Abstract
Long term functional impairments due to spinal cord injury (SCI) in the rat result from secondary apoptotic death regulated, in part, by SCI-induced decreases in protein levels of the anti-apoptotic protein Bcl-xL. We have shown that exogenous administration of Bcl-xL spares neurons 24h after SCI. However, long term effects of chronic application of Bcl-xL have not been characterized. To counteract SCI-induced decreases in Bcl-xL and resulting apoptosis, we used the TAT protein transduction domain fused to the Bcl-xL protein (Tat-Bcl-xL), or its anti-apoptotic domain BH4 (Tat-BH4). We used intrathecal delivery of Tat-Bcl-xL, or Tat-BH4, into injured spinal cords for 24h or 7 days, and apoptosis, neuronal death and locomotor recovery were assessed up to 2 months after injury. Both, Tat-Bcl-xL and Tat-BH4, significantly decreased SCI-induced apoptosis in thoracic segments containing the site of injury (T10) at 24h or 7 days after SCI. However, the 7 day delivery of Tat-Bcl-xL, or Tat-BH4, also induced a significant impairment of locomotor recovery that lasted beyond the drug delivery time. We found that the 7 day administration of Tat-Bcl-xL, or Tat-BH4, significantly increased non-apoptotic neuronal loss and robustly augmented microglia/macrophage activation. These results indicate that the anti-apoptotic treatment targeting Bcl-xL shifts neuronal apoptosis to necrosis, increases the inflammatory response and impairs locomotor recovery. Our results suggest that a combinatorial treatment consisting of anti-apoptotic and anti-inflammatory agents may be necessary to achieve tissue preservation and significant improvement in functional recovery after SCI.
Keywords: Apoptosis, spinal cord injury, inflammation, Tat-Bcl-xL, Tat-BH4, cell death, microglia
INTRODUCTION
Mechanical trauma to the spinal cord triggers events resulting in the death of neurons and glia over several weeks after the initial injury (Liu et al., 1997;Ahn et al., 2006). In the early acute phase, there is a cascade of excitatory amino-acid-induced Ca2+ entry and energy failure, nitric oxide (NO) production, oxidative stress and membrane breakdown that lead to early necrosis (Park et al., 2004;Bao and Liu, 2004;Bao et al., 2006), which is followed by apoptosis of neurons and glia (Beattie et al., 2000;Lu et al., 2000;Park et al., 2004). Neuronal apoptosis begins as early as 4h near the site of impact and persists for the first 24h after trauma, while neuronal and oligodendroglial apoptosis lasts for a couple of weeks in areas away from the injury site (Liu et al., 1997;Shuman et al., 1997;Casha et al., 2001). Since the functional outcome after spinal cord injury (SCI) is in part dependent on the extent of secondary cell death, it has been suggested that the prevention of delayed apoptosis after SCI is likely to have a beneficial effect by reducing the extent of tissue damage (Schwab et al., 2006). With the belief that the final steps of apoptosis are highly conserved and likely to be mediated by a related set of caspases; inhibitors of caspases have been used to prevent SCI-induced apoptosis with different levels of success (Barut et al., 2005;Knoblach et al., 2005;Colak et al., 2005). However, apoptosis is known to be triggered through different pathways, caspase-dependent and caspase-independent, both impinging on mitochondrial function (Yuan et al., 2003). For example, the release of mitochondrial cytochrome c is indispensable for the activation of caspases (Hengartner, 2000); while the release of mitochondrial apoptosis-inducing factor (AIF) leads to DNA fragmentation in a caspase-independent fashion (Yakovlev and Faden, 2004). Main regulators of apoptosis via mitochondria are members of the Bcl-2 family of proteins. The Bcl-2 family of proteins, containing pro-apoptotic (Bax, Bad, Bid) and anti-apoptotic (Bcl-2, Bcl-xL) members, is central to the regulation of both caspase-dependent and caspase-independent apoptosis, by modulating mitochondrial outer membrane permeability (Tsujimoto, 2003;Sharpe et al., 2004). Among the Bcl-2 family, Bcl-xL is the principal anti-apoptotic member in the postnatal and adult central nervous system (Gonzalez-Garcia et al., 1994;Gonzalez-Garcia et al., 1995;Alonso et al., 1997;Parsadanian et al., 1998); where it is highly expressed in neurons and oligodendrocytes in the rat spinal cord (Qiu et al., 2001;Nesic-Taylor et al., 2005).
Manipulation of the levels of Bcl-2 proteins could provide new treatment paradigms that prevent apoptosis associated with SCI. Conditional Bcl-xL overexpression protects postnatal and adult neurons from traumatic hypoxia (Matsuoka et al., 2002;Wen et al., 2002), and metabolic injury (Xu et al., 1999;Shinoura et al., 2000). Furthermore, exogenous Bcl-xL has been shown to be highly effective in protecting against cell injury in response to ischemia (Cao et al., 2002), oxidative stress (Cherbonnel-Lasserre and Dosanjh, 1997), hypoglycemia (Panickar et al., 2005), neurotrophin deprivation (Vander Heiden et al., 1999) and excitotoxicity (Matsuoka et al., 2002). We have found that Bcl-xL levels are significantly reduced after SCI and that the short-term administration of Bcl-xL-fusion protein to the injured spinal cord significantly increases neuronal survival within 24h after spinal injury (Nesic-Taylor et al., 2005). However, the long-term effects of such anti-apoptotic therapy have not been assessed in a rat model of SCI.
In a previous study (Nesic-Taylor et al., 2005), we used a Bcl-xL fusion protein, a construct in which Bcl-xL was fused into a 254 amino acid nontoxic derivative (lethal factor, LFn) of anthrax toxin to render the Bcl-xL cell permeable (Liu et al., 2001). The transduction of LFn-Bcl-xL requires the binding of the LFn domain to another anthrax toxin component, protective antigen (PA)(Liu et al., 2001), which binds to an unidentified cell surface receptor and mediates the transport of the Bcl-xL fusion protein into the cell (Friedlander, 1986). In the present study, we chose Tat-mediated delivery of Bcl-xL because it offered several important advantages over the anthrax-toxin delivery system (Yin et al., 2006). First, Tat-mediated protein transduction in the CNS does not require co-administration of helper proteins. The Tat sequence is only 11 amino acid residues long, which does not substantially increase the size of the fusion protein and thus, is less likely to interfere with the activity of the transduced protein (Cao et al., 2002). Tat-Bcl-xL has been shown to rapidly transduce into mammalian cells (Nagahara et al., 1998) via an endocytosis-mediated, but receptor-independent mechanism (Potocky et al., 2003;Wadia et al., 2004;Kaplan et al., 2005). In addition, the ability of the TAT peptide to bind to ubiquitous targets such as heparan sulfate, chondroitin sulfate, or even phospholipid heads in the lipid bilayer (Wadia and Dowdy, 2005) allows for consistent transduction into multiple cell types (Schwarze et al., 1999). The anti-apoptotic BH4 domain of Bcl-xL has also been fused to the Tat–peptide (Sugioka et al., 2003) , providing an additional tool to asses the anti-apoptotic activity of Bcl-xL. Thus, Tat-Bcl-xL is a useful tool to evaluate the long-term effects of exogenously administered Bcl-xL into the injured rat spinal cords. In the present work, we found that administration of exogenous Bcl-xL (Tat-Bcl-xL) and its anti-apoptotic domain BH4 (Tat-BH4-Bcl-xL) into the injured spinal cord decreased apoptotic cell death 24h and 7 days after SCI. However, long-term administration (7 days) of exogenous Bcl-xL impaired locomotor recovery and increased neuronal losses to a greater extent than SCI alone. Furthermore, long-term administration of Tat-Bcl-xL significantly increased microglial/macrophage levels in injured spinal cords compared to vehicle-treated SCI rats, suggesting that there is an enhanced inflammatory reaction induced by the Tat-Bcl-xL treatment perhaps resulting from increased survival of activated microglia and macrophages. Taken together, these results would suggest that delayed effects of anti-apoptotic therapy may be pro-inflammatory and detrimental over time, although the initial effects 24h after SCI could be beneficial.
METHODS
Expression and purification of Tat-Bcl-xL fusion protein and Tat-BH4 peptide
The P-Tat-HA-Bcl-xL expression vector (a generous gift from Dr. R.Pastori, Miami, FL, with permission of Dr. S.F. Dowdy, St Louis, MO) was generated by cloning the coding region of human Bcl-xL in frame with the TAT peptide into the pTAT-HA bacterial expression vector (Klein et al., 2004). The vector pTAT-HA has an N-terminal 6-histidine leader followed by the 11-amino-acid TAT-protein transduction domain, a hemaglutinin (HA) tag and a polylinker (Nagahara et al., 1998). To produce the fusion protein, the plasmid was transformed into E. coli BL21 competent cells (Novagen, Madison, WI) and incubated overnight on Carbenicillin (100ug/ml, Invitrogen, La Jolla, CA)-selective LB plates. A single colony was inoculated in LB selective medium and protein expression was induced by incubation with IPTG (1mM final concentration, Roche Applied Science, Germany) for 1h. Bacteria were lysed by sonication and denatured in 8M urea. The supernatant was subjected to metal affinity chromatography using a Ni-NTA column (Quiagen, Valencia, CA). Salt was removed by gel filtration and protein identity was confirmed by western blotting using antibodies against Bcl-xL and the HA-tag as described below. This procedure was performed by the protein expression and purification core facility at University of Texas Medical Branch (UTMB). The Tat-BH4 peptide HIV-TAT48–57-β-Ala-Bcl-xL BH44–23 (Calbiochem, La Jolla, CA) containing the conserved N-terminal homology domain (BH4) of Bcl-xL (amino acids 4 – 23) is linked to a 10-amino acid HIV-TAT48–57 sequence with a β-alanine residue as a spacer. Tat-Bcl-xL and Tat-BH4 were dissolved in saline (vehicle) and filtrated throughout a 0.2 um sterile filter.
