Abstract
Many autoimmune diseases are driven by self-reactive T helper cells. Until recently, organ-specific autoimmune diseases were primarily associated with Th1 cells but not Th2 cells. However, the discovery of a number of new effector T cell subsets, like Th17 and Th9 cells, and regulatory T cells, like Tregs and Tr1 cells, has changed the way we view and understand autoimmunity at cellular and molecular levels. In recent years, IL-17 producing Th17 cells have emerged as major players in autoimmunity. The complicated relationship between Th1 and Th17 cells, as well as the intricate balance between Tregs and Th17 cells, provide a basis for understanding the immunological mechanisms that induce and regulate autoimmunity. Here, we give an overview of the interplay between different effector T cell subsets and regulatory T cell subsets, and how they contribute to the development of autoimmunity and tissue inflammation.
Introduction
The immune system has developed to defend the body against a myriad of ever-evolving pathogens, including bacteria, viruses and parasites. This enormous task requires the immune cells to have an almost unlimited repertoire of receptors to ensure that the invading pathogen is recognized. Antigen-specific recognition by receptors on lymphocytes leads to clonal expansion of pathogen-specific lymphocytes, which directly or indirectly clear the invading pathogen. A crucial prerequisite for this process is the ability of the immune system to distinguish self from non-self. Since the receptors are generated in a random process, immune cells with self-reactive receptors need to be eliminated by mechanisms of self-tolerance. Self-reactive immune cells that manage to escape these mechanisms can pose a serious threat to health, as they can lead to the development of autoimmunity. Depending on the expression profile of the antigen, self-reactive cells may induce organ-specific or systemic autoimmune diseases. Type I diabetes is an example for organ-specific autoimmunity because only the insulin-producing β-cells of the pancreas are targeted by self-reactive lymphocytes, resulting in destruction of the β-islets within the pancreas and loss of insulin production. However, in systemic autoimmune diseases like Lupus erythematosus the self-antigen is present throughout the body leading to inflammation and tissue damage in multiple end-organs.
Autoimmune pathogenesis often involves self-reactive T helper cells, whose effector mechanisms include the production of pro-inflammatory cytokines that initiate inflammation and also provide T cell help to autoreactive B cells. Subsequent expansion and maturation of those self-reactive B cells then leads to the production of auto-antibodies which further contribute to tissue inflammation and damage. The ability of autoreactive T cells to induce autoimmunity and tissue inflammation is not only dictated by their specificity for self-antigen but, more importantly, by their effector functions. Upon interaction with the self- or a cross-reactive antigen, T helper cells activate, expand, and differentiate into various effector T cell subsets. Depending on the cytokines they produce, these T cell subsets have very different properties. Thus, T helper cells include the well-defined effector subsets Th1 cells and Th2 cells, as well as the more recently described Th17 and Th9 cells, but also regulatory subsets like induced Tregs and Tr1 cells. How these different T cell subsets can induce or regulate autoimmunity is discussed in this article using the animal model for human Multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE).
Th1 cells
Depending on the cytokines present in the milieu naïve T cells differentiate into one of various T cell subsets. Tim Mosmann and Bob Coffman first defined Th1 and Th2 effector T cell subsets, according to the cytokines that these cells produce [1]. Th1 cells predominantly secrete interferon (IFN)-γ and lymphotoxin, which mainly serve to activate macrophages at the site of inflammation. Th1 cells are important for host defense against intracellular pathogens and induction of delayed type hypersensitivity responses. Uncontrolled Th1 responses against self-antigens, however, can lead to the development of autoimmunity and several lines of evidence pointed to Th1 cells as the major effector T cell responsible for inducing EAE and potentially MS. Thus, early studies showed that encephalitogenic T cells had a Th1 cytokine profile and consistent with this observation IFN-γ was detected in CNS-lesions of mice [2, 3] undergoing EAE and humans with MS [4]. Furthermore, Th1 cells can attract and activate macrophages by producing cytokines including IFN-γ, MIP-1α, MIP-1β, and TCA-3 [5], and activated macrophages infiltrating the CNS can actively contribute to demyelination in both EAE and MS. In addition, Th1-clones specific for myelin antigens were shown to induce EAE upon adoptive transfer [5, 6]. Moreover, administration of IFN-γ exacerbated signs and symptoms of disease in MS patients [7]. These findings led to the conclusion that Th1 cells are the main effector cells involved in CNS autoimmunity. Th1 cells are generated from naïve T helper cells by TCR engagement and STAT1 signaling, induced by activation of the IFN-γR with IFNs. Phosphorylated STAT1 induces expression of the transcription factor T-bet, which then drives Th1 differentiation by transactivating the Th1 signature cytokine IFN–γ and the specific subunit of the receptor for interleukin (IL)-12, IL-12Rβ2. Thus, the cell becomes responsive to IL-12, which is produced by activated APCs, and subsequent IL-12 signaling through STAT4 further stabilizes the Th1 phenotype [8]. Studies showing that mice deficient in the Th1 transcription factors T-bet and STAT4 are resistant to the development of EAE [9, 10] further supported the hypothesis that Th1 cells are responsible for disease induction. Since Th1 effector mechanisms seemed to explain many histopathological and clinical features not only in EAE but also in other autoimmune diseases, including Type I diabetes and rheumatoid arthritis, Th1 cells became the archetypical inducer of organ-specific autoimmunity.
Th2 cells
While Th1 cells were associated with host defense against intracellular pathogens and autoimmunity, Th2 cells, on the other hand, were described to have very different effector functions, as they are essential for clearing extracellular organisms like parasites and helminthes. In addition, Th2 cells also play a very important role in eosinophilic inflammation and IgE production in allergic reactions and asthma [11, 12]. These opposite effector functions of Th1 and Th2 cells are rooted in distinct pathways for the generation of either Th1 or Th2 cells. These pathways counterregulate expansion and function of the other subset at several levels. Differentiation of naïve T cells into Th2 cells is driven by activation of T cells via TCR and IL-4 receptor, which leads to phosphorylation of STAT6. pSTAT6 is critical for the induction of the Th2 transcription factor GATA3, which in turn transactivates Th2-specific cytokines such as IL-4, IL-5, and IL-13, while at the same time down-regulating Th1 related factors such as STAT4 and IL-12Rβ2 [13]. In addition, the transcription factor c-maf also contributes to Th2 differentiation by transactivating IL-4 transcription [14]. The Th2 cytokine IL-4 strongly inhibits Th1 differentiation and, vice versa, the Th1-cytokines IL-12 and IFN-γ inhibit Th2 differentiation.
