Abstract
Short, “one-pot” syntheses of malondialdehyde adducts of deoxyguanosine, deoxyadenosine, and deoxycytidine are described. These syntheses proceed in improved yield and easier purification than previous syntheses and are well suited for the preparation of isotopically labeled nucleoside adducts for biomarker and metabolic studies.
Keywords: Malondialdehyde; DNA adducts; M1dG, biomarkers
INTRODUCTION
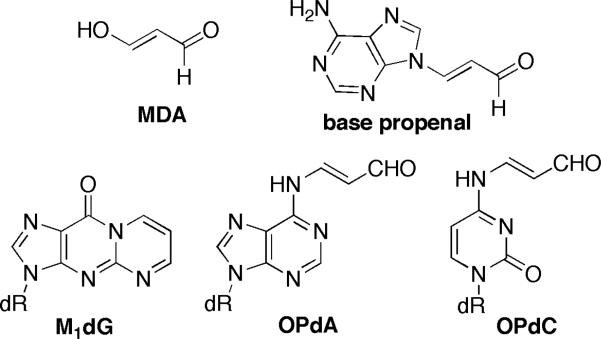
Malondialdehyde (MDA) and its reactive equivalent, base propenal, are products of oxidative damage to polyunsaturated fatty acids and DNA, respectively (Figure 1). They react with proteins and DNA and are cytotoxic and mutagenic.[1] The major product from the reaction of MDA or base propenal with DNA is 3-(2-deoxy-β-D-erythro-pentofuranosyl)-pyrimido[1,2-α]purin-10(3H)-one (M1dG); minor products are N6-(3-oxo-1-propenyl)-2′-deoxyadenosine (OPdA) and N4-(3-oxo-1-propenyl)-2′-deoxycytidine (OPdC).
FIGURE 1.
DNA adducts of MDA and base propenal.
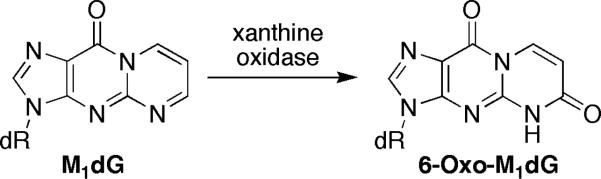
Modified nucleosides are of interest as biomarkers to assess toxicant exposure or to evaluate disease onset and progression.[2] Mass spectrometry is emerging as the method of choice for biomarker identification and quantitation. M1dG is excised from genomic DNA by nucleotide excision repair (NER) and has been detected in the urine of healthy humans.[3,4] Urinary analysis is a convenient, noninvasive approach for determining adduct burden, making M1dG an attractive biomarker for oxidative stress. We recently reported that M1dG is a substrate for cellular oxidases and is rapidly converted to 3-(2-deoxy-β-D-erythro-pentofuranosyl)-3,4-dihydropyrimido[1,2-α]purine-6,10-dione (6-oxo-M1dG) after NER excision from DNA (Scheme 1).[5] Understanding such processes are essential for the development and validation of potential biomarkers.
SCHEME 1.
Metabolic oxidation of M1dG.
We required an efficient synthesis of M1dG that would be amenable to the incorporation of a radioisotope for further metabolic studies. Similarly, the mass spectrometric quantitation of a biomarker can be achieved by the stable isotope dilution method if a labeled standard is available. M1dG was previously synthesized by the direct reaction of dG or guanine with MDA or a reactive equivalent.[6,7] These procedures suffer from very low overall yield and tedious purification of the products. Similarly, OPdA and OPdC were prepared in low yield from the nucleosides.[8,9] The formation of nucleoside adducts of higher oligomers of MDA was a complicating factor to these approaches. More recently, a new strategy for the synthesis of M1dG, OPdA, and OPdC was developed and is exceptionally well suited for the preparation of modified oligonucleotides by a post-synthetic modification strategy;[10,11] however, this approach required several steps from activated nucleoside analogues and is therefore, not ideal for the preparation of isotopically labeled materials. We report here one-pot syntheses of M1dG, OPdA, and OPdC from the corresponding nucleosides that are well suited for such applications.
