Abstract
The primary symptoms of Attention Deficit Hyperactivity Disorder (ADHD) include poor impulse control and impaired regulation of attention. Research has shown that the prefrontal cortex (PFC) is essential for the “top down” regulation of attention, behavior, and emotion, and that this brain region is underactive in many patients with ADHD. The PFC is known to be especially sensitive to its neurochemical environment; relatively small changes in the levels of norepinephrine and dopamine can produce significant changes in its function. Therefore, alterations in the pathways mediating catecholamine transmission can impair PFC function, while medications that optimize catecholamine actions can improve PFC regulation of attention, behavior, and emotion. This article reviews studies in animals showing that norepinephrine and dopamine enhance PFC function through actions at postsynaptic α2A-adrenoceptors and dopamine D1-receptors, respectively. Stimulant medications and atomoxetine appear to enhance PFC function through increasing endogenous adrenergic and dopaminergic stimulation of α2A-receptors and D1-receptors. In contrast, guanfacine mimics the enhancing effects of norepinephrine at postsynaptic α2A-receptors in the PFC, strengthening network connectivity. Stronger PFC regulation of attention, behavior, and emotion likely contributes to the therapeutic effects of these medications for the treatment of ADHD.
Keywords: guanfacine, prefrontal cortex, attention-deficit/hyperactivity disorder, catecholamines, norepinephrine, alpha-2A adrenergic receptors
Basic studies of catecholamine actions in the prefrontal cortex (PFC) have helped us understand how genetic insults in catecholamine signaling pathways may lead to symptoms of Attention Deficit Hyperactivity Disorder (ADHD), and how current medications may alleviate symptoms of weak attention and impulse control in ADHD and related disorders. The following provides a brief review of PFC functions and their modulation by catecholamine signaling pathways.
The Prefrontal Cortex and Attention Deficit Hyperactivity Disorder
The PFC is the most recently evolved region of the brain, subserving our highest order cognitive abilities. The cellular networks of the PFC are able to maintain representations of goals and rules and use remembered information to guide attention, actions, and emotion (Goldman-Rakic, 1995). Current evidence supports the role of the PFC in the regulation of top-down attention, i.e. attention based on relevance (Buschman and Miller, 2007). Extensive projections to the sensory association cortices allow the PFC to suppress processing of irrelevant distractions and enhance processing of meaningful stimuli that may not be inherently captivating (e.g. homework) (Barbas et al., 2005; Yamaguchi and Knight, 1990) (Figure 1). Depending on task demands, the PFC facilitates sustained attention on a single task (Wilkins et al., 1987) or manages rapid shifts in attention to accomplish multiple sequential tasks (Robbins, 2007). In addition to regulating attention, the PFC also regulates behavior and emotions. The right inferior PFC is especially important for reducing impulsive behavior and inhibiting inappropriate actions (Aron et al., 2004), while the orbital and ventromedial PFC is essential for the regulation of emotion, such as the inhibition of aggressive impulses (Best et al., 2002; Izquierdo et al., 2005; Price et al., 1996). These PFC regions act in concert to carry out the executive functions of planning and organizing appropriate actions, thoughts, and emotions.
Figure 1.
