Abstract
Signaling by the γδ T cell receptor (TCR) is required not only for αβ/γδ lineage commitment but also to activate and elicit effector functions in mature γδ T cells. Notably, at both of these stages, the signal delivered by the γδTCR is more robust than the one delivered by either the preTCR or the αβTCR. Recent studies now provide evidence that signaling by the γδTCR is also required at other stages during γδ T cell development. Remarkably, the strength of the γδTCR signal also plays a role at these other stages, as evidenced by the findings that genetic manipulation of γδTCR signal strength affects γδ T cell maturation and effector fate. In this review, we discuss how a strong TCR signal is a recurring theme in γδ T cell development and activation.
Keywords: T cell development, Signal transduction, γδ T cell receptor, Effector function
1. Enhanced signaling proficiency of the γδ T cell receptor
There are two T cell lineages, αβ and γδ, that are defined by the antigen-binding heterodimers contained within their respective antigen receptors. While it is still not known why two T cell lineages have been conserved in all jawed vertebrates, years of study demonstrate that γδ T cells recognize antigens differently than αβ T cells, acquire effector functions faster than αβ T cells, and play specialized roles in immunity (reviewed in Refs. [1–6]). However, despite this knowledge, the signaling properties of the γδ T cell receptor (TCR) remain poorly understood.
To learn more about γδTCR signal transduction, we directly compared the signaling ability of the γδTCR with that of the more extensively studied αβTCR [7]. Because there are no known antigens for murine αβ- and γδTCRs with similar binding kinetics [8], we chose to crosslink the respective TCRs with the same anti-CD3ε monoclonal antibody (mAb) to initiate the TCR signaling cascade. When signal transduction by the two TCR isoforms was compared, the magnitude of the γδTCR signal was found to be greater than that of the αβTCR in assays that measure the mobilization of calcium and activation of the mitogen-activated protein kinase (MAPK) ERK (extracellular signal-regulated kinase) [7]. Importantly, the enhanced signaling proficiency of the γδTCR affected the kinetics of T cell activation, as the proliferative response of stimulated γδ T cells was greater than that of stimulated αβ T cells, regardless of the concentration of anti-CD3 mAb used [7]. This difference in proliferative response was due to the dependence of αβ T cells but not γδ T cells on CD28 costimulation for optimal proliferation. In fact, we found that the proliferative capacity of αβ T cells stimulated by anti-CD3ε and anti-CD28 mAbs was comparable to that of γδ T cells stimulated by anti-CD3ε mAb alone [7]. As would be expected, the discovery of the enhanced signaling proficiency of the γδTCR had a significant impact not only on our perception of γδ T cells but also on our present understanding of T cell development and function.
2. γδTCR signal strength and the αβ/γδ lineage fate decision
Both αβ and γδ T cells arise from a common thymic precursor but diverge into separate lineages early in ontogeny [9]. Once they diverge, αβ and γδ lineage cells follow different developmental pathways. Differentiation along the αβ lineage begins at the immature CD4− CD8− (double negative; DN) stage. Following expression of, and signaling by, the pre-T cell receptor (preTCR), immature αβ lineage cells undergo a strong proliferative burst, transition to the CD4+ CD8+ (double positive; DP) stage and initiate rearrangement at the TCRα locus [10].DP thymocytes that are capable of expressing amature αβTCR then undergo a selection process based on the affinity of their TCRs for self-peptides/self-MHC and eventually emerge as functionally competent CD4+ or CD8+ single positive (SP) thymocytes [11,12]. Differentiation along the γδ lineage also begins at the immature DN stage; however, it does not proceed through developmental stages defined by preTCR expression, CD4/CD8 coreceptor expression or extensive proliferation. Instead, γδ lineage cells express only the mature γδTCR complex, remain DN, and undergo a small proliferative burst relative to αβ lineage (i.e., preTCR+) DN thymocytes [13–15].