Spinal Cord Injury
Weight-matched Sprague Dawley male rats (175–200g, 200–225g) were obtained from Harlan Laboratories (Indianapolis, IN) and housed at UTMB Animal Care facilities until surgery-weight was reached (225–240g). All rats were anesthetized with 35 mg/kg pentobarbital (Nembutal Sodium, Abbott Laboratories, Chicago, IL) intraperitoneally, and subjected to laminectomy over spinal segments T10 and T13-L1. A moderate spinal contusion injury over the spinal segment T10 was performed with the Infinite Horizon spinal cord impactor (single impact, 150 Kdynes producing a 0.8mm displacement) as described previously (Nesic-Taylor et al., 2005).
Avoiding damage to the spinal cord, the dura was raised with an extrafine forceps and cut with fine scissors. Sterilized polyethylene tubing (32G/PE-60; Rethaco, LLC, Allison Park, PA) was inserted into the intrathecal space through the punctured dura at T13-L1 and extended so that the tip of the catheter was directly beneath the T11 vertebrae. The catheter was connected to a primed mini-osmotic pump (Alzet, Durect Co. CA) that was placed in a subcutaneous pocket made over the sacral vertebrae caudal to the incision. The catheter was secured by suture and superglue to both the L1-L2 vertebral junction and the fascia over the paravertebral muscles at the incision margin, the wound was closed by suturing muscle and fascia and the skin closed with surgical staples. Following injury, animals were injected subcutaneously with 5mL of 0.9% sterile saline and placed on a heating pad to maintain body temperature. Animals received prophylactic antibiotic (Baytril, 2.7mg/Kg twice a day for 5 days), analgesic (Buprenorphin, 0.1mg/Kg twice a day for 3 days) and saline (5ml, daily for 5 days) to prevent dehydration. Bladders were voided manually twice a day until normal function returned. Sham-treated animals were exposed to the same procedure without the contusion injury. All procedures complied with the recommendations in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UTMB Animal Care and Use Committee.
Intrathecal delivery of drugs in the rat spinal cord
Tat-Bcl-xL has been shown to cross the blood brain barrier when delivered intravenously (Kilic et al., 2002) or intraperitoneally (Cao et al., 2002;Yin et al., 2006). However, in order to reach an optimal concentration of Tat-Bcl-xL in the brain, those studies used a dose which was not feasible for the size and number of the adult rats used in our SCI-model. Thus, we use intrathecal delivery of Tat-Bcl-xL, Tat-BH4 or vehicle as summarized in Table 1. The specific doses and delivery rate of either drug or vehicle into the spinal cord were achieved by connecting the external catheter (PE-60) to miniosmotic pumps (Alzet, Durect Co. CA) 1003D (for 24h delivery) or 1007 days (for 7 days delivery). To prime the pumps, the interior container was filled with either Tat-Bcl-xL, Tat-BH4 or saline and incubated overnight at 37°C. Animals surviving for 60 days were anesthetized and the catheter was retrieved from the spinal cord by day 7. Post-surgical antibiotic and pain relievers were administered as previously described.
Table 1.
Description of experimental groups
| Time after injury |
Groups | Drug delivery |
n (ELISA, WB) |
n (H,IHC) |
n (BBB) |
Total |
|---|---|---|---|---|---|---|
| 24h | Sham + Vehicle | 1ul/hr for 24h | 4 | 3 | NA | 7 |
| Sham + Tat-Bcl-xL | 10ug/day | 4 | − | NA | 4 | |
| Injury + Vehicle | 5 | 3 | NA | 8 | ||
| Injury + Tat-Bcl-xL | 5 | 3 | NA | 8 | ||
| 7days | Sham + Vehicle | 0.5ul/hr for 7 | 7 | 3 | NA | 10 |
| Injury + Vehicle | days | 7 | 3 | NA | 10 | |
| Injury + Tat-Bcl-xL | 5ug/day. | 7 | 3 | NA | 10 | |
| Injury + Tat-BH4 | 7 | 3 | NA | 10 | ||
| 60 days | Sham + Vehicle | 0.5ul/hr for 7 | 3* | 3 | 10 | 10 |
| Injury + Vehicle | days | 3* | 3 | 10 | 10 | |
| Injury + Tat-Bcl-xL | 5ug/day. | 3* | 3 | 8# | 10 | |
| Injury + Tat-BH4 | 3* | 3 | 10 | 10 | ||
| TOTAL | 48 | 105 | ||||
Only WB analysis but no Cell death ELISA was performed in this group.
An additional injury at the site of catheter implantation was observed at the time of perfusion in two Tat-Bcl-xL treated animals. Data from these animals were omitted from the analysis. n(ELISA, WB), number of animals for which apoptotic cell death was measured by ELISA and analysis of Bcl-xL levels were quantified by western blot. n(H, IHC), number of animals used for histology and immunohistochemistry analysis. n (BBB), number of animals for which BBB scores were obtained. NA, Not applicable
Protein extraction and subcellular fractionation
For protein extractions, rats were intracardially perfused with PBS and a 1cm segment centered at tissue epicenter (T10, site of injury) was removed and immediately frozen in liquid nitrogen. The tissue was homogenized in ice-cold Buffer M (250 mM sucrose, 20 mM Hepes, 1 mM EDTA,1 mM EGTA, 1 mM dithiotheitol, 1 mM PMSF, pH 7.4, protease inhibitor cocktail and phosphatase inhibitor cocktail 2 (Sigma, Saint Louis, MO) using a Dounce homogenizer. To obtain different subcellular fractions the homogenate was centrifuged three times at 800 × g for 20 min (Eppendorf 5810R centrifuge) to collect nuclei and cell debris (pellet). The supernatant was set aside and the pellets collected at each step were pooled and washed two times with 500 µl of Buffer M to separate the nuclei from complete cells and cytosolic proteins. Nuclear pellets were mixed in a vortex plate at 1,400 rpm, 4°C for 20 min in 70µl of nuclear extraction buffer (10 mM Hepes, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 2 µg/mL antipain, 2 µg/mL chymostatin, 2 µg/mL pepstatin, 2 µg/mL leupeptin). After centrifuging at 10,000 × g for 10 minutes, the nuclear proteins contained in the supernatant were aliquoted and the pellet discarded. The supernatant containing cytosolic proteins and organelles other than nuclei was centrifuged at 100.000 × g for 1h. The resultant pellet, containing mitochondria and Endoplasmic reticulum (ER), was resuspended in 100µl of mitochondrial extraction Buffer (Active Motif CN40015, Carlsbad CA). All procedures were performed at 4°C. Protein concentrations were calculated with the BioRad Protein Assay following the recommended protocol of the manufacturer (BioRad, Hercules, CA).
Western Blotting
Protein extracts were boiled for 5 min in Laemmli buffer (100 mM Tris, pH 6.8, 250 mM 2-mercaptoethanol, 4% SDS, 0.01% bromophenol blue, 20% glycerol). Equal amounts of protein (40 µg) were separated by using 10%–15% SDS-polyacrylamide gel electrophoresis and electrotransferred overnight (4°C, 25 volts) onto a Immobilon-P® membrane (Millipore, Billerica, MA). Membranes were then blocked in 5% nonfat milk in PBS and then probed with different antibodies. Endogenous Bcl-xL was detected using a rabbit polyclonal anti-Bcl-xL (1:2000, Cell signaling, Danvers, MA) while exogenous Tat-Bcl-xL was detected by using a rabbit polyclonal anti- HA-tag (1:4000, Abcam, Cambridge, MA) diluted in 1% Blocking buffer for 1h at room temperature. After washing, membranes were incubated with secondary anti rabbit IgG conjugated with HRP (Bio Rad, Hercules, CA) for 1h. Visualization of the proteins was accomplished using an enhanced chemiluminescence detection kit (ECL, Amershan Biosciences, UK). The relative amount of immunoreactive protein in each band was determined by scanning densitometric analysis of the X-ray films. Autoradiographs were scanned (canonScan 4200F) and densitometry was performed with AlphaEasy v5.5 Software. Density readings were normalized against control samples on the same-blot. When membranes were reprobed, the bound antibodies were incubated in stripping buffer (Pearce, Rockford, IL) for 15 min, followed by two washes in TBS for 20 minutes.
Measurement of apoptotic cell death by ELISA
Levels of apoptotic cell death 24h and 7 days after spinal cord injury were examined by commercially available sandwich technique ELISA kit (Roche Applied Science, Germany). The assay measures the amount of oligonuclesomes released to the cytosol, an event that occurs during apoptotic cell death, but not during necrotic processes. Briefly, 80 µg of cytosolic extract from spinal cords were added to ELISA microplates covered with an anti-histone antibody. Complexes formed by the antibody and histones present in cytosolic oligonucleosomes were detected by a second peroxidase-conjugated antibody against DNA. Oxidized peroxidase enzymatic products in the microplate wells were read at 405 nm absorbance in a MRX Microplate Reader (Dynex Technologies, INC).