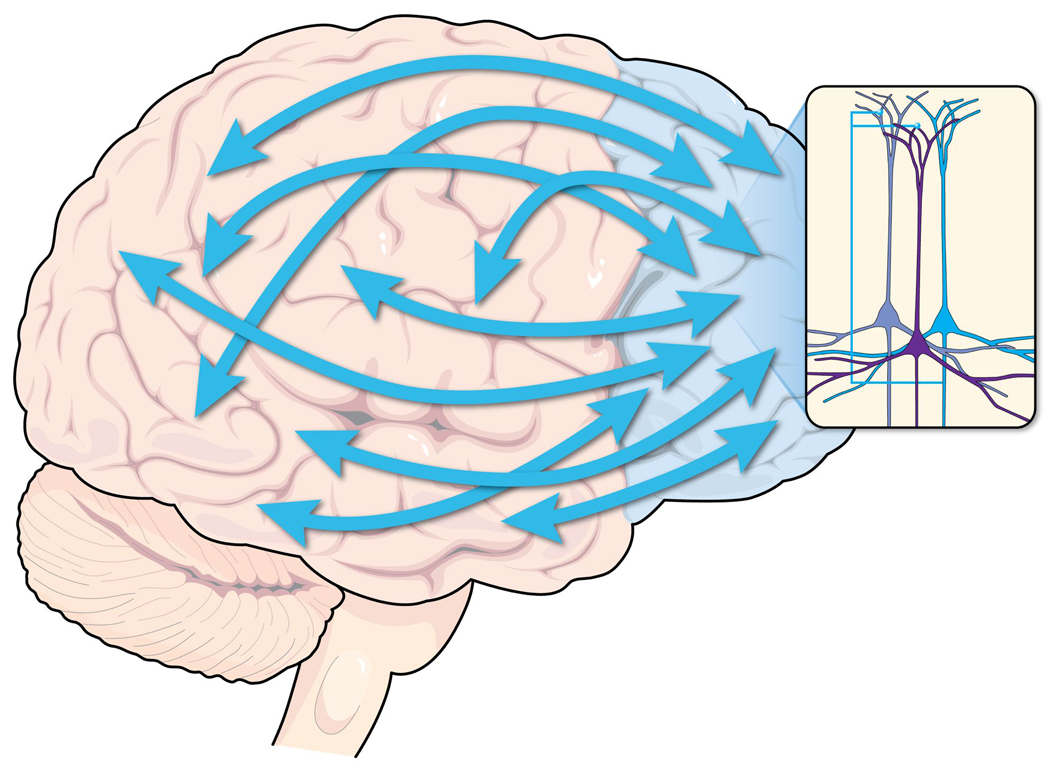
The PFC regulates attention, behavior, and emotion through extensive network connections with other brain regions. Networks of neurons within the PFC (insert) excite each other to maintain representations of goals and rules used to guide attention, behavior, and emotion.
Several imaging studies have shown that the dorsolateral PFC has a smaller volume and reduced activity in patients with ADHD compared with controls (Castellanos et al., 2002; Castellanos et al., 2008; Mostofsky et al., 2002; Rubia et al., 1999; Seidman et al., 2005). Decreased PFC activity is particularly evident in the performance of tasks that require sustained attention or inhibition of inappropriate movement (Rubia et al., 2005). In patients with ADHD, the white matter tracts that link the PFC to other brain regions also appear less well organized (Casey et al., 2007; Makris et al., 2008), and functional connectivity is reduced (Castellanos et al., 2008). Thus, PFC regulation of posterior cortical and subcortical structures is likely to be less effective in patients with ADHD, leading to decreased top-down regulation of attention, behavior, and emotion. In addition, the volumes of other brain regions, such as the caudate and cerebellum, which have reciprocal connections with the PFC, have been reported to be smaller in children with ADHD than in control subjects in some studies (Castellanos et al., 2002). The PFC is slow to mature, reaching adult dimensions in the early 20s (Giedd, 2004). There is evidence of delayed maturation of the PFC in children with ADHD (Shaw et al., 2007). This delayed maturation may vary in degree, which may explain why ADHD continues into adulthood for some individuals, yet may resolve in others. Recent studies have found slower maturation of PFC in typically developing adolescents with weaker impulse control, indicating that this is a dimensional characteristic related to the integrity of PFC gray matter (Shaw et al., 2010).
The PFC regulates attention, behavior, and emotion through networks of pyramidal neurons that interconnect on dendritic spines (Figure 1, inset). PFC networks are able to excite each other in the absence of external environmental stimulation, thus representing information such as goals for behavior, our “mental sketch pad” (Goldman-Rakic, 1995). However, recurrent PFC network activity is fragile, and extremely sensitive to the neurochemical environment. Thus, small changes in the arousal systems can markedly alter the connectivity of PFC networks (Arnsten et al., 2010). In particular, these PFC connections require that catecholamine concentrations be maintained at optimal levels (Arnsten, 2007).