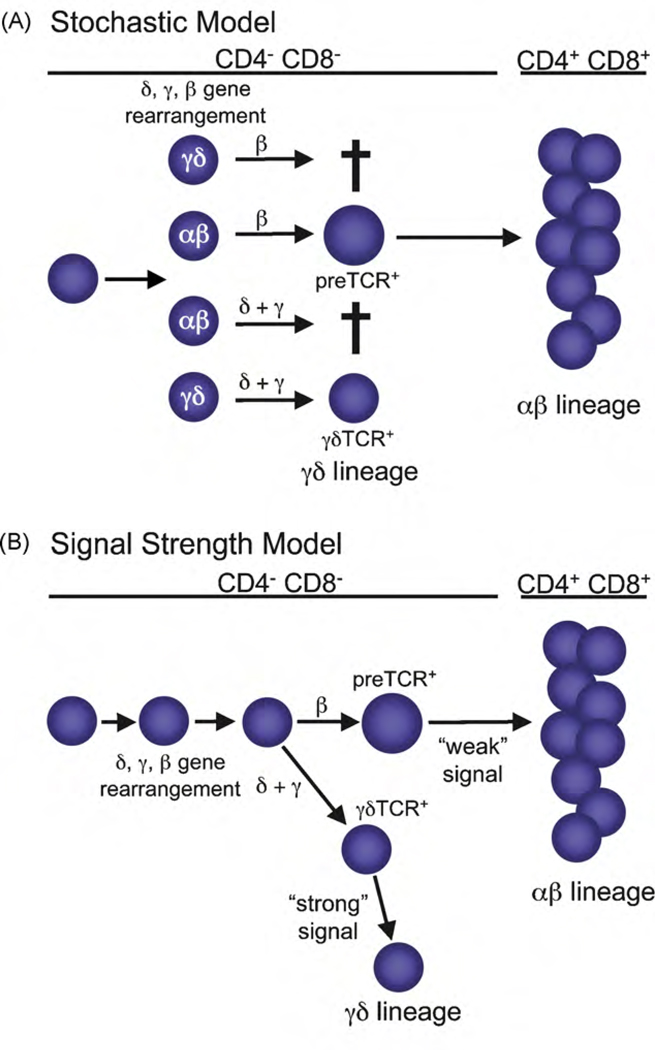
Several models have been proposed to explain the mechanism(s) by which an immature DN thymocyte chooses to become either an αβ or γδ T cell (reviewed in Refs. [16–21]). Of these, the two which are currently in favor are the stochastic model and the signal strength model (Fig. 1). The stochastic model proposes that the fate of the cell is determined randomly prior to expression of a TCR and that the isoform of the TCR must match the predetermined fate of the cell in order for that cell to survive and mature (Fig. 1A). Evidence supporting this model is the heterogeneous expression of intracellular and surface proteins, which include transcription factors and cytokine receptors, in the progenitor pool that gives rise to αβ and γδ T cells [22,23]. This heterogeneity may reflect the activation of αβ or γδ lineage-specific molecular programs prior to the expression of a functional TCR isoform.
Fig. 1.
Current models of αβ/γδ lineage commitment. (A) Stochastic model: in this model, the lineage fate decision occurs randomly prior to the expression of either the preTCR or the γδTCR. Nonetheless, the expressed TCR isoform must match the predetermined fate of the immature thymocyte in order for that cell to mature. If the isoform does not match the predetermined fate, then the immature thymocyte undergoes apoptosis. (B) Signal strength model: in this model, the strength of the TCR signal dictates the fate of the cell, with cells receiving a strong signal choosing the γδ T cell fate and cells receiving a weak signal choosing the αβ T cell fate. In a wild-type thymus, it is usually the γδTCR that delivers a strong TCR signal and the preTCR that delivers a weak TCR signal.