In some systemic autoimmune diseases, like Lupus erythematosus, Th2 cells aid in the production of autoantibodies by providing help to autoreactive B cells, but they are not considered the driving force of autoimmune pathogenesis [15]. In most organ-specific autoimmune diseases Th2 cells are not involved in pathogenesis. Accordingly, it was already shown in 1993 that MBP-specific Th2 clones are not able to transfer EAE unless they were transferred into a lymphopenic host [16, 6]. In addition, we demonstrated that Th2 cells specific for either myelin proteolipid protein (PLP) or myelin oligodendrocyte glycoprotein (MOG) also fail to induce EAE upon adoptive transfer [17-18]. Even more so, in some instances induction of a Th2 response or administration of the Th2 cytokine IL-4 during ongoing autoimmune inflammation can be of therapeutic value, especially considering the potential of Th2 cells to crossregulate the generation of Th1 cells. Thus, glatiramer acetate, a drug used in the therapy of relapsing-remitting MS, is thought to act at least partly by inducing a cytokine shift towards Th2 [19].
Th17 cells
The hypothesis of Th1 cells being solely responsible for induction of organ-specific autoimmunity was challenged when it was shown that animals lacking the Th1 signature cytokine IFN-γ are not resistant, but in fact are more susceptible to multiple autoimmune diseases including EAE [20], experimental autoimmune uveitis (EAU) [21], and collagen-induced arthritis (CIA) [22]. Similarly, loss of several other molecules involved in the Th1 differentiation pathway, including IFN-γR, STAT-1, and IL-12p35 chain led to increased autoimmune disease in experimental models of autoimmunity. While IL-12p35-chain deficient mice were more susceptible to EAE, surprisingly loss of the other chain of IL-12, the p40 chain, made mice highly resistant to EAE. IL-12 is composed of the subunits p35 and p40, but Robert Kastelein showed that p40 is not only essential for forming IL-12, but can also pair up with another subunit called p19, and thus form a novel cytokine called IL-23 [23]. In a seminal study, Cua and colleagues showed that loss of IL-23 made both p40- and p19 deficient animals resistant to EAE, whereas IL-12-p35-deficient animals, which lacked IL-12 and Th1 responses but could still form IL-23, remained susceptible to EAE [24, 25]. These data provided the foundation for the hypothesis that not IL-12 but IL-23 was crucial for the development of autoimmunity. It was later shown that IL-23 may induce a unique subset T cells, which due to their production of the effector cytokine IL-17, were named Th17 cells. Th17 cells produce IL-17A and IL-17F, which belong to the same family and are partly redundant in their effector functions: upon crosslinking of their receptor both cytokines induce pro-inflammatory cytokines like IL-6, IL-1, TNF, and pro-inflammatory chemokines like CXCL1, GCP-2 and IL-8, and thus promote tissue inflammation and recruitment of neutrophils to the site of inflammation [26]. IL-17A and IL-17F promote inflammation on several levels, as their receptors IL-17RA and IL-17RC are expressed on both hematopoietic cells and non-hematopoietic cells. Th17 cells play an important role in mediating host defenses and have a specialized role in clearing pathogens that are not adequately handled by Th1 or Th2 cells, including bacteria like Citrobacter, Klebsiella pneumoniae, and Borrelia burgdorferi, but also fungi such as Candida albicans [26]. Due to an important role of IL-17 in inducing tissue inflammation, Th17 are particularly suited for the promotion of autoimmunity. Accordingly, elevated levels of IL-17 were detected in several autoimmune diseases including Multiple Sclerosis [27], rheumatoid arthritis [28] and psoriasis [29]. Furthermore, it was shown that IL-17 neutralizing antibodies ameliorate EAE [30] and IL-17 deficient animals develop attenuated CIA and EAE [31, 32]. These studies demonstrated the importance of Th17 cells in EAE and other autoimmune diseases, and required a re-evaluation of the role of other effector T cells in the induction of autoimmune tissue inflammation previously thought to be driven by Th1 cells.
Generation and differentiation of Th17 cells
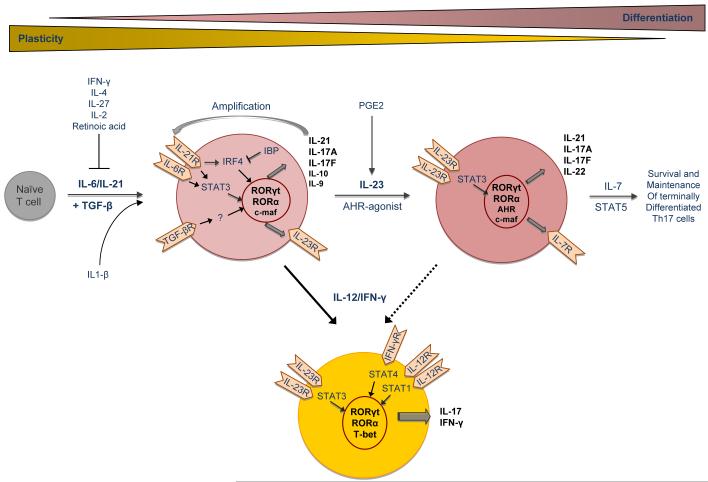
Since IL-23 seemed to have a prominent role in expansion of IL-17 producing T cells, it was initially suggested that IL-23 may be the differentiation factor for Th17 cells. However, since naïve T cells do not express the IL-23 receptor, it was concluded that other cytokines must be responsible for initiating differentiation of Th17 cells from naïve T cells (Figure 1). Subsequently, it was shown independently by three groups that differentiation of Th17 cells is driven by the combination of the cytokines TGF-β and IL-6 [33-35]. IL-6 signaling leads to phosphorylation of STAT3, which is essential for proper Th17 differentiation [36]. In the absence of IL-6, differentiation of Th17 cells can also be induced by TGF-β plus IL-21 [37-39], but comparatively IL-6 is a stronger driver of Th17 responses. In addition, Th17 cells also produce IL-21, which acts in an autocrine fashion to amplify Th17 differentiation. Recent data suggest that both differentiation and self-amplification of Th17 cells by IL-21 is mediated via IRF4 [40], which in turn is regulated by the IRF-binding protein [41, 42]. Aside from the above-mentioned factors, IL-1β can synergize with IL-6 to induce murine Th17 cell differentiation [43]. IL-1β, together with TGF-β, IL-6, and IL-21, has been described as a critical differentiation factor for human Th17 cells as well [44, 45].