Spinal cord processing for histological analysis
Rats were intracardially perfused with 300ml of 0.1M PBS, followed by 500 mL of 4% paraformaldehyde in 0.1 M phosphate buffer. The spinal cords were removed and postfixed in 4% paraformaldehyde for 2 h at 4°C, then rinsed and cryoprotected in 30% sucrose in phosphate buffer for 48 h at 4°C. Spinal cords were cut in 1.5cm segments centered at the lesion site and equivalent segments of different experimental groups were embedded in a single block in OCT (Optima Cutting Temperature) medium (Fisher Scientific, Suwannee, GA). Transverse serial sections (10µm) through the complete segment were mounted on glass-slides and frozen at –20°C.
Immunofluorescence staining
Slides were rinsed three times in Tris-phosphate-buffer 0.3 % Triton-X (TBST), pH 7.4, for 10 min and then blocked with 5% normal goat serum, 1% BSA (Sigma, St. Louis, MO) TBS for 30 min at room temperature (RT). The sections were incubated overnight with IgG primary antibodies diluted in TBST 1% BSA, 1% normal goat serum as indicated. Mouse monoclonal antibody recognizing neurons (NeuN, 1:5000, Chemicon, Temecula, CA), was used in combination with rabbit polyclonal anti HA-tag against exogenous Tat-Bcl-xL (1:1000, Abcam, CA). After rinsing three times in TBS for 10 min, the slides were incubated with secondary anti-rabbit IgG AlexaFluor 568 and anti-mouse IgG AlexaFluor 488 (1:1000, Molecular Probes, Eugene, OR) diluted in TBST for 1h. Sections were coverslipped using mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Negative controls omitting the primary antibodies were performed each time. Imaging was performed using laser scanning confocal microscopy (Nikon Eclipse 800 with Roper CoolSnap FQ monochrome digital camera, Nikon DXM1200 color digital camera and Laser sharp 2000 software).
Microglia and macrophage immunohistochemistry
Frozen sections were dried for 2h at room temperature followed by 2h at 37C. After rinsing with 0.2M PB for 1 min, sections were blocked with 4% horse serum (HS) in 0.1M PBS for 1h at room temperature. Mouse monoclonal antibody against OX-42 (1:400; Serotec) diluted in 0.1M PBS 1% HS was incubated overnight at 4C in humidified chambers. After rinsing, sections were incubated overnight with biotinylated secondary antibody (rat-preabsorbed, 1:400 in 0.1M PBS; Vector Laboratories, Burlingame, CA). After rinsing, endogenous peroxidase acitivity was quenched by incubating with 6% H202/methanol for 15 min. The reaction was visualized with Elite ABC-reagent for 1h (Vector Laboratories, Burlingame, CA) followed by DAB substrate (Vector Laboratories, Burlingame, CA). Sections were dehydrated in ascending alcohols, cleared in xylene and mounted in synthetic resine (Permount).
Quantitative analysis of immunohistochemistry
Neuronal survival was evaluated by counting Neu N staining cells at the dorsal and ventral horn 4mm rostral to the lesion epicenter. Total number of NeuN/DAPI staining cells at the dorsal and ventral horn in a 20X field of two sections spaced by 200µm were counted and averaged per animal.
Microglia/macrophage density analysis was performed by measuring the proportional area of immunoreactive cells relative to the total sample area as reported by Popovich et al (2006). The immunoreactivity expressed in a defined area has been shown to be an accurate measurement for changes in number and size of labeled microglia in the rat spinal cord, and a reliable marker for microglial/macrophage activation (Popovich et al., 1997). Briefly, images of three consecutive sections at the lesion epicenter or 4mm rostral to the epicenter were stained with OX-42 and analyzed using the Image Pro-Express analysis system. At the lesion epicenter, the intensity of OX-42 staining over a 6.25mm2 area (containing all the cross section of the cord) was measured for three consecutive sections per animal. At the rostral sections, intensity of OX-42 staining in a 6.25mm2 area (total OX-42 labeling) or a 0.0625mm2 area was measured at the dorsal horn, ventral horn and lateral funiculus in 3 consecutive sections per animal. The final “area of staining” for each animal, represents the average of values obtained for the 3 consecutive sections at each given area.
White matter sparing analysis
Luxol-fast blue staining was used to distinguish spared myelin from gray matter and lesioned tissue. Serial sections cut over the rostrocaudal extent of the lesion were incubated with 0.1% Luxol for 30 min at 70°C; then differentiated with lithium carbonate and 70% ethanol. After counterstaining with hematoxilin-eosin, slides were dehydrated in increasing alcohols and coversliped in permount mounting medium. The injury epicenter was defined as the site with the least amount of spared white matter. White matter sparing was defined as tissue showing normal myelin appearance and density (lacking cysts, degeneration). The average area of spared myelin was calculated from images of three Luxol-fast blue stained sections containing the lesion epicenter. Images were digitized with an Olympus BX-41 microscope and area calculation was obtained by using an Image analysis system (Image Pro-Express Version 4.0. Media Cybernetics, Silvers spring, CA). To eliminate fixation–dependent artifacts in spinal cord cross-sectional area, spared myelin at the impact site was expressed relative to the area of white matter measured 1 cm rostral to the site of injury (Olby and Blakemore, 1996;Popovich et al., 1997).
Behavioral assessment
The locomotor function of SCI rats was measured using the BBB open field locomotor scale, a 21 point ordinal scale that assign scores for right and left hindlimb performance based in well defined behavioral categories (Basso et al., 1995).) The BBB scale can be analyzed using parametric statistics over a portion of the scale (scores 1–14), when a transformation that pools scores 2–4 and 14–21 is applied in a post-hoc fashion (Scheff et al., 2002;Ferguson et al., 2004). Since the application of such a transformation improves the statistical power of the BBB measurements in the lower part of the scale, corresponding to animals with moderate injuries (Ferguson et al., 2004), we used the transformed BBB scale to measure the effects of Tat-Bcl-xL and Tat-BH4 treatment in the locomotor recovery in SCI-treated rats. BBB locomotor testing was performed daily for the first 14 days after injury and once every two weeks thereafter for 6 weeks. Rats were tested pre-operatively and trained to locomote in an open field. All the animals were coded, and behavioral analyses were performed for an investigator blinded with respect to the treatment groups. The BBB scores for right and left hindlimb per animal were transformed using the above mentioned algorithm (available at http://graulab.tamu.edu/ BBBtransformation.html) (Ferguson et al., 2004) and then averaged to obtain a Combined BBB score. The means of combined BBB scores were tallied by groups and plotted as functions of time after injury.
Statistical analysis
Western blot densitometric values, immunohistochemical and morphometric data were evaluated by using one one-way ANOVA, followed by Tukey’s post- hoc analysis. Changes in BBB scores over time were analyzed using repeated-measures two-ways ANOVA (groups and time after injury as the two factors), with Bonferoni post-hoc corrections. Results were considered statistically significant at P<0.05. All data points represent group mean ± SEM.
RESULTS
1. Intrathecal administration of Tat-Bcl-xL increases total Bcl-xL levels in injured spinal cords
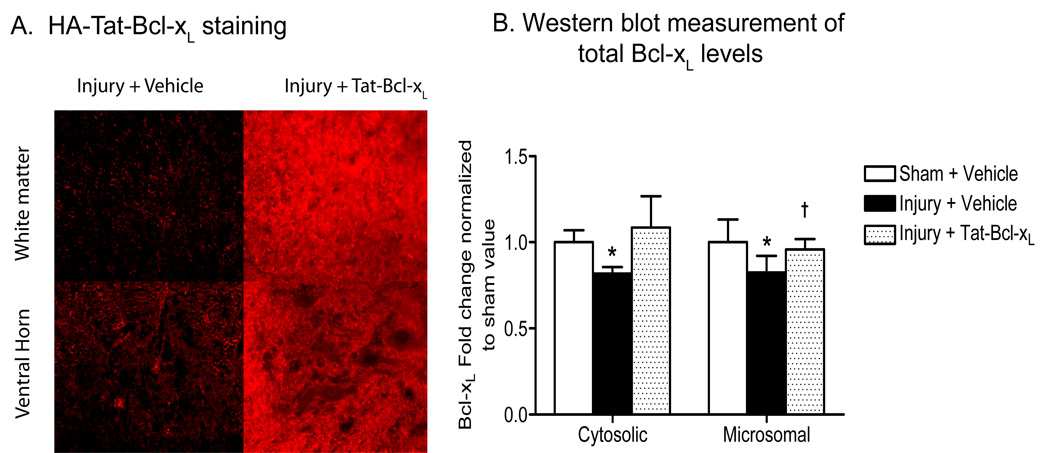
To examine the ability of intrathecally delivered Tat-Bcl-xL to transduce cells located in deep layers in the spinal cord, we delivered 10ug of Tat-Bcl-xL (1µl/hr, over 24h) or vehicle into the intrathecal space of contused-spinal cord rats, and measured the levels of exogenous Tat-Bcl-xL by immunohistochemistry and Western blot assays. Immunofluorescence labeling using an antibody against the hemaglutinin (HA) -tag present in the fusion protein, showed Tat-Bcl-xL to be throughout white and gray matter (Fig. 1 A) in transverse spinal cord sections located 3mm rostral to the lesion epicenter (T10), 24h after trauma.
Fig. 1. Protein transduction of Tat-Bcl-xL in the injured spinal cord 24h after trauma.