SOX13, which encodes a high-mobility-group transcription factor, is one of the genes that is differentially expressed in the progenitor pool (i.e., approximately 50% of immature DN thymocytes express this gene) [23]. Notably, SOX13 is also expressed in γδ thymocytes but not in DP thymocytes, suggesting that SOX13 expression in immature DN thymocytes marks cells committing to the γδ lineage (Table 1) [23]. To determine whether Sox13 plays a role in the αβ/γδ lineage decision, Melichar et al. [23] generated transgenic mice in which Sox13 is expressed in all immature DN thymocytes. In both fetal and adult Sox13 Tg mice, there was a striking decrease in the number of αβ lineage cells (i.e., DP thymocytes) but no concomitant increase in the number of γδ thymocytes. Further analysis demonstrated that, in Sox13 Tg mice, the proliferative status of immature DN thymocytes as well as the viability of both αβ and γδ thymocytes were reduced. Moreover, DP thymocytes from Sox13 Tg mice were found to express TCRγ transcripts, which are normally not detected in wild-type DP thymocytes [23]. Because of this latter finding, the authors concluded that Sox13 is able to impose a γδ lineage-specific molecular program when its expression is enforced in αβ lineage cells [23]. However, since proliferation is required to silence transcription at the TCRγ locus in αβ lineage cells as they transition from the DN to DP stage [24], an alternative explanation for this result, which is consistent with the thymic phenotype of Sox13 Tg mice, is that Sox13 regulates cellular proliferation, in that cells expressing no or low levels of Sox13 have a higher proliferative capacity than those expressing high levels of Sox13. Therefore, considering that αβ lineage cells are more dependent than γδ lineage cells on cellular proliferation for their development, it follows that overexpression of Sox13 would have a greater impact on their generation than on the generation of γδ lineage cells. Interestingly, in the absence of Sox13, the converse phenotype is observed, namely a significant decrease in the number of γδ thymocytes and no change in the number of DP thymocytes compared to wild-type mice [23]. However, it is important to note that because SOX13 is expressed in multiple tissues (reviewed in Ref. [25]) and because Sox13−/− mice die prematurely for an as yet unknown reason [23], it is not clear whether the defects in γδ T cell development that are observed in Sox13−/− fetuses are in fact due to the loss of Sox13 in γδ T cell precursors. Thus, more studies are required to elucidate the functional significance of SOX13 expression in both immature DN thymocytes and γδ lineage cells.
Table 1.
Recently identified genes that play a role in the commitment and/or development of γδ lineage cells.
SOX13, sex-determining region (Sry)-related high-mobility-group (HMG) box; EGR1, early growth response 1; ID3, inhibitor of DNA binding 3.
The signal strength model differs from the stochastic model in that TCR signaling plays a primary (i.e., deterministic), rather than a secondary (i.e., confirmatory), role in the lineage decision process (Fig. 1B). Specifically, it posits that the strength of the signal delivered by the antigen receptor instructs lineage choice, with immature DN thymocytes receiving a strong signal choosing the γδ T cell fate and those receiving a weak signal choosing the αβ T cell fate [21]. Although the signal that directs lineage choice can potentially be delivered by any TCR isoform, under normal conditions, it is the γδTCR that transduces the strong signal and the preTCR that transduces the weak signal [26,27]. Genetic manipulation of the strength of the signal through a single TCR isoform, namely the γδTCR, provides strong evidence in support of this model. First, these studies demonstrated that the critical factor in dictating the αβ/γδ lineage decision is the strength of the TCR signal strength, not the type of TCR expressed [26,27]. Moreover, the genetic manipulations affected lineage choice in a consistent manner, with attenuation of γδTCR signal strength favoring the αβ fate and augmentation of γδTCR signal strength favoring the γδ fate [26,27]. Recently, the extracellular signal-related kinase (ERK)-early growth response gene (Egr)-inhibitor of DNA binding 3 (Id3) or ERK-Egr-Id3 pathway was identified as a signaling pathway that is activated in γδ lineage cells as a consequence of a strong TCR signal [27,28]. Accordingly, alterations in the expression levels of either EGR1 or ID3 in immature DN thymocytes had a significant effect on αβ/γδ lineage commitment (Table 1) [27,28]. Despite the fact that these data support the signal strength model, it is also conceivable that TCR signal strength does not determine lineage fate but instead confirms the fate decision of pre-committed immature DN thymocytes. However, a recent study demonstrated that the progeny of a single thymocyte, expressing either the γδTCR or the preTCR and destined to adopt the αβ lineage fate, can be redirected to the γδ lineage by a strong TCR signal, indicating that pre-commitment does not occur prior to TCR signaling [29]. These results suggest that, regardless of which molecules are expressed in immature thymocytes, or their potential to influence the decision process, the strength of the TCR has the final say.
3. γδTCR signal strength and γδ T cell maturation
The maturation stages of γδ thymocytes are not as well-defined as those of αβ thymocytes and, to date, very few surface antigens have been identified to define their maturation stages. γδTCR surface expression is first detected on DN thymocytes expressing the CD25 and CD24 surface antigens [15,26,30]. Subsequent signaling by the γδTCR is necessary to downregulate CD25 expression, as virtually all TCRγδ+ thymocytes in mice bearing mutations in critical components of the TCR-coupled signaling pathway retain CD25 expression [26,30]. Interestingly, not all γδ thymocytes downregulate CD24 expression in the thymus. Although a small percentage of CD24lo TCRγδ+ cells can be detected in the thymus [15,31,32], the vast majority of γδ thymocytes that are exported to the periphery are CD24hi, suggesting that these recent thymic emigrants undergo some of their maturation in the periphery [31,32].