Figure 1.
Schematic illustrating differentiation process and plasticity of Th17 cells
IL-6 and TGF-β signals ultimately lead to the expression of the transcription factor RORγt, which was first described in lymphoid tissue inducer cells and IL-17 producing T cells in the lamina propria [46-48]. RORγt is believed to transactivate many components essential for differentiation of Th17 cells including IL-17A, IL-17F, and IL-23R [49]. Aside from RORγt the transcription factor RORα is also involved in Th17 differentiation, as deletion of either one of them partially impairs Th17 differentiation, whereas deletion of both transcription factors completely prevents generation of Th17 cells [50]. Another factor that is involved in certain aspects of Th17 cell differentiation is c-maf. Although not required for initial Th17 differentiation, it was shown that c-maf enhances the production of IL-21 in Th17 cells and thereby contributes to the amplification of Th17 cells [51]. Although c-maf was initially described as a transcription factor involved in inducing IL-4 and Th2 differentiation, our studies show that c-maf is expressed at 100 fold higher levels in Th17 cells than Th2 cells. However, the c-maf target genes and signaling in Th17 cells need to be further investigated.
The identification of the Th17-lineage specific transcription factors, together with the observation that transcription factors of the other lineages, such as T-bet, GATA-3, STAT1, and STAT6, are dispensable for Th17 cell differentiation [53-55], established Th17 cells as an independent T cell subset. In addition, as Th1 and Th2 subsets crossregulate each other, Th1 and Th2 cells also appear to regulate the development of Th17 cells, since both IFN-γ and IL-4 inhibit Th17 cell differentiation [53, 54].
The role of IL-23 in the generation of Th17 cells
Differentiation of naïve T cells into Th17 cells depends on TGF-β and IL-6 but does not require IL-23. However, both IL-23 [25] as well as IL-23R deficient mice [56, 57] cannot mount a significant Th17 response in vivo and are resistant to EAE and other autoimmune diseases. Therefore, it was concluded that, although Th17 cells can be generated with IL-6 and TGF-β in the absence of IL-23, its presence is essential for expansion and maintenance of pathogenic Th17 cells. IL-23 is produced by APCs after activation through TLR signaling (especially TLR2 and TLR4), and its production can be further enhanced by certain factors, such as PGE2 [58]. The receptor for IL-23 is composed of IL-12Rβ1 (which is also part of the IL-12 receptor) and the specific chain IL-23R [59]. Induced by IL-6 and TGF-β the IL-23 receptor appears on Th17 cells during ongoing Th17 cell differentiation (Figure 1). IL-23 induces pSTAT3 and expands and stabilizes Th17 response by further up-regulating expression of the specific IL-23R chain in Th17 cells [57]. Thus, developing Th17 cells become more responsive to IL-23 and less responsive to IL-12, since the constitutively expressed IL-12Rβ1 chain is more likely to pair with IL-23R because of its increased expression induced by IL-23. Therefore, IL-23 reinforces the Th17 differentiation program and decreases the chances of dedifferentiation and plasticity in Th17 cells. In addition to enhancing IL-17 production, IL-23 induces de novo production of IL-22 from Th17 cells [60, 61]. The receptor for IL-22 is expressed on the majority of epithelial and parenchymal tissues, and in tissue cells IL-22 promotes epithelial growth and elicits an innate immune response triggering expression of acute-phase reactants and β-defensins [62]. Hence, IL-23 not only stabilizes the Th17 cytokine phenotype, but it also induces more effector cytokines like IL-22 and possibly others and thereby enables Th17 cells to mediate all of its effector functions. Consequently, IL-23 is needed to terminally differentiate Th17 cells and stabilize their effector phenotype. Recent data suggest that IL-23 might facilitate terminal differentiation and expansion/proliferation of pathogenic Th17 cells by driving re-expression of the IL-7R on Th17 cells [56], thus rendering Th17 cells responsive to IL-7, which may be important for survival/expansion of pathogenic Th17 cells in EAE [63].
The reciprocal relationship between Th17 cells and Tregs
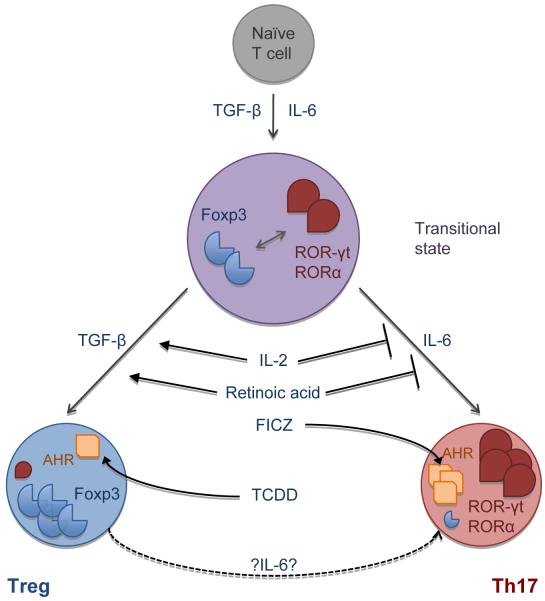
Surprisingly, generation of pro-inflammatory Th17 cells required the presence of an anti-inflammatory cytokine, TGF-β, which together with IL-6 induced Th17 differentiation. TGF-β alone induced the Treg transcription factor Foxp3 and is essential for the development of Tregs in the periphery. However, the presence of pro-inflammatory cytokines like IL-6, which is induced during infection, inflammation, or injury, inhibited the induction of Foxp3+ Tregs and simultaneously promoted Th17 cell differentiation [33]. This led us to hypothesize that there is a reciprocal relationship between pro-inflammatory IL-17 producing Th17 cells and protective Foxp3+ Tregs (Figure 2). Indeed, multiple lines of evidence support this conclusion: 1) At a molecular level, it has now been shown that the Th17 and Treg transcription factors RORγt/RORα and Foxp3 can bind to each other and inhibit each others function [64, 65]. 2) IL-2, a growth factor for Tregs, inhibits Th17 cell differentiation, whereas IL-21, which promotes Th17 differentiation, inhibits Treg expansion [66]. 3) The vitamin A metabolite retinoic acid promotes Treg differentiation, but inhibits Th17 cells [67]. 4) Aryl hydrocarbon receptor (AhR), which is expressed in both Tregs and Th17 cells, can have opposing effects on Th17 and Treg differentiation depending on the ligand. Thus, engagement of AhR with one of the natural ligands, FICZ, promotes Th17 differentiation and enhances IL-22 production [68]. On the other hand, the engagement of AhR with another synthetic ligand, TCDD, seems to primarily expand Tregs by enhancing Foxp3 expression [69].