A. Immunofluorescence staining of a transverse section of spinal cord 3mm rostral to the lesion epicenter, 24h after injury, using an antibody against HA-tag. The HA immunoreactivity was strong in gray and white matter in Tat-Bcl-xL-treated, but not vehicle-treated spinal cords. Scale bar: 50 µm. B. Quantitation of cytosolic and microsomal Bcl-xL levels based on western blot analyses. We used an antibody against a sequence surrounding Asp61 in the loop region of Bcl-xL that recognized both endogenous Bcl-xL and Tat-Bcl-xL. Total Bcl-xL levels at cytosolic and microsomal fractions increased in Tat-Bcl-xL-treated animals to levels comparable to those of sham-treated cords. Bcl-xL expression values were normalized to sham values and presented as mean ± SD. n=4/5 per group as indicated in Table 1. * p<0.05 compared to sham-vehicle group; † p<0.05 compared to the injury-vehicle treated group; Two way ANOVA with Tukey’s post-hoc correction).
We have shown that SCI induces decreases in Bcl-xL levels in cytosol and mitochondria that correlate with apoptotic cell death of neurons occurring 24 h after trauma (Nesic-Taylor et al., 2005). To determine if the delivery of exogenous Tat-Bcl-xL counteracts SCI-induced decreases in Bcl-xL, we performed Western blot analysis of Bcl-xL levels in cytosolic and microsomal extracts (containing mitochondria and endoplasmic reticulum) of 1 cm long spinal cord segments that contained the site of injury T10 (Fig. 1 B). We analyzed spinal cords from three groups of rats: sham-treated (sham) rats that received vehicle for 24h, SCI-treated (SCI) rats that received vehicle, and SCI-treated rats also treated with Tat-Bcl-xL. As expected, SCI induced decreases in Bcl-xL protein levels, while Tat-Bcl-xL treatment restored Bcl-xL levels in SCI-treated rats to levels compared to those of sham-treated rats, in both cytosolic and microsomal fractions (Fig. 1 B).
2. Anti-apoptotic effects of Tat-Bcl-xL
A) 24h after SCI
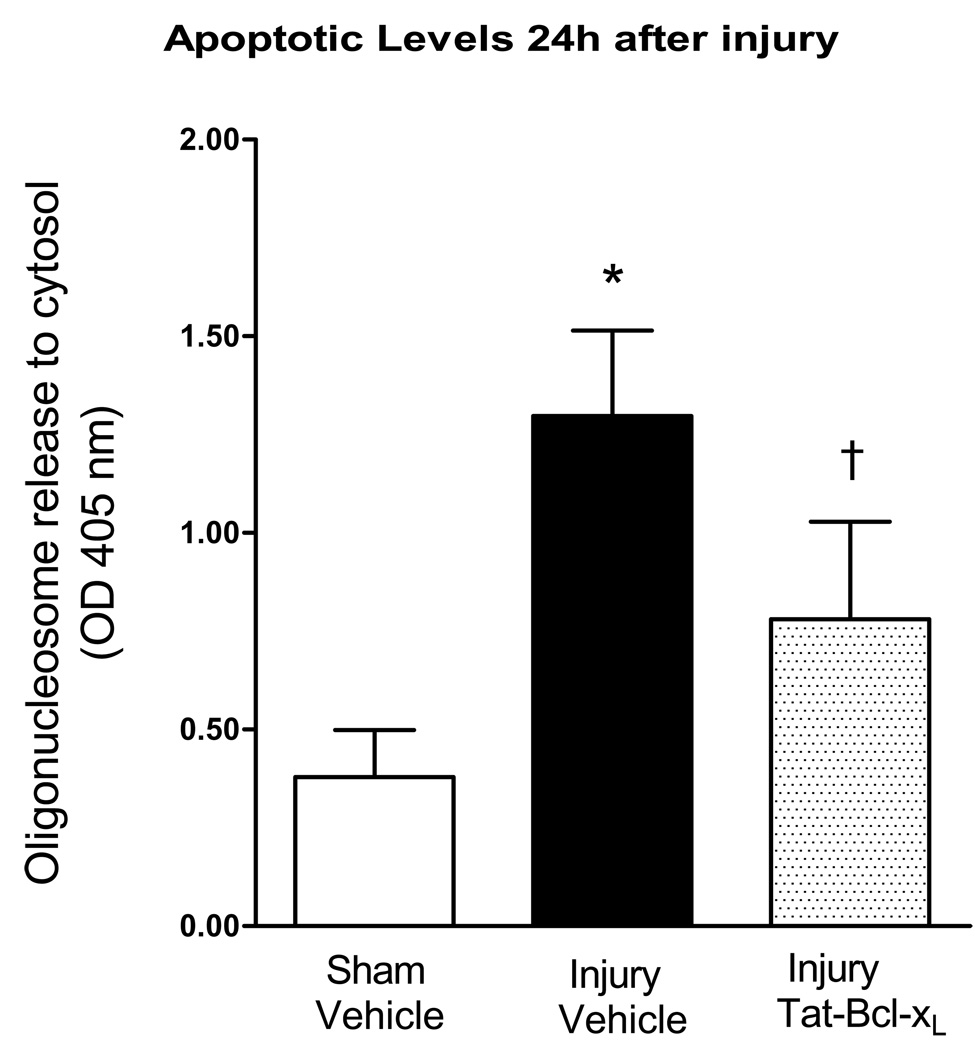
To examine the antiapoptotic activity of Tat-Bcl-xL, we measured the levels of oligonucleosomes in the cytosol of uninjured and injured spinal cords, using an Elisa cell death assay. A total of 10µg of Tat-Bcl-xL, or vehicle, was intrathecally delivered over 24h after SCI (1µl/hr, 24µl). The presence of cytosolic oligonucleosomes was tested in protein extracts of thoracic spinal cords segments (1 cm long) containing the site of injury (T10). Vehicle-treated injured spinal cords showed significant increases in cytosolic oligonucleosomes when compared to sham rats treated with vehicle (Fig. 2), in agreement with our earlier reports (Nesic et al., 2001;Qiu et al., 2001;Nesic-Taylor et al., 2005) that showed that significant apoptotic cell death occurs during the first 24 h after injury. As expected, Tat-Bcl-xL treatment significantly decreased levels of cytosolic oligonucleosomes, confirming the anti-apoptotic effectiveness of Tat- Bcl-xL.
Fig. 2. Tat-Bcl-xL decreased apoptosis 24h after injury.
A. The release of oligonucleosomes to the cytosol during the first 24h after trauma was evaluated in cytoplasmic extracts from spinal cords isolated from three groups of rats: (a) sham-and vehicle treated (n=4); (b) SCI+vehicle (n=5); or (c) SCI+Tat-Bcl-xL (n=5). In all measurements we used 1cm-long thoracic segments containing the site of injury, T10. Apoptotic cell death was significantly reduced in Tat-Bcl-xL treated rats in comparison to vehicle-treated rats. Data represent mean ± SD. * p<0.05 compared to sham-vehicle group; † p<0.05 compared to the injury-vehicle treated group; Two way ANOVA with Tukey’s post-hoc correction).
B) Seven days after SCI
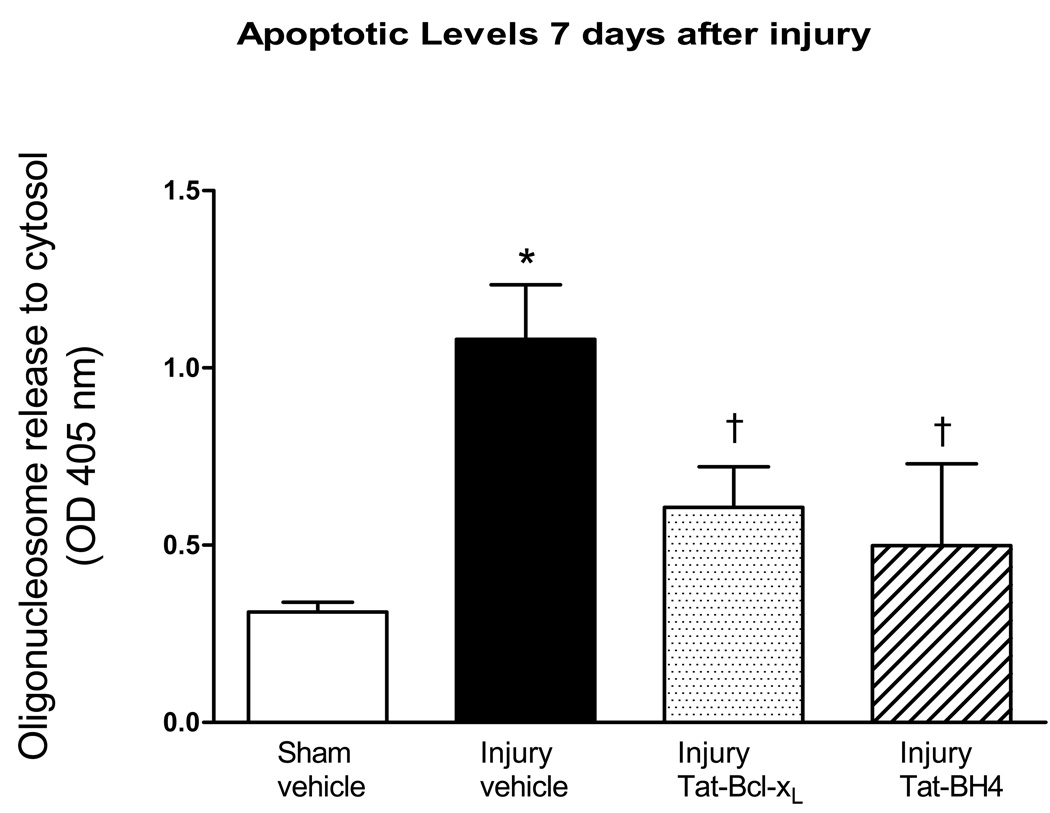
To assess the effects of longer lasting administration of Tat-Bcl-xL to counteract late SCI-induced Bcl-xL decreases, we intrathecally delivered 35ug of Tat-Bcl-xL at a rate of 0.5ul/hr for 7 days (5ug/day). Cytosolic fractions were extracted from the 1 cm-spinal cord segments containing the epicenter of the lesion (T10). In agreement with our previous results (Fig. 1), Tat-Bcl-xL administration significantly increased cytosolic levels of Bcl-xL at 7 days (1.67±0.32 fold increase in comparison with SCI-vehicle treated cords, p<0.05; n=7 per group). As shown in Fig. 3, cytosolic oligonucleosomal levels were significantly reduced after Tat-Bcl-xL treatment.