RESULTS
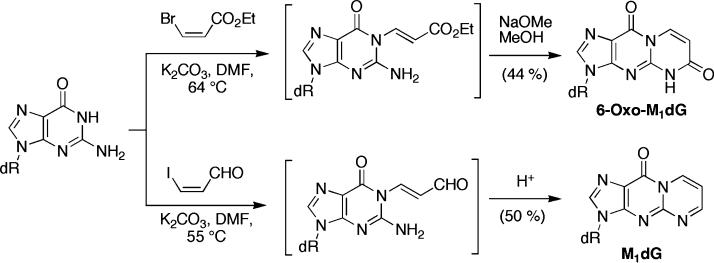
Selective alkylation of dG at the N1-position can often be achieved by forming the N1-anion.[12,13] We took advantage of this reactivity for the synthesis of 6-oxo-M1dG by alkylating dG with commercially available ethyl cis-3-bromoacrylate (K2CO3, DMF) through an addition-elimination pathway to afford N1-(3-carboxyethyl-1-propenyl)-dG (Scheme 2).[5] Subsequent treatment with sodium methoxide in methanol provided 6-oxo-M1dG. We reasoned that this strategy could be extended to the lower oxidation state homologue M1dG by using 3-iodoacrolein[14] as the electrophile. 3-Iodoacrolein was condensed with dG as described above and after neutralization with dilute acetic acid,[15] M1dG was obtained in 50% isolated yield (Scheme 2).
SCHEME 2.
One-pot syntheses of 6-oxo-M1dG and M1dG.
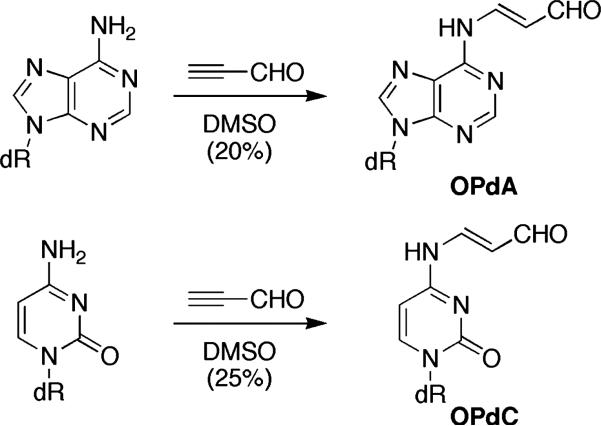
The reaction of 2-iodoacrolein with dA and dC under the same conditions gave OPdA and OPdC in low yields (<10%) with few by-products. Prolonged heating lead to increased by-products without an improvement in yield. When propargyl aldehyde[16] was used in place of 3-iodoacrolein and the reaction stirred in DMSO, OPdA, and OPdC were obtained in 20 and 25% isolated yield, respectively (Scheme 3). Once again, purification was simplified by the presence of few byproducts. Attempts to improve the yield by heating or use of a microwave reactor resulted in undesired byproducts, presumably from the polymerization of propargyl aldehyde. Condensation of propargyl aldehyde with dG under either reaction conditions gave M1dG in low yield.
SCHEME 3.
One-pot syntheses of OPdA and OPdC.
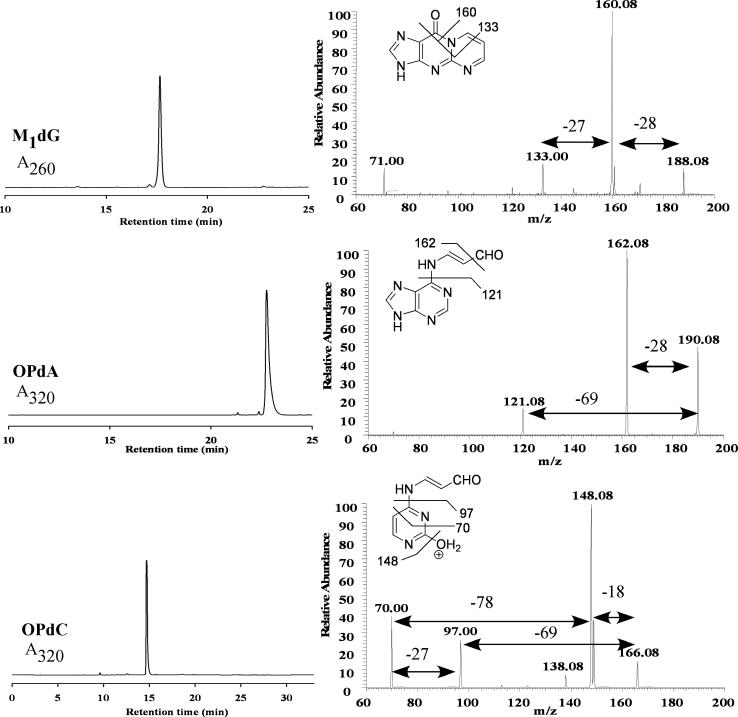
Nucleoside adducts can be identified by comparison of their mass spectrum to an authentic standard. The purified nucleosides were analyzed by tandem mass spectrometry and the MS3 spectra for each are shown in Figure 2. Each nucleoside showed a neutral loss of the deoxyribose unit (−116 Da) upon collision-induced dissociation (CID), which is characteristic of nucleosides. The major transition for M1dG (m/z 188 → 160) corresponds to loss of CO (−28 Da); the MS4 spectrum (not shown) demonstrated further loss of HCN (−27 Da, m/z 160 → 133). The major transitions for OPdA (m/z 190 → 162) and OPdC (m/z 166 → 148) represented the loss of CO and dehydration, respectively. In addition, each showed the loss of 69 Da assigned to the N6-oxopropenyl (m/z 190 → 121) an N4-oxopropenyl (m/z 166 → 97) groups. The MS4 spectrum of OPdC showed that the fragment ion at m/z 70 could arise from either the m/z 166 (−96 Da) or 97 (−27 Da) ions.
FIGURE 2.
LC analysis (left) and MS3 spectra (right) for M1dG, OPdA, and OPdC.