Notably, decreasing, not increasing, γδTCR signal strength has an effect on γδ T cell maturation [27,33]. Lck is a positive regulator of both αβ- and γδTCR signaling [33–36]; therefore, γδTCR signal strength can be attenuated by reducing or eliminating the expression of Lck. When γδ T cell maturation was examined in γδTCR Tg Lck+/− or Lck−/− mice, significant decreases in the numbers of both CD24lo γδ thymocytes [27] and peripheral CD24hi γδ T cells [33] were observed. Interestingly, augmenting γδTCR signal strength, by reducing the expression levels of the negative regulator Fyn [33,37], had no effect on γδ T cell maturation as evidenced by the wild-type numbers of CD24hi γδ T cells in the periphery of γδTCR Tg Fyn+/− mice [33]. Together, these data indicate that a relatively strong γδTCR signal is required following γδ T cell commitment for the maturation, survival and/or export of thymic γδ T cells.
4. γδTCR signal strength and acquisition of effector fates
Recent studies have demonstrated that γδ T cells have the potential to adopt multiple effector fates and, importantly, that these effector fates are predetermined in the thymus [38–40]. For at least some of these effector fates, there is evidence that interactions between the γδTCR and endogenous self-ligand are required for their fate selection [38,40]. Accordingly, because of this dependence on ligand-induced signaling, these effector fate decisions are sensitive to alterations in γδTCR signal strength. However, as ligand may play a role in the αβ/γδ lineage decision [27], it is not clear whether these effector fate decisions occur concurrently with or subsequent to the γδ lineage fate decision (Fig. 2).
Fig. 2.
Proposed scenarios by which thymic γδ T cells acquire effector functions. γδ T cells acquire the potential to differentiate into effectors, which are able to produce IL-17, IFNγ or both IFNγ and IL-4, in the thymus. As it is not known whether the acquisition of effector fate occurs concurrently with or subsequent to commitment to the γδ lineage, we have designated the cell that has the potential to give rise to these different effectors by a “?”. Moreover, based on current data, we have ordered the different effector fates relative to one another according to their dependence on ligand- and cytokine-induced signaling. (A) In the first scenario, all effector fates represent different lineages, with the resulting effector fate depending on the specificity of theγδTCR in addition to the availability of self-ligands and various cytokines. (B) In the second scenario, CD27+ γδ thymocytes, which all have the potential to become IFNγ-producers, represent a single effector lineage that can give rise to CD122+ CD27+ γδ thymocytes following encounter with self-antigen. (C) In the last scenario, we have grouped NKT-like, CD122+ CD27+ and CD122− CD27+ γδ subsets into one lineage based on their potential to produce IFNγ. As NKT-like and CD122+ CD27+ γδ subsets require interactions with self-antigen, we propose that it is the strength of the signal delivered by this interaction that dictates effector fate, with cells receiving the stronger signal becoming NKT-like γδ T cells.
The Vγ1+ Vδ6.3/6.4+ γδ T cell subset shares properties with NKT cells, in that they express promyelocytic leukemia zinc finger protein or PLZF, a transcription factor required for the development of functional NKT cells, and they produce IL-4 and/or IFNγ following TCR engagement [40–44]. The restricted expression of PLZF to a γδ T cell subset with limited Vγ and Vδ usage suggests that TCR specificity plays a critical role in the development of these NKT-like cells [39]. Consistent with this idea is the finding that the expression of a Vγ1/Vδ6.4 γδ TCR transgene supports the generation of PLZF+ γδ T cells [39]. Remarkably, PLZF expression can also be induced in immature γδ thymocytes with a diverse repertoire (i.e., extensive Vγ and Vδ usage) following TCR cross-linking [40]. Together, these results demonstrate that a strong TCR signal, delivered either by the interaction of the Vγ1+ Vδ6.3/6.4+ γδTCR with self-ligands or by treatment with anti-TCRγδ or specific anti-Vγ mAbs, is required for the development of this γδ T cell subset [40]. Paradoxically, when TCR signal strength is attenuated, such as in mice deficient for the Tec kinase Itk or in mice expressing a signaling mutant form of LAT or SLP-76, the development of Vγ1+ Vδ6.3/6.4+ PLZF+ γδ T cells is favored [30,45–47]. One possible explanation for these data is that the signal generated by the interaction between the Vγ1+ Vδ6.3/6.4+ γδTCR and self-ligands is strong enough to surmount an attenuated TCR signal and to promote the development of this γδ T cell subset. It would be interesting to examine the development of PLZF+ γδ T cells in mice in which γδTCR signal strength has been augmented to determine whether the generation of Vγ1+ Vδ6.3/6.4+ PLZF+ γδ T cells is affected and whether PLZF expression can be induced in mature γδ thymocytes bearing Vγ and Vδ gene segments other than Vγ1 and Vδ6.3/6.4.
