Survey (FPAS) and Florida Shock Acceptance Survey (FSAS), will also be administered.[38,39] QOL responses will be analyzed utilizing multi-level mixed models methodology.
Substudies
Biological Markers and Genetics Substudy: PRE-DETERMINE:
Blood samples will be collected and stored for future genetic and biomarker analyses from all patients enrolled in the randomized trial and the observational cohort study. At the end of the study, the predictive value of promising protein, genetic, and metabolic biomarkers on risk of arrhythmic events will be examined in the 6850 patients with LV ejection fraction >35% enrolled in both the observational cohort study and in the parent DETERMINE randomized trial. The goal of these analyses will be to identify a series of markers that alone or in combination with CMR imaging specifically predict risk of arrhythmic death as compared to other causes of mortality among this at-risk, but under-studied, population of CAD patients. Such markers may serve as relatively inexpensive methods to identify those at risk for SCD. The public health impact of identifying such markers could be quite substantial and lead to 1) more efficient utilization of ICDs and 2) advances in our understanding of mechanisms that could ultimately lead to novel therapeutic approaches.
Statistical Methods
Death from any cause-Primary endpoint
A two tailed test will be utilized to evaluate the primary endpoint.
-
H0
Rate of all-cause mortality in ICD group = rate of all-cause mortality in control group
-
H1
rate of all-cause mortality in ICD group ≠ rate of all-cause mortality in control group
This hypothesis tests the effects of ICD implantation on all-cause mortality in the study population. The null hypothesis will be rejected if the observed value of the log-rank statistic for testing the null hypothesis is greater than the critical value that corresponds to the two-tailed 5% significance level.
Sample Size
Based on the recent trials [8,9,16,21,25,40,41] the mortality of the control patients is estimated to be 25% over an average of 3.5 years of follow-up. ICDs are expected to reduce the overall risk of death by 28%. Patients will be randomized to each study arm in equal numbers. We estimate that patients will be uniformly enrolled for 3 years and, after the last patient is enrolled, all patients will be followed for an additional 2 years. It is estimated that 5% percent of the control group patients and 1% of the ICD arm will cross over. After crossover, patients’ mortality rate will become that of their new study arm. Crossovers were modeled to occur at a constant rate. Two interim analyses will be performed after 60% and 80% of events have occurred. O’Brien-Fleming alpha spending function with asymmetric upper and lower bounds was incorporated in the sample size derivation. The benefit boundary will be more conservative in the early stages of the study (Power family with rho=7). After the adjustment, the final analysis will be conducted at 4.2% two-sided significance level. Patients cannot be randomized until 40 days after a MI or 90 days after revascularization. Given those exclusions, no evidence is available to suggest that ICD effect will change during the course of the trial; therefore, proportional hazards assumption was adopted. Under these conditions, the number of events required to detect the difference that corresponds to HR=0.6969 with 89.4% power and 5% two-sided significance level will be 330 and 1550 patients will be needed to reach this number of endpoints.
Primary Analysis
Total survival will be evaluated when all surviving patients have been seen for their 2-year follow-up visit. The survival time for patients who die during the study will be calculated as the number of the days from randomization to death. Analyses of survival times will be based on the log-rank test for comparison of survival curves using Kaplan-Meier methodology and implemented via the current version of SPSS software.
Interim analyses will take place after 198 and 264 deaths will have occurred. If the Z-value of the test statistic exceeds the upper boundary at any interim analysis (3.195 and 2.570 for interim analyses 1 and 2), the trial will be stopped in favor of the alternative hypothesis (for ICD superiority). If the Z-value is below the lower boundary at any interim analysis (-2.669 and -2.289 for interim analyses 1 and 2) the trial will be stopped in favor of the alternative hypothesis (for ICD inferiority). If the number of primary endpoint events is not accumulated at the time of the final analysis the DSMB will make a recommendation regarding extending the trial until all surviving patients will be followed for a new fixed minimum amount of time. ICD efficacy will be demonstrated if the observed value of the log-rank statistic will exceed the critical value that corresponds to the 4.2% two-sided significance level. Poolability across sites will be examined for demographic and clinical variables as well as for time to death. In addition, interaction between treatment and site will also be assessed.