Fig. 3. Tat-Bcl-xL and Tat-BH4 decreased apoptosis 7 days after injury.
Elisa assays were use to measure the levels of oligonucleosomes in the cytosol 7 days after SCI in cords isolated from four groups of rats: (a) sham-and vehicle treated (n=7); (b) SCI+vehicle (n=5); or (c) SCI+Tat-Bcl-xL (n=5); and (d) SCI+Tat-BH4 (n=5). In all measurements we used 1cm-long thoracic segments containing the site of injury, T10. Both Tat-Bcl-xL and Tat-BH4 treatment significantly decreased apoptosis compared to vehicle-treated animals. Data represent mean ± SD. * p<0.05 compared to sham-vehicle group; † p<0.05 compared to the injury-vehicle treated group; Two way ANOVA with Tukey’s post-hoc correction).
C) Tat-Bcl-xL vs. Tat-BH4
We have shown that SCI induces phosphorylation of endogenous Bcl-xL, and thus possibly inactivates anti-apoptotic effects of Bcl-xL (unpublished results). Therefore, we hypothesized that some fraction of the exogenous Tat-Bcl-xL may also undergo phosphorylation and thus prevent its full anti-apoptotic effect. To assess whether phosphorylation diminishes the anti-apoptotic effect of Tat-Bcl-xL, we used a Tat-BH4 peptide, a construct that contains only the BH4 (Bcl-2 homology 4) antiapoptotic domain of Bcl-xL; and measured its ability to prevent apoptosis in the injured spinal cords. A total of 35ug of Tat-BH4 was intrathecally delivered at a rate of 0.5ul/hr (5ug/day) for 7 days and cytosolic fractions extracted as previously described (see Fig. 2). As shown in Fig. 3, Tat-BH4 induced decreases in cytosolic oligonucleosomes levels to a similar extent to the Tat-Bcl-xL treatment. This result would suggest that significant phosphorylation of Tat-Bcl-xL is unlikely, and that the full anti-apoptotic effect of the exogenously applied Bcl-xL was achieved.
3. Effect of Tat-Bcl-xL and Tat-BH4 on locomotor recovery
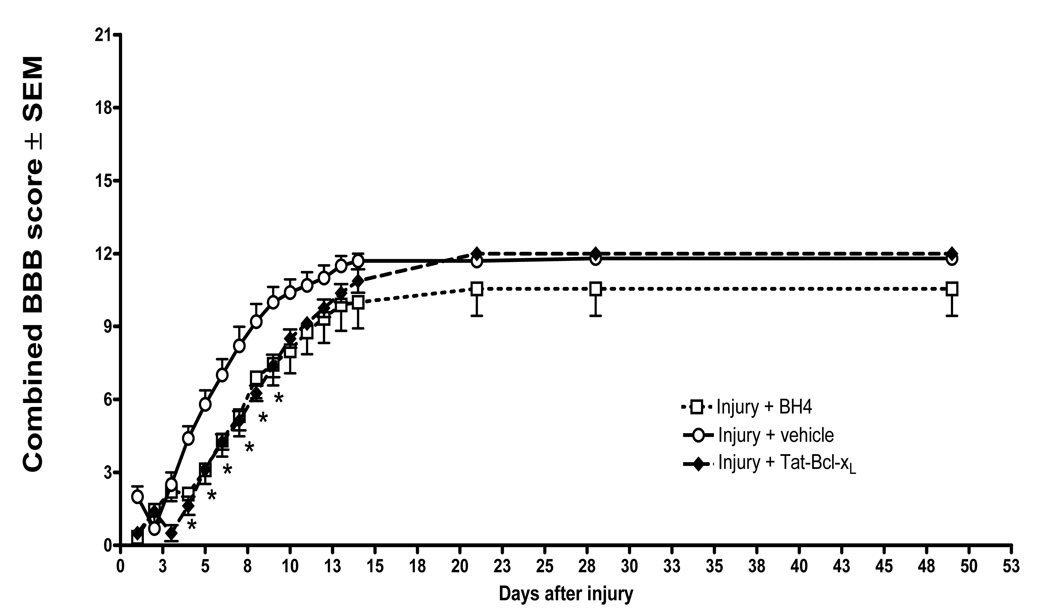
It is known that treatments that significantly spare spinal cord tissue after SCI also improve locomotor recovery (Pearse et al., 2004;Colak et al., 2005;Yates et al., 2006). To evaluate whether antiapoptotic activity of Tat-Bcl-xL and Tat-BH4 had an effect on hindlimb locomotor recovery after SCI, we intrathecally administered Tat-BH4 or Tat-Bcl-xL (5ug/day) to injured spinal-cords for 7 days after SCI. Locomotor function was measured daily for 14 days, and then biweekly for 60 days. Vehicle-treated sham rats did not show significant impairments in locomotor function at any time. Consistent with published reports, an injury induced with 150 Kdynes impact force caused complete paralysis of the hind limbs in the first days after SCI that partially improved over time, as reflected in the increased BBB scores over a two month period (Fig. 4). However, locomotor recovery of SCI-rats treated with either Tat-Bcl-xL or Tat-BH4 did not improve, but rather worsened in comparison to vehicle-treated SCI rats. As shown in Fig. 4, BBB scores were significantly lower from day four to day nine (P<0.05) in both Tat-Bcl-xL and Tat-BH4 treated animals.
Fig. 4. Impaired locomotor recovery in Tat-Bcl-xL and Tat-BH4-treated rats.
Transformed BBB scores for left and right hindlimb per animal were averaged and analyzed as combined score. Plotted data represent the mean ±SEM per time point of combined BBB score of Tat-Bcl-xL (n=8); Tat-BH4 (n=9) and vehicle treated (n=9) injured rats. Rats treated with either Tat-Bcl-xL or Tat-BH4 showed decreased locomotor recovery during the first 10 days after injury in comparison to vehicle treated animals. (*) p<0.05 compared to vehicle –treated group. Transformed data were analyzed using Two-ways Repeated measures ANOVA with Bonferoni post-hoc corrections).
4. Effect of Tat-Bcl-xL and Tat-BH4 on microglia/macrophage activation
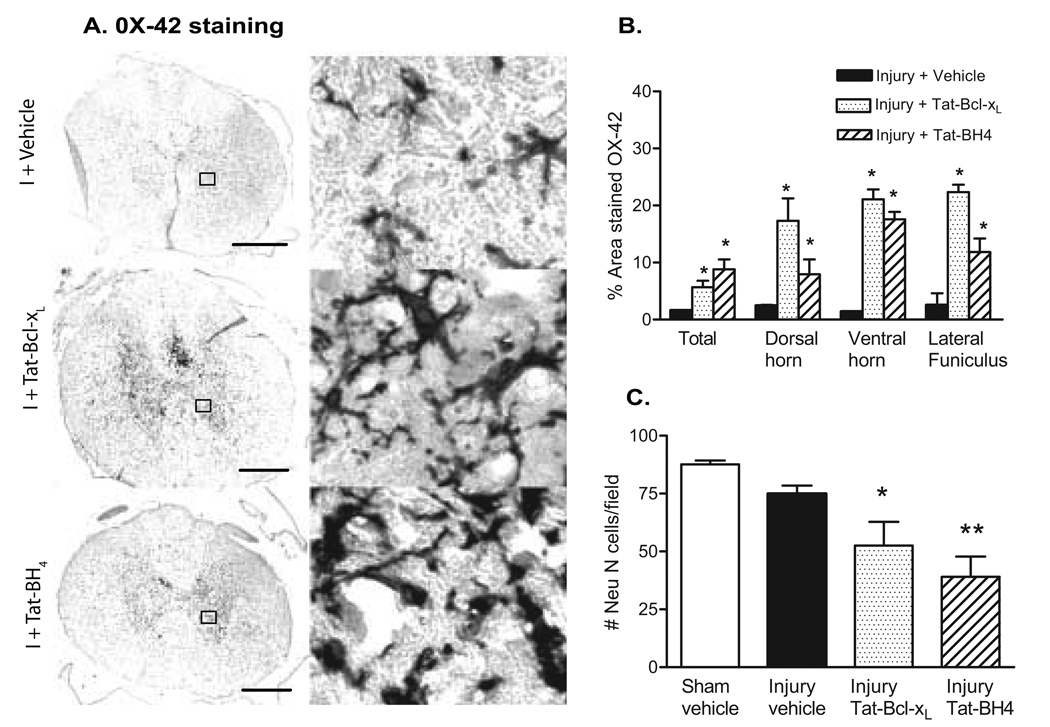
To test the hypothesis that both Tat-Bcl-xL and Tat-BH4 induced increased inflammatory responses and additional tissue damage/worsening of functional recovery, we measured the density of microglia/macrophages 4 mm rostral to the lesion epicenter (the same region where we counted the number of neurons), by measuring the proportional area of cells expressing OX-42, corresponding to the area of tissue occupied by immunohistochemically-stained cellular profiles within a defined target area (Popovich et al., 1997). As shown in Fig. 5 A , B (left panel), SCI rats treated with either Tat-Bcl-xL or Tat-BH4 showed a robust and significant increase in the total intensity of OX-42 staining in a 6.25 mm2 area (containing all the cross section of the cord) in comparison to vehicle-treated injured spinal cords, indicating an increased inflammatory reaction in Tat-Bcl-xL and Tat-BH4 treated SCI rats. Furthermore, consistent with the spatial and temporal profile of microglial/macrophage activation/infiltration after rat SCI (Popovich et al, 1997), an increased OX-42 immunolabeling in a 0.0625 mm2 area at the dorsal horn, ventral horn and lateral funiculus was observed rostral to the lesion epicenter 7 days after injury (Fig. 5 A). However, OX-42 immunolabeling was significantly higher in Tat-Bcl-xL and Tat-BH4 treated SCI rats. Intense OX-42 labeling in gray matter was observed surrounding neurons in the damaged spinal cords. In treated cords, OX-42 labeling stained hypertrophic cell bodies with short pseudopodic processes or round cells presenting morphology of activated microglia/macrophages (Fig. 5 B, right panel).