A second effector response of γδ T cells is the production of IFNγ. IFNγ-producing effectors are defined by the expression of the tumor necrosis factor receptor member CD27, with a subset of these CD27+ γδ T cells expressing CD122 [38,39]. CD122+ CD27+ γδ T cells are generated in the thymus through interactions with self-ligands [38].When stimulated by TCR cross-linking, these CD122+ CD27+ γδ T cells are capable of rapidly producing IFNγ [33,38]. As encounter with self-antigen is required for their development, alterations in γδTCR signal strength have been shown to affect the generation of CD122+ CD27+ γδ T cells. Significantly, both weakening and strengthening the γδTCR signal, by reducing the cellular levels of Lck and Fyn, respectively, resulted in fewer peripheral CD122+ CD27+ γδ T cells compared to wild-type mice [33]. These results suggest that selection of CD122+ CD27+ γδ T cells, at least for those bearing the Vγ6/Vδ1 γδTCR transgene, occurs over a relatively narrow signaling range.
CD122− CD27+ γδ T cells, which also have the potential to be IFNγ-producers, differ from CD122+ CD27+ γδ T cells in many ways. First, CD122− CD27+ γδ T cells require several days following TCR engagement to differentiate into IFNγ-producing effectors [39]. Second, although they also arise in the thymus, there is no evidence that CD122− CD27+ γδ T cells are selected by interactions with self-ligands [39]. However, there is evidence that their development is dependent on interactions with a quorum of DP thymocytes capable of producing cytokines such as lymphotoxin [39,48]. As would be predicted, alterations in γδTCR signal strength have no apparent effect on the generation of CD122− CD27+ γδ T cells. This is evidenced by the comparable numbers of CD122− CD27+ γδ T cells in wild-type, Lck+/− and Fyn+/− mice [32]. These results indicate that the selection of CD122− CD27+ γδ T cells, compared to that of CD122+ CD27+ γδ T cells, is less dependent on TCR signaling.
The third known effector fate of γδ T cells is to produce IL-17. γδ T cells destined to be IL-17 producers express the IL-23 receptor [49–51] but not CD122 and CD27 [38,39]. Current data indicate that encounter with self-antigens in the thymus is not required for the generation of this γδ T cell effector subset [38]. Accordingly, when γδTCR signal strength is either attenuated by reducing Lck levels or augmented by reducing Fyn levels, the expression of IL23R and IL12RB1 (subunits of the IL-23 receptor [52]) is unaffected [33]. A recent report demonstrates that the acquisition of IL-17-producing ability requires TGF-β signaling, as mice deficient in TGFβ1 and Smad3, an intermediate in the TGF-β signaling pathway, have a selective impairment in the generation of IL-17-producing γδ T cells [53]. Thus, these results suggest that signals other than those delivered through the γδTCR play a role in shaping the γδ effector repertoire.
5. Concluding remarks
Data is accumulating to support a model in which TCR signal strength has a critical and deterministic role at multiple points in γδ T cell development, affecting lineage choice, maturation and acquisition of effector functions. An intriguing concept emerging from these findings is that TCR signal strength may be utilized to achieve entirely different outcomes in αβ and γδ lineage cells. In the αβ lineage, it is well established that the strength/duration of signal regulates the outcome of thymocyte selection, with relatively weak signals promoting positive selection and continued development, whereas strong signals promote cell death by negative selection. In striking contrast, strong or sustained TCR signals appear to be required for efficient γδ lineage commitment and maturation. It is tempting to speculate that these differential signaling requirements may reflect a hierarchical and functional relationship between αβ and γδ T cells. γδ T cells recognize unprocessed antigen, are not necessarily dependent on costimulation by APCs, and appear to require strong interaction with self-ligands for their maturation. These properties may be optimized for the development of rapidly responding T cell populations with a limited TCR repertoire, which represent a first line of defense against common insults. In contrast, αβ T cells are self-MHC restricted, require weak interactions with self-ligand for development (and in fact are deleted by strong interactions) and are strictly dependent upon costimulation for their full activation. This latter system favors the formation of a highly diverse TCR repertoire that requires appropriate antigen processing and presentation as well as costimulation for full activation in order to prevent autoimmunity. Further investigation into the role of TCR signaling potential in the γδ T cell developmental program should provide further insights into the shared and unique aspects of αβ and γδ T cell maturation.