Figure 2.
Schematic illustrating the reciprocal relationship between IL-17 producing Th17 cells and Foxp3+ Tregs.
Interplay of Th1 and Th17 cells in CNS autoimmunity
It is now well established that Th17 cells are important effector cells in the development of autoimmunity, however, the role of Th1 cells in autoimmune pathogenesis cannot be disregarded. After all, bonafide Th1 cells with specificity for myelin antigens induce EAE upon adoptive transfer, and after active immunization with myelin antigens both IFN-γ producing Th1 cells and IL-17 producing Th17 cells infiltrate the CNS. Whether only one subset or both initiate disease, was not entirely clear because it is difficult to study the pathogenic potential of Th17 cells and Th1 cells independently of each other. To address this issue, we recently developed a protocol to generate pure Th1 and Th17 cells derived from naïve T cells bearing a transgenic MOG35-55 specific TCR. Adoptive transfer of these subsets allowed us to study the pathogenicity of Th1 and Th17 cells separately, and it was clear that both subsets are able to induce EAE independently of each other [18]. Therefore, Th17 cells per se do not need Th1 cells to overcome the blood-brain-barrier and induce EAE as has been suggested by others [70]. However, in an inflammatory setting that generates both Th1 and Th17 cells one subset may migrate to the target organ first, and facilitate infiltration or even attract the other subset. Accordingly, kinetic experiments from our group have shown that after active immunization the frequency of Th17 cells peaked earlier, whereas IFN-γ producing Th1 cells reached highest levels later at the peak of clinical disease, suggesting that Th17 cells may infiltrate the CNS before Th1 cells, which then propagate tissue inflammation [71]. Supporting this concept, it has been demonstrated that during a Mycobacterium tuberculosis challenge Th17 cells infiltrate the lung first and subsequently attract Th1 cells [72]. They do so by inducing the chemokines CXCL9, CXCL10 and CXCL11, whose receptor CXCR3 is highly expressed on Th1 cells [73]. Migration of Th17 cells themselves to the target organ might be regulated by the chemokine CCL20, as Th17 cells express its receptor CCR6 [74]. However, whether Th17 cell migration is also modulated by other factors remains to be determined. The natural entry sequence of Th1 and Th17 cells into the target organ and the chemoattractants might differ depending on the target organ itself, the method of disease induction, and the antigen specificity of the T cells involved in inducing disease. However, it is very important to address this question in every model, as those cells that first reach the target organ and subsequently induce infiltration of other cells might be the most promising therapeutic target for regulating autoimmunity.
Dissection of Th1 and Th17 induced effects is further complicated by T helper cell plasticity. Overall, Th17 cells seem to be more plastic than Th1 cells. Thus, IL-17+ cells can become IFN-γ producers when cultured with IL-12 in vitro [75] (Figure 1), and several reports have shown that Th17 cells acquire IFN-γ production relatively easy upon in vivo transfer [76-78]. In fact, in our adoptive transfer study, almost all Th17 cells converted to IFN-γ producing cells in vivo when they were exposed to IL-2 and not IL-23 in vitro. On the other hand, Th17 cells cultured in the presence of IL-23 in vitro maintained their IL-17 production in vivo, but a small percentage (about 10%) also began to produce IFN-γ [18]. These data confirm that IL-23 is required for stabilization and terminal differentiation of Th17 cells, as Th17 cells readily begin to produce IFN-γ when generated in the absence of IL-23. The mechanism by which IL-23 stabilizes the Th17 phenotype might include up-regulation of IL-23R and transcription factors required for Th17 differentiation such as RORγt. Importantly, Lexberg and coworkers have shown that Th17 cells can indeed reach the stage of terminal differentiation, as Th17 memory cells isolated ex vivo do have a stable cytokine profile and do not produce IFN-γ even when cultured with IL-12 [75]. Nevertheless, T cells producing both IL-17 and IFN-γ are clearly present in the CNS during EAE and have also been observed in EAU [76, 79]. Considering that Th17 are more plastic than Th1 cells and that IL-23R deficient mice not only have less IL-17+ but also less IL-17+IFN-γ+ T cells, it is possible that double producers are derived from Th17 cells. Whether Th17 cells start to produce IFN-γ as part of their pathogenic effector mechanisms, or whether IFN-γ is actually up-regulated to limit further Th17 cell differentiation and thus protect the tissue from excessive damage, has not been resolved yet. In addition, it cannot be excluded that IL-17+IFN-γ+ double producers are derived from previously uncommitted cells or even from Th1 cells. Unraveling the origin and pathogenicity of double producers will shed light on the dynamics of autoimmune pathogenesis involving both Th1 and Th17 responses.