Fig. 5. Neuronal and microglial/macrophage densities after Tat-Bcl-xL and Tat-BH4 treatment.
A. Semi-quantitative analysis of OX-42 staining 4mm rostral to lesion epicenter in Tat-Bcl-xL- (n=3); Tat-BH4- (n=3) and vehicle-treated rats (n=3) 7 days after SCI. Data represent the proportional area of OX-42 staining in a 6.25mm2 region (total OX-42 labeling) or a 0.0625mm2 region at the dorsal horn, ventral horn and lateral funiculus. (See methods for details of quantification methods). B. Representative example of OX-42 staining of microglia and macrophages 4mm rostral to the lesion epicenter, 7 days after injury. Left panel: cross sectional OX-42 staining showing increased microglial/macrophage activation in Tat-Bcl-xL- and Tat-BH4- treated injured spinal cords in comparison to vehicle-treated injured spinal cords. Scale bar: 350µm Right panel: high magnification of the area marked in the left panel as squares, shows intense labeling of OX-42 in long processes and round cell bodies surrounding gray matter cells, likely neurons, in Tat-Bcl-xL- and Tat-BH4-treated injured spinal cords, and a moderate OX-42 labeling in vehicle-treated injured spinal cords. C. Quantitative analysis of NeuN-labeled cells in the dorsal and ventral horn. Neurons were counted 4mm rostral to the lesion epicenter in sections of Tat-Bcl-xL(n=3), Tat-BH4 (n=3) and vehicle-treated rats (n=3) 7 days after SCI. The number of Neu-N-positive cells was significantly lower in the Tat-Bcl-xL- and Tat-BH4-treated injured spinal cords in cord (*p<0.05). Sections are adjacent to those used for OX-42 analysis.
5. Effect of Tat-Bcl-xL on neuronal loss
To evaluate whether increased microglial activation in Tat-Bcl-xL-or Tat-BH4 -treated SCI rats, affected neuronal loss, we counted the number of neurons labeled with the neuronal specific marker, NeuN in sections located 4 mm rostral to the lesion epicenter. As shown in Fig. 5C, the number of neurons was significantly lower in the Tat-Bcl-xL- and Tat-BH4-treated SCI rats, compared to the vehicle-treated-SCI rats. This result suggests that while anti-apoptotic treatment protected neurons from apoptotic cell death, it did not prevent them from dying, likely due to necrosis. Thus, it is possible that long term exposure to Tat-Bcl-xL or Tat-BH4 shifted neuronal death from apoptosis to necrosis, and thus amplified neuronal death due to necrosis-induced inflammatory reactions.
6. Effect of Tat-Bcl-xL and Tat-BH4 on white matter sparing
Given that Tat-Bcl-xL and Tat-BH4 increased inflammation/microglial activation and neuronal loss, we further evaluated whether Tat-Bcl-xL and Tat-BH4 also affected white matter sparring at the lesion epicenter, as described in methods. As shown in Table 2, neither Tat-Bcl-xL nor Tat-BH4 treatment had a significant effect on the amount of spared white matter when compared to vehicle-treated spinal cords, at both 7 and 60 days post-injury; suggesting that Tat-Bcl-xL and Tat-BH4-induced worsening of the locomotor function does not result from more extensive white matter damage.
Table 2.
Spared white matter after Tat-Bcl-xL and Tat-BH4 treatment.
| Time after Injury |
Injury + Vehicle (n=3) |
Injury + Tat-Bcl-xL (n=3) |
Injury + Tat-BH4 (n=3) |
P | |
|---|---|---|---|---|---|
|
Spared White matter * |
7 days | 0.230 ± 0.05 | 0.28±0.03 | 0.32±0.04 | 0.110 |
| 60 days | 0.291±0.12 | 0.32±0.15 | 0.248±0.06 | 0.068 |
White matter sparing was defined as tissue showing normal myelin appearance and density (lacking cyst, degeneration). To eliminate fixation –dependent artifacts in spinal cord cross-sectional area, spared myelin at the impact site was expressed relative to the area of white matter measured 1 cm rostral to the site of injury.
DISCUSION
Anti-apoptotic Tat-Bcl-xL and Tat-BH4 impaired functional recovery after SCI
Using intrathecal delivery, we demonstrated that Tat-Bcl-xL restored Bcl-xL levels in both cytosolic and microsomal fractions of SCI rats during the 24h or 7 days delivery period, thus confirming that our chosen dose and delivery method of Tat-Bcl-xL were effective. To confirm that the anti-apoptotic effect of Tat-Bcl-xL was due to its role in preserving mitochondrial permeability, we used Tat-BH4 peptide. Bcl-2 and Bcl-xL possesses four conserved Bcl-2 homology (BH) domains, designated BH1 through BH4 (Aritomi et al., 1997;Petros et al., 2004). The BH4 domain of Bcl-xL is essential for the prevention of apoptotic mitochondrial changes (Shimizu et al., 2000;Sugioka et al., 2003). Our results showed that both the Tat-Bcl-xL and Tat-BH4 treatment significantly decreased levels of cytosolic oligonucleosomes to a similar extent, thus confirming that anti-apoptotic effects of Tat-Bcl-xL in injured spinal cords was solely due to its known protective role in mitochondria. We also used the BH4 construct because Tat-BH4 is not susceptible to phosphorylation or cleavage, two processes capable of reducing the anti-apoptotic effects of Bcl-xL (Kharbanda et al., 2000;Brichese et al., 2002;Simizu et al., 2004;Tamura et al., 2004). Bcl-xL possesses an unstructured loop between BH3 and BH4 that contains recognition sites for phosphorylation and caspase-mediated cleavage, mechanisms that appear to regulate the function of Bcl-xL after different insults in multiple cell lines (Fujita et al., 1998;Ojala et al., 2000;Figueroa, Jr. et al., 2003). We have also shown that SCI induces phosphorylation of endogenous Bcl-xL and thus possibly inactivates its anti-apoptotic effect (Cittelly et al., 2005). Therefore, it was possible that a fraction of the exogenous Tat-Bcl-xL undergoes phosphorylation in injured spinal cords, and thus prevents its full anti-apoptotic effect. Our results showed that both Tat-Bcl-xL and Tat-BH4 treatment significantly decreased levels of cytosolic oligonucleosomes to the same extent, suggesting that phosporylation of Tat-Bcl-xL did not occur and that the Tat-Bcl-xL treatment increased local levels of functional Bcl-xL. Thus, the full anti-apoptotic effect of the exogenous Bcl-xL was achieved.
In agreement with other reports (Dietz et al., 2002;Kilic et al., 2002;Hotchkiss et al., 2006), Tat-Bcl-xL significantly reduced total apoptotic death at 24h and 7 days after SCI, thus suggesting that the recovery of functions may be improved in Tat-Bcl-xL or Tat-BH4-treated SCI rats. This expectation was also based on reports on other anti-apoptotic treatments that target Bcl-2 and Bcl-xL and showed beneficial effects on functional recovery after CNS trauma (Cao et al., 2002;Yin et al., 2006). Surprisingly, the recovery of locomotor function of SCI-rats treated with Tat-Bcl-xL or Tat-BH4 did not improve during the first 14 days, but rather worsened in comparison to vehicle-treated SCI rats. After day 14, SCI rats in all groups reached BBB scores above 14, which can not be analyzed with the transformation applied (Ferguson et al., 2004). To the best of our knowledge, this is the first report showing negative effects of long-term anti-apoptotic treatments after SCI.
Tat-Bcl-xL and Tat-BH4 increased neuronal loss and microglial activation without affecting white matter sparing
We have shown that there are significant early decreases in Bcl-xL expression in neurons after SCI (Cittelly et al, in press) and that Bcl-xL administration increases motoneuron survival 24h after injury (Nesic-Taylor et al, 2005). Therefore, we expected that the long term effect of Tat-Bcl-xL administration should protect more effectively neurons thus further increasing their survival. However, we found that the 7 day administration of Tat-Bcl-xL resulted in additional neuronal losses and did not enhance neuronal sparing. Since both Tat-Bcl-xL and Tat-BH4 treatments decreased SCI-induced apoptotic levels at 7 days (Fig. 2), additional neuronal losses are likely due to necrotic cell death, which is directly linked to increased inflammation. It has been shown that necrotic neuronal death in excitotoxic models of SCI results from increased microglial activation in gray matter (Gomes-Leal et al., 2004). Thus, it is possible that the anti-apoptotic activity of Tat-Bcl-xL and Tat-BH4 shifted neuronal death from apoptosis to necrosis, and possibly amplified neuronal death due necrosis-induced inflammatory reactions. Consistent with this hypothesis we found increases in neuronal death in Tat-Bcl-xL- (30%) and Tat-BH4- (48%) treated injured spinal cords compared to vehicle-treated injured spinal cords. Although, double label immunohistochemical analysis of cell type and expression levels of necrotic or apoptotic markers (e.g. TUNEL staining) would be required to confirm our hypothesis, we do have evidence that supports it. In our recent report (Cittelly et al., in press) we showed Bcl-xL expression in neurons and oligodendrocytes, but not other glial cells, in uninjured spinal cords. Furthermore, SCI induced decreases in Bcl-xL expression in neurons, but not in oligodendrocytes. Interestingly, activated microglia/macrophages showed robust expression of Bcl-xL in injured spinal cords. Therefore, it is likely that exogenous administration of Tat-Bcl-xL primarily affects neurons and microglia/macrophage population, consistent with our hypothesis.