Acknowledgments
This research was supported in part by the Hendricks Fund for Medical Research (to S.M.H.) and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH (to P.E.L.).
References
- 1.Chien Y-H, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 2.Boismenu R, Havran WL. γδ T cells in host defense and epithelial cell biology. Clin Immunol Immunopathol. 1998;86:121–133. doi: 10.1006/clin.1997.4468. [DOI] [PubMed] [Google Scholar]
- 3.Hayday AC. γδcells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 4.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 5.Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nat Rev Immunol. 2003;2:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 6.Konigshofer Y, Chien Y-H. γδ T cells-innate immune lymphocytes? Curr Opin Immunol. 2006;18:527–533. doi: 10.1016/j.coi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Hayes SM, Love PE. Distinct structure and signaling potential of the γδTCR complex. Immunity. 2002;16:827–838. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 8.Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, et al. A population of murine γδ T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 9.Petrie HT, Scollay R, Shortman K. Commitment to the T cell receptor-αβ or -γδ lineages can occur just prior to the onset of CD4 and CD8 expression among immature thymocytes. Eur J Immunol. 1992;22:2185–2188. doi: 10.1002/eji.1830220836. [DOI] [PubMed] [Google Scholar]
- 10.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 11.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 12.Sebzda E, Mariathasan S, Ohteki T, Jones R, Bachmann MF, Ohashi PS. Selection of the T cell repertoire. Annu Rev Immunol. 1999;17:829–874. doi: 10.1146/annurev.immunol.17.1.829. [DOI] [PubMed] [Google Scholar]
- 13.Pardoll DM, Fowlkes BJ, Lew AJ, Maloy WL, Weston MA, Bluestone JA, et al. Thymus-dependent and thymus-independent developmental pathways for peripheral T cell receptor-γδ-bearing lymphocytes. J Immunol. 1988;140:4091–4096. [PubMed] [Google Scholar]
- 14.Kang J, Fehling HJ, Laplace C, Malissen M, Cado D, Raulet DH. T cell receptor γ gene regulatory sequences prevent the function of a novel TCRγ/pTα pre-T cell receptor. Immunity. 1998;8:713–721. doi: 10.1016/s1074-7613(00)80576-2. [DOI] [PubMed] [Google Scholar]
- 15.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of γδ T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 16.Kang J, Raulet DH. Events that regulate differentiation of αβTCR+ and γδTCR+ T cells from a common precursor. Semin Immunol. 1997;9:171–179. doi: 10.1006/smim.1997.0069. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald HR, Wilson A. The role of the T-cell receptor (TCR) in αβ/γδ lineage commitment: clues from intracellular TCR staining. Immunol Rev. 1998;165:87–94. doi: 10.1111/j.1600-065x.1998.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 18.Robey E, Fowlkes BJ. Theαβversus γδ T-cell lineage choice. Curr Opin Immunol. 1998;10:181–187. doi: 10.1016/s0952-7915(98)80247-1. [DOI] [PubMed] [Google Scholar]
- 19.Fehling HJ, Gilfillan S, Ceredig R. αβ/γδ lineage commitment in the thymus of normal and genetically manipulated mice. Adv Immunol. 1999;71:1–76. [PubMed] [Google Scholar]
- 20.Hayday AC, Barber DF, Douglas N, Hoffman ES. Signals involved in γδ T cell versus αβ T cell lineage commitment. Semin Immunol. 1999;11:239–249. doi: 10.1006/smim.1999.0180. [DOI] [PubMed] [Google Scholar]
- 21.Hayes SM, Shores EW, Love PE. An architectural perspective on signaling by the pre-, ββ and γδ T cell receptors. Immunol Rev. 2003;191:28–37. doi: 10.1034/j.1600-065x.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 22.Kang J, Volkmann A, Raulet DH. Evidence that γδ versus αβ T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med. 2001;193:689–698. doi: 10.1084/jem.193.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, et al. Regulation of γδ versus αβ T lymphocyte differentiation by the transcription factor Sox13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 24.Ferrero I, Mancini SJC, Grosjean F, Wilson A, Otten L, MacDonald HR. TCRγ silencing during αβ T cell development depends upon preTCR-induced proliferation. J Immunol. 