In summary, one-pot syntheses of the modified nucleosides M1dG, OPdA, and OPdC have been developed. The syntheses start from the corresponding 2′-deoxynucleosides and utilized readily available reagents. A key benefit to the procedures is that few side products were formed, which greatly simplified the purification. The yields of OPdA and OPdC are still somewhat low; however, they are improved when compared to previous preparations and the simplicity of the present method is attractive. These procedures should be readily amenable to the preparation of the modified nucleosides with radioisotopes (i.e., 14C) for metabolic studies or stable isotopes (i.e., 15N) for mass spectrometric quantitation from biological samples. Such studies are currently ongoing in our labs.
EXPERIMENTAL
3-(2-deoxy-β-D-erythro-pentofuranosyl)-pyrimido[1,2-α]purin-10(3H)-one (M1dG)
Anhydrous dG (previously dried under vacuum at 78°C overnight) (1.6 g, 6 mmol) and anhydrous K2CO3 (0.93 g, 6.75 mmol) were stirred at 55°C in anhydrous DMF (18 mL) until the dG was dissolved. A solution of cis-3-iodoacrolein[14] (1.64 g, 9 mmol) in anhydrous DMF (16.4 mL) was prepared; 1 mL of this solution was added to the reaction every 15 minutes for 4 hours. The reaction was stirred for an additional 2 hours then cooled and filtered. The filtrate was evaporated under high vacuum and the residue was dissolved in water (4 mL) and 5% acetic acid (4 mL). The product was purified by reversed-phase (C18) medium pressure liquid chromatography with a Biotage SP1 apparatus (Biotage, Charlottesville, VA, USA) using a pre-packed 25 × 75 mm Biotage FLASH 25+S cartridge (KP-C18-HS) and a FLASH 25 C18 samplet for sample loading. The samplet and the column were pre-activated with 4 column volumes each of 100% methanol, and 100% acetonitrile, followed by preequilibration with 4 column volumes of 1% acetonitrile in water before sample loading. The manufacturer's suggested protocols were followed and the following gradient used at a flow rate of 25 mL/minute: 1% acetonitrile, isocratic for 2 column volumes; 1–10% acetonitrile, linear gradient for 40 column volumes; 10–20% acetonitrile, linear gradient over 8 column volumes; 20–100% acetonitrile, linear gradient for 5 column volumes; 100% acetonitrile, isocratic for 5 column volumes. The combined fractions were evaporated and lyophilized to afford M1dG (0.92 g, 50.6%). 1H NMR (D2O) δ 9.27 (dd, 1H, J = 7.2, 2.1 Hz), 8.92 (dd, 1H, J = 4.0, 2.2 Hz), 8.28 (s, 1H), 7.25 (dd, 1H, J = 7.2, 4.0 Hz), 6.43 (t, 1H, J = 7.0 Hz), 4.60 (m, 1H), 4.02 (m, 1H), 3.67 (m, 2H), 2.77 (m, 1H), 2.48 (m, 1H). 13C-NMR (D2O): δ 163.3, 154.3, 150.2, 149.9, 142.7, 138.4, 118.7, 112.3, 88.3, 85.3, 72.0, 62.5, 39.9
Propargyl aldehyde
was prepared as previously described.[16] 1H NMR (CDCl3) δ 9.2 (1H, s); 3.5 (1H, s).