Necrosis initiates inflammatory responses via activation of microglia and macrophages, which then release soluble factors, including nitric oxide, free radicals, proteolytic enzymes, arachidonic acid metabolites, tumor necrosis factor, interleukin-1, cyclooxygenase-2 and prostaglandins (Schnell et al., 1999;Popovich et al., 2002;Beattie, 2004;Ahn et al., 2006). A large body of evidence suggests that all these inflammatory agents released by microglia can stimulate neuronal death (Beattie, 2004;Skaper et al., 2006;Gibbons and Dragunow, 2006), and in turn, promote further microglial activation (Gomes-Leal et al., 2004). As shown in Fig 5A, an enhanced labeling of OX-42 in rounded cells and hypertrophic cells with thin processes, is indicative of activated macrophages and microglia in perineuronal spaces surrounding neurons throughout gray matter in the Tat-Bcl-xL- and Tat-BH4-treated SCI rats, compared to vehicle-treated SCI rats. This supports our hypothesis that both anti-apoptotic agents triggered a positive feedback loop involving neuronal necrosis and microglial activation.
Alternatively, it is also possible that Tat-Bcl-xL and Tat-BH4 treatments directly affected microglial/macrophage survival in injured spinal cords. We have found that activated microglia/macrophages robustly expressed Bcl-xL 7 days after SCI (Cittelly et al., in press), and it is known that SCI-induced microglial activation peaks at 7 days after SCI when microglia undergo apoptotic cell death (Popovich et al., 1997). Thus, it is possible that Tat-Bcl-xL and Tat-BH4 decreased microglial/macrophage apoptosis, and increased microglial presence after injury, which may have increased inflammation and thus decreased neuronal survival in the sub-chronic phase after SCI
Decreased neuronal numbers in Tat-Bcl-xL and Tat-BH4-treated SCI rats may reflect increased inflammation, and not be a direct cause for the deterioration of locomotor recovery reported here. Given that locomotor recovery mainly depends on the preservation of myelin and axons in white matter, we performed analysis of white matter sparing (WMS) at the lesion epicenter. Our results showed that neither Tat-Bcl-xL nor Tat-BH4 treatment had a significant effect on WMS compared to vehicle-treatment, both at 7 and 60 days post-injury (Table 2). Although we cannot rule out the possibility that Tat-Bcl-xL or Tat-BH4 treatment affected survival of oligodendrocytes, our results showing unaffected WMS suggest that these treatments did not affect oligodendrocyte function in preserving myelination and axonal survival after SCI, and thus is indirect evidence that the Tat-Bcl-xL treatment did not significantly affect oligodendrocyte populations in injured spinal cords. However, cell- specific analysis of the glial populations dying by apoptosis vs. necrosis before and after treatment should be performed.
Since the white matter damage was not affected by the Tat-Bcl-xL treatments, the alternative explanation for Tat-Bcl-xL- or Tat-BH4-inducud worsening of locomotor recovery may be the increased production of scar tissue, consistent with the increased inflammation, observed here. There are numerous reports of increased production of scar tissue directly related to locomotor impairment in SCI-treated rats (Silver and Miller, 2004;Klapka et al., 2005). For example, Schwab’s group showed that creatine-treated SCI rats showed significant improvement in locomotor recovery (BBB scores) although WMS was not affected, but the scar tissue was significantly reduced (Hausmann et al., 2002), suggesting that treatment that modulates locomotor recovery after SCI may affect scar formation, but it does not have to affect white matter damage. The effect of Tat-Bcl-xL or Tat-BH4 on the formation of scar tissue in injured spinal cords remains to be determined.
Our results may cast doubt on therapeutic strategies relying on anti-apoptotic targeting using Bcl-2 proteins. However, we believe that the successful outcome of anti-apoptotic strategies depends on the severity and type of initial injury. In contrast to the model of neonatal hypoxia or ischemia in which Tat-Bcl-xL treatment has been shown to be beneficial (Cao et al., 2002;Yin et al., 2006), SCI is accompanied by massive vasculature disruption and hemorrhage that markedly amplify the inflammatory response triggered by the initial injury (Zhang and Guth, 1997;Popovich et al., 1999). As shown in numerous reports, inflammatory reactions after SCI significantly extend the initial damage (Popovich et al., 1999;Beattie, 2004;Norenberg et al., 2004). Furthermore, anti-inflammatory agents are, among all tested treatment strategies, the most effective in sparing gray and white matter and improving recovery after SCI (Gonzalez et al., 2003;Beattie, 2004;Jones et al., 2005). Apoptosis triggered by a severe CNS injury, and thus followed by robust inflammatory reactions, may help to block a vicious cycle involving necrosis and inflammation, and, as a result, may limit more extensive damage. We therefore propose that the outcomes of anti-apoptotic treatments will depend on the balance between necrosis –inflammation-apoptosis, which is directly related to the extent of injury-induced inflammatory reactions.
Consistent with this hypothesis, previous work has shown that anti-apoptotic treatments targeting caspase inhibition are beneficial; because they decreased not only apoptosis, but also inflammation (Li et al., 2000; Knoblach et al., 2005). For example, caspase inhibitors modulate production of cytokines, key regulators of inflammation (Friedlander et al., 1996;Li et al., 2000;Barut et al., 2005;Nhan et al., 2006). Taken together, our results would suggest that only a combinatorial treatment consisting of anti-apoptotic and anti-inflammatory agents may be necessary to achieve tissue preservation and significant improvement in functional recovery after SCI. To the best of our knowledge this is the only study that reports deleterious effects of long-term anti-apoptotic treatments of CNS injury. Further studies are necessary to identify mechanisms underlying damaging effects of chronic anti-apoptotic Bcl-xL or any other anti-apoptotic treatments in SCI. Those studies will reveal cell-specific effects of anti-apoptotic treatments, and delineate a time window during which different cells respond to these treatments, which should help in designing more effective anti-apoptotic treatments.