Histopathologic differences between Th1 and Th17 induced autoimmune inflammation
As Th1 and Th17 cells produce different cytokines and employ different effector mechanisms, we specifically began to analyze whether there were histopathologic differences between Th1 and Th17 induced tissue inflammation. Indeed, Th1 and Th17 induced EAE have been shown to differ in respect of lesion localization, clinical manifestations, and lesion composition (Table 1). Several groups have investigated whether Th1 and Th17 cells induce lesions in different parts of the CNS. Interestingly, Th17 cells seem to promote formation of lesions in the brainstem and cerebellum which often cause atypical signs of EAE including severe balance defects and ataxia, whereas Th1 cells induce lesions in the spinal cord causing only classical signs of EAE. Thus, it has been described that adoptive transfer of T cells into IFN-γR deficient recipients leads to development of atypical EAE, suggesting that IFN-γ signaling protects brainstem and cerebellum but not the spinal cord from inflammation [80]. On the other hand, it has also been shown that inflammation in brainstem/cerebellum depends on a high Th17:Th1 ratio and can be abolished by treatment with IL-17-neutralizing antibody [81]. Confirming these results, we also observed that recipients of MOG-specific Th17 cells often developed atypical signs of EAE, which seemed to be caused by IL-17 itself since transfer of Th17 cells into IL-17RA deficient recipient animals exclusively produced classical signs of EAE [18] (and unpublished observation). These data highlight the very different and in some aspects even opposing roles of Th1 and Th17 cells in EAE pathogenesis, since IL-17 appears to promote inflammation of brainstem and cerebellum whereas IFN-γ serves to protect these regions from inflammation. Inflammation in the spinal cord, however, can be induced by both Th1 and Th17 cells.
Table 1.
Characteristics of EAE induced by different T cell subsets
| Th1 | Th17 | Th9 | |
|---|---|---|---|
| Severity | +++ | +++ | ++ |
| Effector cytokines | IFN-γ |
IL-17, IFN-γ, IL-22, IL-21 |
IFN-γ, IL-9, IL-10, IL-4, IL-17 |
| Lesion localization |
Spinal cord, brain |
Cerebellum/brain stem, spinal cord |
Spinal cord, brain |
| Clinical signs | Classical | Atypical and classical | Classical |
| Secondary cells | Macrophages | Neutrophils, B cells?, macrophages |
? Mast cells? |
| Special features | Lymphoid-follicle like structures |
Peripheral neuritis, extensive demyelination |
Aside from lesion localization, Th1 and Th17 cells also appear to induce different types of CNS lesions. In our adoptive transfer model Th1 cells induced typical meningeal and parenchymal perivascular infiltrates with demyelination in the CNS [18], reminiscent of the classical MS-lesion containing mostly activated macrophages, microglia and T cells. In contrast, many lesions in Th17 recipients were characterized by large organized aggregates of lymphocytes that were attached to the leptomeninges and often extended into the parenchyma. Similar structures have also been detected in the leptomeninges of MS patients with severe progressive but not relapsing-remitting disease and were shown to consist of B cells, plasma cells, T cells and dendritic cells [82]. Interestingly, these lymphoid aggregates were only induced by Th17 cells cultured with IL-23, suggesting that IL-23 induces certain cytokines and/or surface molecules in Th17 cells enabling them to promote formation of these lymphoid aggregates. It is also possible that stable expression of Th17 cytokines and reduced plasticity in IL-23 exposed-Th17 cells are crucial for the development of lymphoid aggregates. Since Th17 cells seem particularly suited to induce formation of ectopic follicle-like structures, it is interesting to consider the similarities between Th17 cells and follicular T helper cells (TFH), a specialized T cell subset which is absolutely essential for the germinal center reaction in the lymph node. Both Th17 cells and TFH cells express the costimulatory molecule ICOS on their surface, which enhances IL-21 production via the transcription factor c-maf [51]. IL-21 promotes B cell differentiation and isotype switching [83] in the germinal center reaction. In addition, IL-21 contributes not only to Th17 cell differentiation, but is also required for the generation of TFH cells [84]. Furthermore, TFH cells can express the Th17 cytokine IL-17 [51]. These similarities suggest that Th17 and TFH cells may be related, however, whether Th17 can convert into TFH cells, or vice versa, or whether these two subsets merely share some properties, remains to be determined. Further analyzing the cellular composition of CNS infiltrates in Th1 versus Th17 induced EAE, other studies have shown that Th1 induced lesions in the CNS primarily contain macrophages and monocytes, whereas Th17 induced lesions had considerable numbers of neutrophils [85]. Analogous observations were reported for EAU [79] and CIA [86].
In summary, these data show that, although Th1 and Th17 cells can both induce autoimmune inflammation, they probably employ very different mechanisms and attract different effector and innate cells to do so (Table 1). It is possible that in some patients Th1 and Th17 cells both contribute to pathogenesis, whereas in other patients the disease is either Th1 or Th17 dominated. This could manifest itself in different lesion localization and composition, and secondary cells that are attracted to the site of inflammation, resulting in diverse clinical symptoms and disease courses, which consequently would require distinctive therapeutic strategies. In fact, it has been shown recently, that MS patients with high serum levels of IL-17 do not respond to IFN-β therapy, whereas patients with low levels of IL-17 benefit from IFN-β therapy, suggesting that one might be able to differentiate between ‘Th1- and Th17-MS’ [87] and tailor therapies depending on the involvement of effector T cells in the disease process.