N6-(3-oxo-1-propenyl)-2′-deoxyadenosine (OPdA)
In a flame-dried flask under an argon atmosphere, dA•H2O (1.84 g, 6.83 mmol) was added to anhydrous DMSO (6 mL). Neat propargyl aldehyde (100 μL, 93 mg, 1.73 mmol, 0.25 eq.) was added to the stirred solution; additional propargyl aldehyde was added every 8 hours over a 72-hour period. The solvent was removed under vacuum. The residue was dissolved in a minimum amount of water and loaded onto a 25S C18 BIOTAGE column for purification as described above to give OPdA (417 mg, 20%). 1H NMR (D2O): δ 9.18 (d, 1H, J = 8.7 Hz), 8.60 (d, 1H, J = 13.2 Hz), 8.40 (s, 1H), 8.35 (s, 1H), 6.40 (t, J = 7.0 Hz, 1H), 5.90 (dd, 1H, J = 13.5 Hz, 8.7 Hz), 4.60 (m, 1H), 4.10 (m, 1H) 3.75 (m, 2H), 2.78 (m, 1H), 2.52 (m, 1H). 13C NMR (D2O): δ 196.0, 152.7, 152.2, 151.5, 149.8, 143.5, 121.8, 111.9, 88.3, 85.6, 72.0, 62.4, 39.9.
N4-(3-oxo-1-propenyl)-2′-deoxycytidine (OPdC)
was prepared in 25% yield by the same procedure as OPdA. 1H NMR (D2O): δ 9.25 (d, 1H, J = 8.6 Hz, aldehyde-H), 8.20 (d, 1H, J = 13.7 Hz), 8.10 (d, 1H, J = 7.4 Hz), 6.22 (d, 1H, J = 7.4 Hz), 6.18 (t, J = 6.3 Hz, 1H), 5.83 (dd, 1H, J = 13.6, 8.6 Hz), 4.39 (m, 1H, H-3′), 4.05 (m, 1H), 3.80 (1H, m), 3.70 (1H, m), 2.49 (m, 1H), 2.25 (m, 1H). 13C NMR (D2O): δ 196.0, 163.0, 161.0, 149.0, 144.2, 112.0, 97.0, 87.0, 70.1, 60.9, 40.0.
Mass spectrometric analyses
were performed by direct infusion on a ThermoElectron LTQ linear ion trap instrument. A solution of the nucleosides in deionized water was directly injected into the ESI source at a flow rate of 10 μl/min. The ESI source was set up in positive ion mode as follows: capillary temperature was 325°C, source spray voltage was 4.0 kV, source current was 6.5 μA, sheath was 28 units and auxiliary gas was 23 units. Characteristic fragment ions were observed with a collision energy of 26 eV.
Analytical HPLC analysis
of M1dG, OPdA, and OPdC were performed by reversed-phase HPLC with an Atlantis C18 colum (5 μM, 4.6 × 250 mm) with the following solvent gradient (deionized water/acetonotrile) at a flow rate of 1.5 mL/min. Eluents: A: deionized water, B: acetonitrile. Initial conditions, 1% acetonitrile, then a linear gradient to 10% acetonitrile over 15 minutes, then a linear gradient to 20% acetonitrile over 5 minutes, then isocratic at 20% acetonitrile for 8 minutes, then a linear gradient to 80% acetonitrile over 2 minutes, then isocratic at 80% acetonitrile for 3 minutes, then a linear gradient back to the initial conditions over 2 minutes.
Acknowledgments
The National Institutes of Health supported this work through research grants CA87819 and ES05355 and center grant ES00267. CGK was supported by NIEHS pre-doctoral traineeship ES007028.