ACKNOWLEDGMENTS
We would like to thank Dr. Karin High, Dr. Ying Yu, and Linda Muehlberger for their assistance in the neuronal counting and histological preparations. We thank Dr. Ricardo Pastori at University of Miami for the p-HAT-Tat-Bcl-xL plasmid. This work was supported by grants by NINDS NS39161, Mission Connect of TIRR-Houston, and Sealy Smith Endowment Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahn YH, Bae Yeon Y, Lee G, Kang Mee K, Kang SK. Molecular insights of the injured lesions of rat spinal cords: Inflammation, apoptosis, and cell survival. Biochemical and Biophysical Research Communications. 2006;348:560–570. doi: 10.1016/j.bbrc.2006.07.105. [DOI] [PubMed] [Google Scholar]
- Alonso G, Guillemain I, Dumoulin A, Privat A, Patey G. Immunolocalization of Bcl-xL/S in the central nervous system of neonatal and adult rats. Cell Tissue Res. 1997;288:59–68. doi: 10.1007/s004410050792. [DOI] [PubMed] [Google Scholar]
- Aritomi M, Kunishima N, Inohara N, Ishibashi Y, Ohta S, Morikawa K. Crystal structure of rat Bcl-xL. Implications for the function of the Bcl-2 protein family. J Biol Chem. 1997;272:27886–27892. doi: 10.1074/jbc.272.44.27886. [DOI] [PubMed] [Google Scholar]
- Bao F, John SM, Chen Y, Mathison RD, Weaver LC. The tripeptide phenylalanine-(D) glutamate-(D) glycine modulates leukocyte infiltration and oxidative damage in rat injured spinal cord. Neuroscience. 2006;140:1011–1022. doi: 10.1016/j.neuroscience.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Bao F, Liu D. Hydroxyl radicals generated in the rat spinal cord at the level produced by impact injury induce cell death by necrosis and apoptosis: protection by a metalloporphyrin. Neuroscience. 2004;126:285–295. doi: 10.1016/j.neuroscience.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Barut S, Unlu YA, Karaoglan A, Tuncdemir M, Dagistanli FK, Ozturk M, Colak A. The neuroprotective effects of z-DEVD.fmk, a caspase-3 inhibitor, on traumatic spinal cord injury in rats. Surg Neurol. 2005;64:213–220. doi: 10.1016/j.surneu.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Li Q, Bresnahan JC. Cell death and plasticity after experimental spinal cord injury. Prog Brain Res. 2000;128:9–21. doi: 10.1016/S0079-6123(00)28003-5. [DOI] [PubMed] [Google Scholar]
- Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends in Molecular Medicine. 2004;10:580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Brichese L, Barboule N, Heliez C, Valette A. Bcl-2 phosphorylation and proteasome-dependent degradation induced by paclitaxel treatment: consequences on sensitivity of isolated mitochondria to Bid. Exp Cell Res. 2002;278:101–111. doi: 10.1006/excr.2002.5563. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Cherbonnel-Lasserre C, Dosanjh MK. Suppression of apoptosis by overexpression of Bcl-2 or Bcl-xL promotes survival and mutagenesis after oxidative damage. Biochimie. 1997;79:613–617. doi: 10.1016/s0300-9084(97)82011-1. [DOI] [PubMed] [Google Scholar]
- Cittelly D, Nesic-Taylor O, Hulsebosch CE, Perez-Polo JR. Effect of Spinal Cord Injury on Bcl-xL Expression Levels and their Subcellular Distributions. 2005:1164. [Google Scholar]
- Colak A, Karaoglan A, Barut S, Kokturk S, Akyildiz AI, Tasyurekli M. Neuroprotection and functional recovery after application of the caspase-9 inhibitor z-LEHD-fmk in a rat model of traumatic spinal cord injury. J Neurosurg Spine. 2005;2:327–334. doi: 10.3171/spi.2005.2.3.0327. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Kilic E, Bahr M. Inhibition of neuronal apoptosis in vitro and in vivo using TAT-mediated protein transduction. Mol Cell Neurosci. 2002;21:29–37. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Hook MA, Garcia G, Bresnahan JC, Beattie MS, Grau JW. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J Neurotrauma. 2004;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr, Sauerwald TM, Oyler GA, Hardwick JM, Betenbaugh MJ. A comparison of the properties of a Bcl-xL variant to the wild-type anti-apoptosis inhibitor in mammalian cell cultures. Metab Eng. 2003;5:230–245. doi: 10.1016/s1096-7176(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- Friedlander RM, Gagliardini V, Rotello RJ, Yuan J. Functional role of interleukin 1 beta (IL-1 beta) in IL-1 beta-converting enzyme-mediated apoptosis. J Exp Med. 1996;184:717–724. doi: 10.1084/jem.184.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T. Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene. 1998;17:1295–1304. doi: 10.1038/sj.onc.1202065. [DOI] [PubMed] [Google Scholar]
- Gibbons HM, Dragunow M. Microglia induce neural cell death via a proximity-dependent mechanism involving nitric oxide. Brain Res. 2006;1084:1–15. doi: 10.1016/j.brainres.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Gomes-Leal W, Corkill DJ, Freire MA, Picanco-Diniz CW, Perry VH. Astrocytosis, microglia activation, oligodendrocyte degeneration, and pyknosis following acute spinal cord injury. Exp Neurol. 2004;190:456–467. doi: 10.1016/j.expneurol.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia M, Garcia I, Ding L, O'Shea S, Boise LH, Thompson CB, Nunez G. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc Natl Acad Sci U S A. 1995;92:4304–4308. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia M, Perez-Ballestero R, Ding L, Duan L, Boise LH, Thompson CB, Nunez G. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development. 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- Hausmann ON, Fouad K, Wallimann T, Schwab ME. Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord. 2002;40:449–456. doi: 10.1038/sj.sc.3101330. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GP, Bahr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- Jones TB, McDaniel EE, Popovich PG. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr Pharm Des. 2005;11:1223–1236. doi: 10.2174/1381612053507468. [DOI] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Kilic E, Dietz GP, Hermann DM, Bahr M. Intravenous TAT-Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann Neurol. 2002;52:617–622. doi: 10.1002/ana.10356. [DOI] [PubMed] [Google Scholar]
- Klapka N, Hermanns S, Straten G, Masanneck C, Duis S, Hamers FP, Muller D, Zuschratter W, Muller HW. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 2005;22:3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- Klein D, Ribeiro MM, Mendoza V, Jayaraman S, Kenyon NS, Pileggi A, Molano RD, Inverardi L, Ricordi C, Pastori RL. Delivery of Bcl-XL or its BH4 domain by protein transduction inhibits apoptosis in human islets. Biochem Biophys Res Commun. 2004;323:473–478. doi: 10.1016/j.bbrc.2004.08.116. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Huang X, VanGelderen J, Calva-Cerqueira D, Faden AI. Selective caspase activation may contribute to neurological dysfunction after experimental spinal cord trauma. J Neurosci Res. 2005;80:369–380. doi: 10.1002/jnr.20465. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, Stieg PE, Lipton SA, Friedlander RM. Functional role and therapeutic implications of neuronal caspase-1 and −3 in a mouse model of traumatic spinal cord injury. Neuroscience. 2000;99:333–342. doi: 10.1016/s0306-4522(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Liu XH, Collier RJ, Youle RJ. Inhibition of axotomy-induced neuronal apoptosis by extracellular delivery of a Bcl-XL fusion protein. J Biol Chem. 2001;276:46326–46332. doi: 10.1074/jbc.M108930200. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine. 2000;25:1859–1866. doi: 10.1097/00007632-200007150-00022. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Ishii K, Akimoto M, Hamada H, Hashimoto N, Miyatake S. Overexpression of basic fibroblast growth factor and Bcl-xL with adenoviral vectors protects primarily cultured neurons against glutamate insult. Neurosurgery. 2002;50:857–862. doi: 10.1097/00006123-200204000-00032. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001;18:947–956. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- Nesic-Taylor O, Cittelly D, Ye Z, Xu GY, Unabia G, Lee JC, Svrakic NM, Liu XH, Youle RJ, Wood TG, McAdoo D, Westlund KN, Hulsebosch CE, Perez-Polo JR. Exogenous Bcl-xL fusion protein spares neurons after spinal cord injury. J Neurosci Res. 2005;79:628–637. doi: 10.1002/jnr.20400. [DOI] [PubMed] [Google Scholar]
- Nhan TQ, Liles WC, Schwartz SM. Physiological functions of caspases beyond cell death. Am J Pathol. 2006;169:729–737. doi: 10.2353/ajpath.2006.060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol. 2000;2:819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Nonner D, Barrett JN. Overexpression of Bcl-xl protects septal neurons from prolonged hypoglycemia and from acute ischemia-like stress. Neuroscience. 2005;135:73–80. doi: 10.1016/j.neuroscience.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Parsadanian AS, Cheng Y, Keller-Peck CR, Holtzman DM, Snider WD. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van RN, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Potocky TB, Menon AK, Gellman SH. Cytoplasmic and Nuclear Delivery of a TAT-derived Peptide and a {beta}-Peptide after Endocytic Uptake into HeLa Cells. J Biol Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nesic O, Ye Z, Rea H, Westlund KN, Xu GY, McAdoo D, Hulsebosch CE, Perez-Polo JR. Bcl-xL expression after contusion to the rat spinal cord. J Neurotrauma. 2001;18:1267–1278. doi: 10.1089/089771501317095304. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma. 2002;19:1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ. Experimental strategies to promote spinal cord regeneration--an integrative perspective. Prog Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. PNAS. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N, Satou R, Yoshida Y, Asai A, Kirino T, Hamada H. Adenovirus-mediated transfer of Bcl-X(L) protects neuronal cells from Bax-induced apoptosis. Exp Cell Res. 2000;254:221–231. doi: 10.1006/excr.1999.4751. [DOI] [PubMed] [Google Scholar]
- Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Simizu S, Tamura Y, Osada H. Dephosphorylation of Bcl-2 by protein phosphatase 2A results in apoptosis resistance. Cancer Sci. 2004;95:266–270. doi: 10.1111/j.1349-7006.2004.tb02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Culbert AA, Evans NA, Chessell I, Davis JB, Richardson JC. P2X(7) receptors on microglial cells mediate injury to cortical neurons in vitro. Glia. 2006;54:234–242. doi: 10.1002/glia.20379. [DOI] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, Funatsu T, Tamagawa H, Sawa Y, Kawakami T, Tsujimoto Y. BH4-domain peptide from Bcl-xL exerts anti-apoptotic activity in vivo. Oncogene. 2003;22:8432–8440. doi: 10.1038/sj.onc.1207180. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Simizu S, Osada H. The phosphorylation status and anti-apoptotic activity of Bcl-2 are regulated by ERK and protein phosphatase 2A on the mitochondria. FEBS Letters. 2004;569:249–255. doi: 10.1016/j.febslet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195:158–167. doi: 10.1002/jcp.10254. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J Neurosci Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- Xu L, Koumenis IL, Tilly JL, Giffard RG. Overexpression of bcl-xL protects astrocytes from glucose deprivation and is associated with higher glutathione, ferritin, and iron levels. Anesthesiology. 1999;91:1036–1046. doi: 10.1097/00000542-199910000-00024. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Faden AI. Mechanisms of neural cell death: implications for development of neuroprotective treatment strategies. NeuroRx. 2004;1:5–16. doi: 10.1602/neurorx.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Heyes MP, Blight AR. 4-chloro-3-hydroxyanthranilate reduces local quinolinic acid synthesis, improves functional recovery, and preserves white matter after spinal cord injury. J Neurotrauma. 2006;23:866–881. doi: 10.1089/neu.2006.23.866. [DOI] [PubMed] [Google Scholar]
- Yin W, Cao G, Johnnides MJ, Signore AP, Luo Y, Hickey RW, Chen J. TAT-mediated delivery of Bcl-xL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A. Diversity in the Mechanisms of Neuronal Cell Death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Guth L. Experimental spinal cord injury: Wallerian degeneration in the dorsal column is followed by revascularization, glial proliferation, and nerve regeneration. Exp Neurol. 1997;147:159–171. doi: 10.1006/exnr.1997.6590. [DOI] [PubMed] [Google Scholar]