Patient Group
All randomized patients will be included in the analysis of the secondary endpoint on an “intention-to-treat” basis. “Worst-case” analysis will also be performed, in which all randomized patients whose vital status is not confirmed will be presumed dead due to arrhythmic causes at the time of withdrawal, while control patients will be presumed alive. All deaths among patients who withdraw but no controls lost to follow-up. In addition, a “best-case” analysis will be carried out where patients whose vital status is not confirmed at the time of withdrawal will be assumed to be alive at the time of withdrawal and control patients will be presumed dead.
Covariate Analysis
The impact of the following pre-specified set of covariates - age, sex, race, time from the first MI, history of revascularization, descriptors of co-morbidity (diabetes and hypertension), NYHA functional class, number of diseased coronary arteries, LV ejection fraction, atrial fibrillation, QRS duration and infarct mass - on the endpoint analysis will also be examined in a multivariate Cox regression. These baseline patient characteristics will be entered into the model ahead of the treatment assignment as a single block. Subsequently, the predictive power of ICD implantation will be assessed with the likelihood ratio test. Two-sided alpha level of 10% will be used for all analyses.
Discussion and Conclusion
Several recent clinical trials have established the ICD as an important therapeutic modality for primary and secondary prevention of death in post-MI patients with severe LV dysfunction. [3–5,8,10] However, any strategy for primary prevention based on ejection fraction alone has major limitations. For instance, the vast majority of patients who die suddenly following a MI have ejection fractions greater than 30 percent.[9,40] Although these patients are at lower relative risk for SCD, they account for the majority of patients who die suddenly. Conversely, not all patients with low ejection fractions will experience arrhythmic death. Thus, treating all patients with a depressed ejection fraction with an ICD may not be cost-effective. Infarct size determined by CMR in patients with known CAD has been found to be an independent predictor of overall mortality. [15,16,21] Thus, CMR may be useful in risk stratification in patients with a range of ejection fractions. DETERMINE will test this hypothesis.
References
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 2.Mehta D, Curwin J, Gomes JA, Fuster V. Sudden death in coronary artery disease: Acute ischemia versus myocardial substrate. Circulation. 1997;96:3215–3223. doi: 10.1161/01.cir.96.9.3215. [DOI] [PubMed] [Google Scholar]
- 3.Bolick DR, Hackel DB, Reimer KA, Ideker RE. Quantitative analysis of myocardial infarct structure in patients with ventricular tachycardia. Circulation. 1986;74:1266–1279. doi: 10.1161/01.cir.74.6.1266. [DOI] [PubMed] [Google Scholar]
- 4.Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 5.Adhar GC, Larson LW, Bardy GH, Greene HL. Sustained ventricular arrhythmias: Differences between survivors of cardiac arrest and patients with recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1988;12:159–165. doi: 10.1016/0735-1097(88)90369-5. [DOI] [PubMed] [Google Scholar]
- 6.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, Barrett J. Racial differences in the incidence of cardiac arrest and subsequent survival. The cpr chicago project. N Engl J Med. 1993;329:600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 7.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, Zalenski R, Becker LB, Schron EB, Proschan M. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351:637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 8.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 9.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 10.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 11.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved mr imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 12.Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk: Distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol. 2000;36:1985–1991. doi: 10.1016/s0735-1097(00)00958-x. [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of mri delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 14.Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, Carr JC, Holly TA, Lloyd-Jones D, Klocke FJ, Bonow RO. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: Prospective cohort study. Heart. 2008;94:730–736. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 15.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, Kadish AH, Goldberger JJ. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Bello D, Kaushal R, Fieno DS, Radin M, Shaoulian E, Narula J, Goldberger J, Kadish A, Shivkumar K. Cardiac mri: Infarct size is an independent predictor of mortality in patients with coronary artery disease. J Am Coll Cardiology. 2005;45:288A. doi: 10.1016/j.mri.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, Cotts WG, Klocke FJ, Bonow RO, Judd RM, Gheorghiade M, Kim RJ. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–1953. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 18.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed q-wave and non-q-wave myocardial infarction. Lancet. 2001;357:21–28. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the framingham study. N Engl J Med. 1984;311:1144–1147. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 20.Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 21.Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, Boersma E, van der Wall EE, Fleck E, de Roos A, Nagel E, Bax JJ. Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–936. doi: 10.1016/j.amjcard.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 23.Myerburg RJ, Mitrani R, Interian A, Jr, Castellanos A. Interpretation of outcomes of antiarrhythmic clinical trials: Design features and population impact. Circulation. 1998;97:1514–1521. doi: 10.1161/01.cir.97.15.1514. [DOI] [PubMed] [Google Scholar]
- 24.(CMS) CfMMS. Implementation for implantable defibrillators (cag-00157r3) Baltimore, MD: Centers for Medicare & Medicaid Services; 2005. [Google Scholar]
- 25.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 26.Al-Khatib SM, Sanders GD, Mark DB, Lee KL, Bardy GH, Bigger JT, Buxton AE, Connolly S, Kadish A, Moss A, Feldman AM, Ellenbogen KA, Singh S, Califf RM. Implantable cardioverter defibrillators and cardiac resynchronization therapy in patients with left ventricular dysfunction: Randomized trial evidence through 2004. Am Heart J. 2005;149:1020–1034. doi: 10.1016/j.ahj.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 28.Ingkanisorn WP, Rhoads KL, Aletras AH, Kellman P, Arai AE. Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J Am Coll Cardiol. 2004;43:2253–2259. doi: 10.1016/j.jacc.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. Acc/aha guidelines for the management of patients with st-elevation myocardial infarction--executive summary: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the national cholesterol education program adult treatment panel iii guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 31.Kacere RD, Pereyra M, Nemeth MA, Muthupillai R, Flamm SD. Quantitative assessment of left ventricular function: Steady-state free precession mr imaging with or without sensitivity encoding. Radiology. 2005;235:1031–1035. doi: 10.1148/radiol.2353030995. [DOI] [PubMed] [Google Scholar]
- 32.Ichikawa Y, Sakuma H, Kitagawa K, Ishida N, Takeda K, Uemura S, Motoyasu M, Nakano T, Nozaki A. Evaluation of left ventricular volumes and ejection fraction using fast steady-state cine mr imaging: Comparison with left ventricular angiography. J Cardiovasc Magn Reson. 2003;5:333–342. doi: 10.1081/jcmr-120019422. [DOI] [PubMed] [Google Scholar]
- 33.Fieno DS, Jaffe WC, Simonetti OP, Judd RM, Finn JP. Truefisp: Assessment of accuracy for measurement of left ventricular mass in an animal model. J Magn Reson Imaging. 2002;15:526–531. doi: 10.1002/jmri.10107. [DOI] [PubMed] [Google Scholar]
- 34.Carr JC, Simonetti O, Bundy J, Li D, Pereles S, Finn JP. Cine mr angiography of the heart with segmented true fast imaging with steady-state precession. Radiology. 2001;219:828–834. doi: 10.1148/radiology.219.3.r01jn44828. [DOI] [PubMed] [Google Scholar]
- 35.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 36.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 37.Epstein AE, Carlson MD, Fogoros RN, Higgins SL, Venditti FJ., Jr Classification of death in antiarrhythmia trials. J Am Coll Cardiol. 1996;27:433–442. doi: 10.1016/0735-1097(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 38.Burns JL, Serber ER, Keim S, Sears SF. Measuring patient acceptance of implantable cardiac device therapy: Initial psychometric investigation of the florida patient acceptance survey. J Cardiovasc Electrophysiol. 2005;16:384–390. doi: 10.1046/j.1540-8167.2005.40134.x. [DOI] [PubMed] [Google Scholar]
- 39.Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: An initial evaluation of the florida shock anxiety scale. Pacing Clin Electrophysiol. 2006;29:614–618. doi: 10.1111/j.1540-8159.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 40.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 41.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]