Th9 cells
The pleiotropic effects of TGF-β appear to be very important for T cell differentiation: not only can TGF-β differentiate Tregs, but in combination with IL-6 it can also drive differentiation of pro-inflammatory Th17 cells. In 2007 we proposed the hypothesis, that TGF-β in combination with other cytokines might be able to induce other novel T cell subsets [88]. Indeed, testing of this hypothesis resulted in the identification of a novel T cell subset, which predominantly produced IL-9 and was therefore named Th9 cells. Th9 cells are induced by TGF-β plus IL-4 and are characterized by the secretion of both IL-9 and IL-10, effector cytokines that were previously associated with Th2 cells. However, Th9 cells produce much larger quantities of IL-9 than Th2 cells while secreting only small amounts of other Th2-related cytokines such as IL-4, IL-5 and IL-13. In addition, Th9 cells also do not express the Th2 transcription factor GATA-3, nor do they express RORγt or Foxp3, transcription factors associated with Th17 and Treg cells [89, 90]. These findings support the concept that Th9 cells are an independent T cell subset, however, no Th9-specific transcription factor has been identified so far. This raises the issue of whether Th9 cells really constitute a separate T cell subset, or whether they are a specialized form of Th2 cells. IL-9 was originally reported to be a T cell growth factor and most of its effector functions were described in the context of Th2-responses. Thus, IL-9 plays a role in the defense against certain helminth infections [91] as well as in asthma and allergy [92]. CD4 T cells are the main source of IL-9 and a recent study has shown that IL-25 can significantly enhance the production of IL-9 by Th9 cells and thus emerges as a new regulator of IL-9 [93]. The receptor for IL-9 is expressed on many immune cell types including subsets of T and B cells, mast cells, macrophages and dendritic cells, but also on other cells, such as airway epithelial cells [94] and immature neurons [95].
Since IL-10 produced by Tregs and Tr1 cells has anti-inflammatory effects and since Tregs can also produce IL-9, it was initially unclear whether Th9 cells are a regulatory or an effector T cell subset. However, it has been shown that Th9 cells vigorously proliferate in vitro and that they are not able to suppress T cell proliferation in vitro. In addition, our in vivo studies showed that co-transfer of effector cells together with Th9 cells in a colitis model exacerbated disease, strongly suggesting that Th9 cells do not have regulatory properties but may be effector T cells [89]. Consistent with this data, we were also able to demonstrate that in vitro generated MOG-specific Th9 cells can induce EAE in an adoptive transfer model [18]. Interestingly, we found that CNS-lesions induced by Th9 cells differed from classical lesions: we observed more pronounced demyelination compared to lesions induced by other T cell subsets and also peripheral neuritis in the dorsal nerve roots, suggesting that Th9 cells induce EAE using different mechanisms than other T cell subsets. Even more so than Th17 cells, Th9 cells proved to be quite plastic in our adoptive transfer model of EAE as they began to produce large amounts of IFN-γ in the CNS while maintaining the production of their original cytokines IL-9, IL-10, and IL-4. In the colitis model on the other hand, Th9 cells produced mainly IL-17 upon adoptive transfer [89], suggesting that depending the cytokine milieu present in the target organ Th9 cells can shift their cytokine profile more towards Th1 or Th17 cells. In this context it should be noted that other T cells may produce IL-9. Thus, Th17 cells can produce significant amounts of IL-9 upon exposure to IL-2, which seems to enhance IL-9 production by Th17 cells, whereas IL-23 has been described as a negative regulator of IL-9 in Th17 cells [18, 96].
The exact effect of IL-9 in the pathogenesis of EAE seems to be quite complex. Two groups have addressed this question using IL-9R-deficient mice: Elyaman and colleagues show that IL-9R deficient mice immunized with a suboptimal dose of MOG develop more severe EAE than WT mice and attribute this difference to a loss of suppressive function in IL-9R deficient Tregs [96]. On the other hand, Nowak et al. report that immunization with a high dose of MOG results in attenuated EAE in IL-9R deficient mice, characterized by less Th17 cells and less IL-6 positive macrophages in the CNS, as well as reduced number of mast cells in the lymph nodes [97]. These studies accentuate the pleiotropic effects of IL-9. On the one hand, IL-9 can enhance the suppressive function of Tregs and thus might be important to control autoreactive T effector cells. On the other hand, the pro-inflammatory effects of IL-9 include promotion of differentiation of Th17 cells – a function that is quite redundant as both IL-6 and IL-21 in combination with TGF-β can induce Th17 cells. In addition to T cell differentiation, IL-9 can also directly contribute to EAE pathogenesis. It is well established that IL-9 activates mast cells and it has been demonstrated that mast cells can significantly contribute to EAE development and severity [98]. Considering that Nowak et al. found a reduced number of mast cells present in peripheral lymph nodes of IL-9R-/- mice during EAE [97], one can speculate that one of the effector functions of IL-9 produced by Th9 or Th17 cells during EAE is the accumulation/activation of mast cells. Intriguingly, mast cells can degrade myelin by secreting a number of proteolytic enzymes, including tryptase whose receptor PAR2 is expressed on dorsal nerve roots [99, 100]. Thus, mast cells could potentially be responsible for both the increased demyelination and the peripheral neuritis we observed in Th9-recipient mice.
Whether anti- or pro-inflammatory effects of IL-9 are ultimately dominant in EAE or MS pathogenesis remains to be determined, however, it is clear that Th9 cells can transfer EAE and that they probably do so by employing different effector mechanisms than other T cell subsets which is reflected by the pathology of CNS lesions induced by Th9 cells (Table 1). Thus, Th9 cells might contribute to the heterogeneity of CNS lesions observed in different patients and different phases of disease.