REFERENCES
- 1.Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma RA, Farmer PB. Biological relevance of adduct detection to the chemoprevention of cancer. Clin. Cancer Res. 2004;10:4901–4912. doi: 10.1158/1078-0432.CCR-04-0098. [DOI] [PubMed] [Google Scholar]
- 3.Fink SP, Reddy GR, Marnett LJ. Mutagenicity in Escherichia coli of the major DNA adduct derived from the endogenous mutagen malondialdehyde. Proc. Natl. Acad. Sci. USA. 1997;94:8652–8657. doi: 10.1073/pnas.94.16.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoberg AM, Otteneder M, Marnett LJ, Poulsen HE. Measurement of the malondialdehyde-2′-deoxyguanosine adduct in human urine by immuno-extraction and liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. J. Mass Spectrom. 2004;39:38–42. doi: 10.1002/jms.547. [DOI] [PubMed] [Google Scholar]
- 5.Otteneder MB, Knutson CG, Daniels JS, Hashim M, Crews BC, Remmel RP, Wang H, Rizzo C, Marnett LJ. In vivo oxidative metabolism of a major peroxidation-derived DNA adduct, M1dG. Proc. Natl. Acad. Sci. USA. 2006;103:6665–6669. doi: 10.1073/pnas.0602017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basu AK, O'Hara SM, Valladier P, Stone K, Mols O, Marnett LJ. Identification of adducts formed by reaction of guanine nucleosides with malondialdehyde and structurally related aldehydes. Chem. Res. Toxicol. 1988;1:53–59. doi: 10.1021/tx00001a010. [DOI] [PubMed] [Google Scholar]
- 7.Chapeau MC, Marnett LJ. Enzymatic synthesis of purine deoxynucleoside adducts. Chem. Res.Toxicol. 1991;4:636–638. doi: 10.1021/tx00024a006. [DOI] [PubMed] [Google Scholar]
- 8.Stone K, Ksebati MB, Marnett LJ. Investigation of the adducts formed by reaction of malondialdehyde with adenosine. Chem. Res. Toxicol. 1990;3:33–38. doi: 10.1021/tx00013a006. [DOI] [PubMed] [Google Scholar]
- 9.Stone K, Uzieblo A, Marnett LJ. Studies of the reaction of malondialdehyde with cytosine nucleosides. Chem. Res.Toxicol. 1990;3:467–472. doi: 10.1021/tx00017a013. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Marnett LJ, Harris TM, Rizzo CJ. A novel synthesis of malondialdehyde adducts of deoxyguanosine, deoxyadenosine and deoxycytidine. Chem. Res. Toxicol. 2004;17:144–149. doi: 10.1021/tx034174g. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Kozekov ID, Kozekova A, Tamura P, Marnett LJ, Harris TM, Rizzo CJ. Site-specific synthesis of oligonucleotides containing malondialdehyde adducts of deoxyguanosine and deoxyadenosine via a post-synthetic modification strategy. Chem. Res. Toxicol. 2006;19:1467–1474. doi: 10.1021/tx060137o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Johnson F. Regioselective 1-alkylation of 2′-deoxygunaosine. Nucleosides, Nucleotides, Nucleic Acids. 2002;21:435–447. doi: 10.1081/NCN-120014816. [DOI] [PubMed] [Google Scholar]
- 13.Goodenough AK, Kozekov ID, Zang H, Choi J-Y, Guengerich FP, Harris TM, Rizzo CJ. Site-specific synthesis and polymerase bypass of oligonucleotides containing a 6-hydroxy-3,5,6,7-tetrahydro-9H-imidazo[1,2-a]purin-9-one base, an intermediate in the formation of 1,N2-etheno-2′-deoxyguanosine. Chem. Res. Toxicol. 2005;18:1701–1714. doi: 10.1021/tx050141k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer C, Marek I, Normant J. (E)- or (Z)-β-Iodoacrolein. A novel versatile synthon. Synlett. 1993:386–388. [Google Scholar]
- 15.Riggins JN, Pratt DA, Voehler M, Daniels JS, Marnett LJ. Kinetics and Mechanism of the general-acid-catalyzed ring-closure of the malondialdehdye-DNA adduct, N2-(3-oxo-1-propenyl)deoxyguanosine (N2OPdG–), to 3-(2′-deoxy-β-D-erythro-pentofuranosyl)pyrimido[1,2-α]purin-10(3H)-one (M1dG). J. Am. Chem. Soc. 2004;126:10571–10581. doi: 10.1021/ja040010q. [DOI] [PubMed] [Google Scholar]
- 16.Sauer JC. Propiolaldehyde. Org. Synth. 1956;36:66–68. [Google Scholar]