2006;177:6038–6043. doi: 10.4049/jimmunol.177.9.6038. [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre V. The SoxD transcription factors - Sox5, Sox6, and Sox13 - are key cell fate modulators. Int J Biochem Cell Biol. 2010;42:429–432. doi: 10.1016/j.biocel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes SM, Li LQ, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:582–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, et al. Attenuation of γδTCR signaling efficiency diverts thymocytes to theαβlineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Lauritsen JPH, Wong GW, Lee S-Y, Lefebvre JM, Ciofani M, Kappes DJ, et al. Marked Induction of the helix-loop-helix protein Id3 promotes the γδ T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed αβ versus γδ lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuñez-Cruz S, Aguado E, Richelme S, Chetaille B, Mura AM, Richelme M, et al. LAT regulates γδ T cell homeostasis and differentiation. Nat Immunol. 2003;4:999–1008. doi: 10.1038/ni977. [DOI] [PubMed] [Google Scholar]
- 31.Zorbas M, Scollay R. Development of γδ T cells in the adult murine thymus. Eur J Immunol. 1993;23:1655–1660. doi: 10.1002/eji.1830230739. [DOI] [PubMed] [Google Scholar]
- 32.Tough DF, Sprent J. Lifespan of gamma/delta T cells. J Exp Med. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laird RM, Hayes SM. Roles of the Src tyrosine kinases Lck and Fyn in regulating γδTCR signal strength. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008899. e8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legname G, Seddon B, Lovatt M, Tomlinson P, Sarner N, Tolaini M, et al. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity. 2000;12:537–546. doi: 10.1016/s1074-7613(00)80205-8. [DOI] [PubMed] [Google Scholar]
- 35.Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, Zamoyska R. Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol Cell Biol. 2006;26:8655–8665. doi: 10.1128/MCB.00168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeostatic proliferation of Naïve T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 37.Filby A, Seddon B, Kleczkowska J, Salmond R, Tomlinson P, Smida M, et al. Fyn regulates the duration of TCR engagement needed for commitment to effector function. J Immunol. 2007;179:4635–4644. doi: 10.4049/jimmunol.179.7.4635. [DOI] [PubMed] [Google Scholar]
- 38.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, et al. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of γδ T cells with restricted TCR diversity. Proc Natl Acad Sci U S A. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult γδ thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 42.Azuara V, Lembezat MP, Pereira P. The homogeneity of the TCRδ repertoire expressed by the Thy-1dull γδ T cell population is due to cellular selection. Eur J Immunol. 1998;28:3456–3467. doi: 10.1002/(SICI)1521-4141(199811)28:11<3456::AID-IMMU3456>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Gerber DJ, Azuara V, Levraud JP, Huang SY, Lembezat MP, Pereira P. IL-4-producing γδ T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- 44.Azuara V, Grigoriadou K, Lembezat MP, Nagler-Anderson C, Pereira P. Strain-specific TCR repertoire selection of IL-4-producing Thy-1 dull γδ thymocytes. Eur J Immunol. 2001;31:205–214. doi: 10.1002/1521-4141(200101)31:1<205::AID-IMMU205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in γδ T cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci U S A. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, et al. Enhanced development of CD4+ γδ T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, et al. Development of promyelocytic zinc finger and ThPOK-expressing innate γδ T cells is controlled by strength of TCR signaling and Id3. J Immunol. 2010;184:1268–1279. doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennington DJ, Silva-Santos B, Shires J, Theodoridis E, Pollitt C, Wise EL, et al. The inter-relatedness and interdependence of mouse T cell receptor γδ+ and αβ+ cells. Nat Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 49.Awasthi A, Riol-Blanco L, Jäger A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 53.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, et al. Cutting edge: spontaneous development of IL-17-producing γδ T cells in the thymus occurs via a TGF-beta1-dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]