Foxp3+ Tregs
While effector T cells promote inflammation, regulatory T cells serve to control it. Therefore, Tregs play a very important role in autoimmune pathogenesis by maintaining self-tolerance and by controlling expansion and activation of autoreactive CD4+ T effector cells. Regulatory T cells can be identified by expression of the transcription factor Foxp3, and include both naturally occurring Tregs (nTregs) generated in the thymus, as well as induced Tregs (iTregs), whose differentiation from naïve T helper cells in the periphery is driven by TGF-β. So far, natural Tregs cannot be distinguished from induced Tregs since both populations express Foxp3 and both suppress effector T cell responses, although gene expression profiling has shown that iTregs differ from nTregs, as they lack modules of genes that are expressed in nTregs. The importance of Foxp3+ Tregs in the control of autoimmunity is exemplified in the Foxp3-deficient Scurfy mouse, which develops autoimmune inflammation in multiple organs resulting from hyper-proliferative CD4+ T cells that produce high levels of effector cytokines [101]. Similarly, humans with mutations in the Foxp3 gene suffer from X-linked IPEX [102], a syndrome in which patients develop multi-organ inflammatory disease that includes insulin dependent diabetes mellitus, psoriasis-like dermatitis and enlarged secondary lymphoid organs. In addition, a reduction of Treg numbers and/or a loss of Treg function have been observed in many human autoimmune diseases. Thus, SLE-patients have normal levels of Tregs in the blood; however, those Tregs show compromised immunosuppressive function [103]. Similarly, in EAE our group has described that regulatory T cells isolated from the CNS are not able to suppress proliferation of CNS-derived effector T cells, because the presence of the proinflammatory cytokines IL-6 and TNF protects effector T cells from Treg mediated suppression [71]. The therapeutic potential of regulatory T cells is subject of intense investigations since in vivo induction of Tregs or transfer of in vitro generated Tregs might provide a means to control autoreactive effector T cells. However, recent studies raise the question whether Tregs are too plastic for development into a therapy. First of all, Tregs and Th17 cells have a reciprocal relationship, as they can be generated from the same cell with TGF-β or TGF-β plus IL-6 [33]. In fact, during differentiation one cell can express both Foxp3 and RORγt at the same time [64] and it has also been demonstrated that Tregs begin to produce IL-17 after treatment with IL-6 in vitro [104] and in vivo [105, 106]. Similarly, Tregs can also acquire expression of T-bet and begin to produce IFN-γ [107]. Secondly, a study by Bluestone and colleagues using Foxp3-Cre fate-mapping mice, demonstrated that many effector T cells are derived from Tregs [108]. These findings show that regulatory T cells can be quite plastic and can not only loose their immunosuppressive functions but also become effector T cells, a possibility that needs to be carefully considered if Treg cells are to be used in therapy of autoimmune diseases.
Tr1 cells
Expanding the list of T cell subsets that require TGF-β for their generation are the IL-10 producing Tr-1 cells, which are induced by TGF-β and IL-27 [109-111]. The transcription factor c-maf further promotes Tr-1 cell differentiation by inducing IL-21, which acts as an autocrine growth factor for Tr1 cells [52]. Although Tr1 cells do not express Foxp3 [112] they have potent immunosuppressive properties and produce the cytokines IL-10 and IFN-γ. Importantly, it has been demonstrated that animals deficient in IL-27 signaling develop exacerbated EAE with increased frequency of Th17 cells [113]. This phenotype may be caused both directly by lack of IL-27 mediated inhibition of Th17 cell differentiation, and indirectly by lack of immunoregulation by Tr-1 cells. Thus, IL-27 mediated inhibition of Th17 cells and induction of Tr-1 cells might prove to be beneficial in therapy of autoimmune diseases. In fact, Gagliani and colleagues have already shown that adoptive transfer of antigen-specific Tr1 cells can induce tolerance in an islet transplant model [114].
Concluding remarks
Several organ-specific autoimmune diseases are driven by autoreactive T helper cells, and Th1 cells and, more recently, Th17 cells have been shown to play a role in their pathogenesis. Many autoimmune diseases like MS have a very heterogeneous spectrum such that disease courses differ from patient to patient and, in addition, the disease goes through different phases within the same patient. Recent studies have shown that among the T effector cells not only Th1, but also Th17 cells and Th9 cells contribute to pathogenesis of autoimmune diseases. Every subset probably employs different mechanisms for induction of tissue inflammation, since each subset produces different effector cytokines and attracts different secondary cells into the target organ. Importantly, defects in the regulatory mechanisms can also contribute to pathogenesis and the type of disease that is induced. It is possible that pathogenesis is not equal between patients but instead might depend on the immunological history of the patient. Thus, one patient might have a Th1-dominated or a Th17-dominated disease course, while in others both Th1 and Th17 cells might drive the disease together. In addition, various effector cells might have different predilection for the target organ, for example symptoms resulting from lesions in the brainstem/cerebellum might be primarily due to Th17 cell activity, whereas spinal cord disease might be induced by both Th1 and Th17 cells. Since T cell subsets crossregulate each other and are further controlled by various antigen-specific regulatory T cells, in the end, disease phenotype, including sub-anatomical tissue involvement, will depend on the balance and predominance of specific effector T cell subsets promoting tissue inflammation. In due course, it may even be possible to identify the predominant effector T cell involved in the disease process, and tailor-make a regulatory T cell specifically endowed with the ability to suppress the inciting effector cell.
Acknowledgments
This work was supported by grants from the NIH (R01NS045937, R01NS035685, R37NS030843, R01A1044880, P01A1039671, P01NS038037 and a Javits Neuroscience Investigator Award to V.K.K.) and the National Multiple Sclerosis Society (RG-2571 to V.K.K.). A.J. is the recipient of a Ph.D. scholarship by the Boehringer Ingelheim Fonds. A.J. is a graduate student jointly supervised by Professor Dr. Rolf Heumann (Ruhr-University Bochum, Germany) and Professor Vijay K. Kuchroo (Brigham and Women’s Hospital, Harvard Medical School, Boston, USA).