Catecholamine Effects on PFC Function
The release of the catecholamines norepinephrine (NE) and dopamine (DA) in the PFC is related to arousal state (Figure 2). Low arousal conditions are associated with very low levels of NE cell firing (Aston-Jones et al., 2000; Foote et al., 1980). In contrast, under conditions of alert interest, there is moderate tonic firing, and increased phasic firing of NE and DA to relevant stimuli (Aston-Jones et al., 2000; Aston-Jones et al., 1994; Finlay et al., 1995; Foote et al., 1980; Schultz, 1998). Under stressful conditions, there are high levels of catecholamine release in PFC (Deutch and Roth, 1990; Finlay et al., 1995), which may arise from high, tonic firing of NE neurons (Aston-Jones et al., 2000; Aston-Jones et al., 1994), and DA neurons that respond to aversive events (Matsumoto and Hikosaka, 2009). Thus, the level and timing of catecholamine release in PFC can coordinate arousal state and PFC function.
Figure 2.
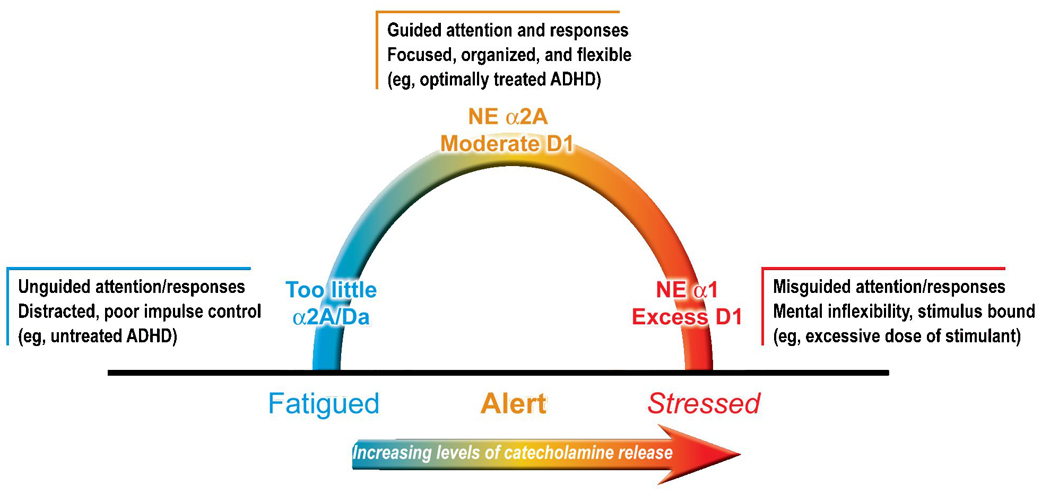
The PFC is very sensitive to its neurochemical environment; both insufficient and excessive catecholamine release impair PFC function. The catecholamines norepinephrine (NE) and dopamine (DA) are released in the PFC according to arousal state: very little during fatigue (and boredom?), a moderate amount of phasic release to relevant stimuli during alert, nonstressed waking, and high tonic release under stressful conditions. Moderate levels of NE engage postsynaptic α2A-receptors to improve PFC function, while higher levels engage α1- and β-receptors, which impair PFC function. Thus, optimal regulation of PFC function depends on postsynaptic α2A- and moderate D1-receptor stimulation. Animal studies suggest that therapeutic doses of stimulants improve PFC function by increasing endogenous noradrenergic and dopaminergic stimulation of α2A- and D1-receptors, respectively. ADHD = attention-deficit/hyperactivity disorder.