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Merrill JE, Kono DH, Clayton J, Ando DG, Hinton DR, Hofman FM. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci U S A. 1992 Jan 15;89:574–8. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989 Nov;124:132–43. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 4.Traugott U, Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann Neurol. 1988 Aug;24:243–51. doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- 5.Kuchroo VK, Martin CA, Greer JM, Ju ST, Sobel RA, Dorf ME. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol. 1993 Oct 15;151:4371–82. [PubMed] [Google Scholar]
- 6.Baron J, Madri J, Ruddle N, Hashim G, Janeway C., Jr. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987 Jul;37:1097–102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Murphy TL, Ouyang W, Murphy KM. Induction of interferon-gamma production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur J Immunol. 1999 Feb;29:548–55. doi: 10.1002/(SICI)1521-4141(199902)29:02<548::AID-IMMU548>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004 Jul 5;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitnis T, Najafian N, Benou C, et al. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001 Sep;108:739–47. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002 Dec;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 12.Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell. 2002 Apr;109(Suppl):S109–20. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 13.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997 May 16;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 14.Ho IC, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996 Jun 28;85:973–83. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- 15.Singh RR. IL-4 and many roads to lupuslike autoimmunity. Clin Immunol. 2003 Aug;108:73–9. doi: 10.1016/s1521-6616(03)00145-1. [DOI] [PubMed] [Google Scholar]
- 16.Lafaille JJ, Keere FV, Hsu AL, et al. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med. 1997 Jul 21;186:307–12. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das MP, Nicholson LB, Greer JM, Kuchroo VK. Autopathogenic T helper cell type 1 (Th1) and protective Th2 clones differ in their recognition of the autoantigenic peptide of myelin proteolipid protein. J Exp Med. 1997 Sep 15;186:867–76. doi: 10.1084/jem.186.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009 Dec 1;183:7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrempf W, Ziemssen T. Glatiramer acetate: mechanisms of action in multiple sclerosis. Autoimmun Rev. 2007 Aug;6:469–75. doi: 10.1016/j.autrev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996 Jan 1;156:5–7. [PubMed] [Google Scholar]
- 21.Jones LS, Rizzo LV, Agarwal RK, et al. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997 Jun 15;158:5997–6005. [PubMed] [Google Scholar]
- 22.Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Billiau A. Anti-IL-12 antibody prevents the development and progression of collagen-induced arthritis in IFN-gamma receptor-deficient mice. Eur J Immunol. 1998 Jul;28:2143–51. doi: 10.1002/(SICI)1521-4141(199807)28:07<2143::AID-IMMU2143>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000 Nov;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 24.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003 Feb 13;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 25.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005 Jan 17;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008 Jun 19;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matusevicius D, Kivisakk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999 Apr;5:101–4. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 28.Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999 Feb 1;162:1246–51. [PubMed] [Google Scholar]
- 29.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998 Oct;111:645–9. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 30.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005 Oct;237:123–30. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003 Dec 1;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 32.Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009 Jan;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006 May 11;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 34.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006 Feb;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-b induces development of Th17 lineage. Nature. 2006 April 30;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 36.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007 Mar 30;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 37.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007 Jul 26;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007 Jul 26;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007 Sep;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 40.Huber M, Brustle A, Reinhard K, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008 Dec 30;105:20846–51. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brüstle A, Heink S, Huber M, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007 Sep;8:958–66. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 42.Chen Q, Yang W, Gupta S, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008 Dec 19;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009 Apr 17;30:576–87. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008 Jun;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008 Jul 17;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004 Jul 9;305:248–51. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 47.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004 Jan;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 48.Ivanov II, McKenzie BS, Zhou L, et al. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17(+) T Helper Cells. Cell. 2006 Sep 22;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009 Apr;21:146–52. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008 Jan;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009 Feb;10:167–75. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pot C, Jin H, Awasthi A, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009 Jul 15;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005 Nov;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005 Nov;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Laurence A, Kanno Y, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006 May 23;103:8137–42. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009 Mar;10:314–24. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Awasthi A, Riol-Blanco L, Jäger A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009 May 15;182:5904–8. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boniface K, Bak-Jensen KS, Li Y, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009 Mar 16;206:535–48. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002 Jun 1;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 60.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006 Oct 2;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007 Feb 8;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 62.Wolk K, Witte E, Witte K, et al. Biology of interleukin-22. Semin Immunopathol. 2010 Mar;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 63.Liu X, Leung S, Wang C, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. Feb;16:191–7. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 64.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008 May 8;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du J, Huang C, Zhou B, Ziegler SF. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol. 2008 Apr 1;180:4785–92. doi: 10.4049/jimmunol.180.7.4785. [DOI] [PubMed] [Google Scholar]
- 66.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007 Mar;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007 Jul 13;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 68.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008 May 1;453:106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 69.Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008 May 1;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 70.O’Connor RA, Prendergast CT, Sabatos CA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008 Sep 15;181:3750–4. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007 Apr;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007 Apr;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 73.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998 Feb 15;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirota K, Yoshitomi H, Hashimoto M, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007 Nov 26;204:2803–12. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lexberg MH, Taubner A, Forster A, et al. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008 Oct;38:2654–64. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 76.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008 Nov 15;181:7205–13. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bending D, De La Pena H, Veldhoen M, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 Feb 2; doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009 Jan 16;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008 Apr 14;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008 Oct 27;205:2633–42. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008 Mar;14:337–42. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004 Apr;14:164–74. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 84.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008 Jul 18;29:138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008 Jul 7;205:1535–41. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008 Oct;29:479–86. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Axtell RC, de Jong BA, Boniface K, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. Apr;16:406–12. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007 Apr;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 89.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008 Dec;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008 Dec;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 91.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997 Oct;27:2536–40. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 92.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009 Aug;127:450–8. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. Mar;11:250–6. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Longphre M, Li D, Gallup M, et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest. 1999 Nov;104:1375–82. doi: 10.1172/JCI6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fontaine RH, Cases O, Lelievre V, et al. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death Differ. 2008 Oct;15:1542–52. doi: 10.1038/cdd.2008.79. [DOI] [PubMed] [Google Scholar]
- 96.Elyaman W, Bradshaw EM, Uyttenhove C, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009 Aug 4;106:12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nowak EC, Weaver CT, Turner H, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009 Aug 3;206:1653–60. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000 Mar 6;191:813–22. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson D, Seeldrayers PA, Weiner HL. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988 Mar 15;444:195–8. doi: 10.1016/0006-8993(88)90929-8. [DOI] [PubMed] [Google Scholar]
- 100.O’Brien PJ, Molino M, Kahn M, Brass LF. Protease activated receptors: theme and variations. Oncogene. 2001 Mar 26;20:1570–81. doi: 10.1038/sj.onc.1204194. [DOI] [PubMed] [Google Scholar]
- 101.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001 Jan;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 102.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001 Dec;13:533–8. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 103.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006 Sep;27:110–8. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 104.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008 Jul 18;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lochner M, Peduto L, Cherrier M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008 Jun 9;205:1381–93. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009 Mar 24;106:4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oldenhove G, Bouladoux N, Wohlfert EA, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009 Nov 20;31:772–86. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009 Sep;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007 Dec;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 110.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007 Dec;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 111.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007 Dec;8:1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 112.Vieira PL, Christensen JR, Minaee S, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004 May 15;172:5986–93. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 113.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006 Sep;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 114.Gagliani N, Jofra T, Stabilini A, et al. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. Feb;59:433–9. doi: 10.2337/db09-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]