The effects of NE and DA on arousal, mood, and behavior are mediated through interactions with an extensive range of receptors that demonstrate varying affinities for these catecholamines. DA acts at both the D1 (D1- and D5-receptors) and D2 (D2-, D3-, and D4-receptors) families of receptors. These receptors have distinct localizations in primate PFC. D2-receptors in the PFC are concentrated in layer V neurons where they also increase response-related firing (Wang et al., 2004). DA also acts at D4-receptors in the PFC, where it inhibits interneurons (Mrzljak et al., 1996; Wang et al., 2002). However, the most prominent dopaminergic actions in the PFC arise from actions at D1-receptors, which are found in both superficial and deep layers of the primate PFC (Lidow et al., 1991). D1-receptors have fundamental excitatory effects on PFC function, but also modify inputs to pyramidal cells (Vijayraghavan et al., 2007).
NE acts chiefly at α1-, α2-, and β-receptors; the α2-receptors are further subdivided as α2A, α2B, and α2C. These subtypes are located presynaptically on noradrenergic neurons, dendrites, or axon terminals, and post-synaptically on neurons receiving noradrenergic input. Although presynaptic receptors are the most recognized, the majority of α2-receptors in the brain are postsynaptic (U'Prichard et al., 1979). Among noradrenergic receptors, NE has the highest affinity for α2-receptors, with lower affinity for α1- and β-receptors (Arnsten, 2000). Studies in animals indicate that NE engages predominantly α2-receptors in the PFC when the subject is alert and interested (Li and Mei, 1994), while actions at α1-receptors and possibly β-receptors are predominant under stressful conditions (Birnbaum et al., 2004; Ramos et al., 2005). Among the α2-receptors, the α2A subtype is the most prevalent in the PFC and is found both presynaptically on noradrenergic terminals and postsynaptically on the dendritic spines of PFC pyramidal cells that receive network inputs (Aoki et al., 1998; Wang et al., 2007). Although previous research focused on presynaptic receptor actions, research has established that the beneficial effects of α2-agonists arise from stimulation of postsynaptic α2A-receptors in the PFC (Arnsten and Goldman-Rakic, 1985; Cai et al., 1993).
The effectiveness of PFC network connections relies on noradrenergic stimulation of α2A receptors on the spines of PFC pyramidal cells (Wang et al., 2007). These α2A-receptors are localized on the dendritic spines near ion channels that control the impact of synaptic inputs on the spine (Wang et al., 2007). When these ion channels are open, nearby synaptic inputs are diverted and the incoming information escapes, weakening the synaptic connection (Figure 3). Alternatively, noradrenergic stimulation of α2A-receptors initiates a cascade of chemical events that close these ion channels, strengthening the synaptic connection. The strengthened synaptic connections enable the PFC to regulate attention, behavior, and emotion more effectively. Blockade of α2A-receptors in the monkey PFC with local yohimbine infusions markedly impairs PFC regulation of attention and behavior, inducing poor impulse control and locomotor hyperactivity (Li and Mei, 1994; Ma et al., 2005; Ma et al., 2003). In humans, lower activity of dopamine β-hydroxylase, the enzyme that synthesizes NE, is associated with poor sustained attention (Greene et al., 2009), poor executive function (Kieling et al., 2008), and impulsiveness (Hess et al., 2009), demonstrating that endogenous NE is important for proper PFC regulation.
Figure 3.
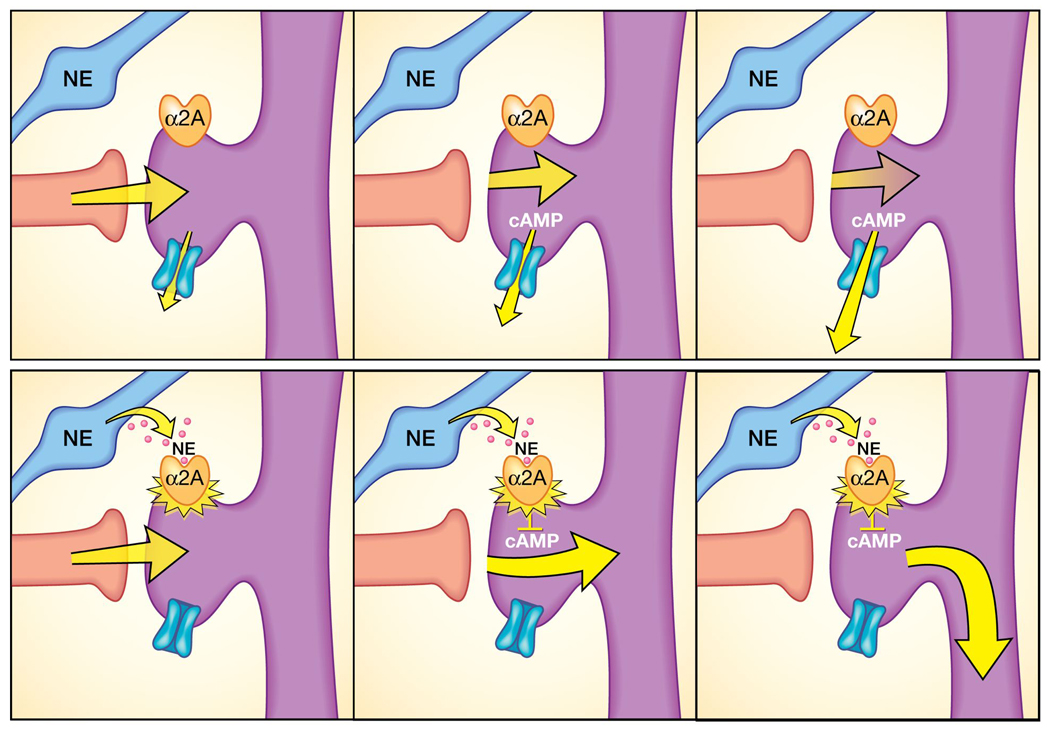
Stimulation of post-synaptic, α2A-receptors on PFC neurons by norepinephrine (NE) or guanfacine strengthens the functional connections between prefrontal cortex (PFC) neurons. Many α2A-receptors are found on the dendritic spines where PFC neurons form network connections. Top row: When there is no α2A-receptor stimulation, cyclic adenosine monophosphate (cAMP) levels are high, potassium channels open, weakening nearby synaptic inputs. As a result, PFC network firing decreases, and there is weakened capability to regulate attention, behavior, or emotion. Bottom row: When α2A-receptors are stimulated by NE or by guanfacine, they close nearby potassium channels, increasing the efficacy of network inputs, and facilitating PFC function.
In the PFC, DA plays a role complementary to that of NE, decreasing PFC neuronal activity in response to irrelevant stimuli. (Vijayraghavan et al., 2007). Dopaminergic stimulation of D1-receptors opens ion channels on a set of dendritic spines that receive inputs irrelevant to focused working memory and attention. Opening channels on these spines weakens irrelevant network connections, reducing noisy input to the neuron and enhancing the efficiency of PFC function. However, diminishing these connections excessively may be harmful in situations that require broad attention or creative solutions. In these instances, a wider range of synaptic inputs may be helpful. In addition, DA D1-receptor overstimulation (eg, under a stressful condition) may lead to disconnection of all network inputs and the cell may stop firing. As a result, PFC neurons require a specific amount of DA to function optimally (Arnsten et al., 2009; Vijayraghavan et al., 2007).
PFC function is also impaired by excessive NE release. High levels of NE release, such as those encountered during a stressful condition, engage α1-receptors that suppress PFC cell firing (Birnbaum et al., 2004). α1-Receptor stimulation impairs working memory by activating protein kinase C intracellular signaling, which is thought to be overactive in patients with bipolar disorder (Birnbaum et al., 2004).
Likely Mechanisms of Action of ADHD Medications
All medications currently approved by the US Food and Drug Administration to treat ADHD enhance catecholamine transmission in the PFC. The stimulants methylphenidate and amphetamine block reuptake at NE and DA transporters, while atomoxetine more selectively targets the NE transporter. However, as the NE transporter clears both NE and DA in the PFC, atomoxetine increases both DA and NE in the rat PFC (Bymaster et al., 2002).
Stimulants enhance both DA and NE actions in PFC
Historically, studies of stimulant actions in ADHD have focused on the effects on DA (Swanson and Volkow, 2002). This focus has arisen, in part, from the fact that positron emission tomography (PET) imaging studies can visualize DA receptors in the striatum, but these studies are unable to visualize changes in NE or DA in the PFC. This view is also based on older biochemical studies in rats that used high doses of stimulants, which increased locomotor activity and had pronounced effects on DA release throughout the brain. However, more recent biochemical studies in rats have shown that lower doses of stimulants, which produce blood levels similar to those in patients with ADHD, preferentially increase NE in the PFC (Berridge et al., 2006). These lower doses in rats improve PFC attention and working memory function, enhance PFC neuronal response, and reduce locomotor hyperactivity in juveniles (Arnsten and Dudley, 2005; Berridge et al., 2006; Kuczenski and Segal, 2002). Importantly, these doses have less effect on subcortical DA release in areas such as the nucleus accumbens (Berridge et al., 2006), which may explain why they do not cause addiction when they are used as prescribed. Animal studies have shown that therapeutic doses of methylphenidate and atomoxetine improve PFC function by increasing endogenous NE stimulation of α2-receptors and DA stimulation of D1-receptors in rats (Arnsten and Dudley, 2005) and monkeys (Gamo et al., 2010). These agents produce an inverted U dose response, whereby higher doses can actually impair PFC cognitive performance in monkeys (Gamo et al., 2010). Stimulant medications might also exert therapeutic effects through actions in striatum (e.g. caudate), and in the posterior association cortices in addition to the PFC. PET imaging studies have shown that therapeutic doses of stimulant medications engage DA receptors in striatum (Swanson and Volkow, 2002), consistent with the small but significant increases in DA release measured in rodent striatum (Berridge et al., 2006). Since PET imaging is unable to detect catecholamine actions in the cortex, drug actions in the sensory association cortices remain speculative. However, excessive doses of stimulants impair PFC function, which may account for the cognitive inflexibility observed when patients with ADHD are administered these doses of stimulant medication (Dyme et al., 1982).
Contrary to common views on stimulant medications, the effects of stimulants for the treatment of ADHD are not paradoxical. When administered in appropriate doses, these medications improve PFC function both in healthy human subjects (Elliott et al., 1997) and in animals (Arnsten and Dudley, 2005), including the reduction of locomotor hyperactivity in juvenile rats (Kuczenski and Segal, 2002). Higher doses of stimulants induce hyperactivity and addiction in rat models, but these doses exceed those given clinically (Segal and Kuczenski, 1987). Indeed, imaging studies have shown that methylphenidate enhances the efficiency of PFC activity both in healthy college students (Mehta et al., 2000) and in subjects with ADHD (Bush et al., 2008). These measureable positive effects of stimulants on attention in subjects without ADHD reflect the sensitivity of the PFC to relatively small changes in catecholamine levels. Interestingly, recent data have shown that stimulant medication can normalize gray matter volume in the developing PFC of patients with ADHD (Shaw et al., 2009). This finding counters fears that stimulant medications could interfere with brain development.
Atomoxetine enhances NE and DA transmission in PFC
Atomoxetine increases extracellular availability of both NE and DA in the rat PFC (Bymaster et al., 2002). Atomoxetine improves measures of PFC cognitive function in rats (Newman et al., 2008; Robinson et al., 2008; Seu et al., 2009), monkeys (Gamo et al., 2010; Seu et al., 2009), and humans (Chamberlain et al., 2007; Chamberlain et al., 2006). Recent studies in monkeys performing a working memory task show that atomoxetine produces an inverted U dose-response, similar to that seen with stimulants (Gamo et al., 2010). Thus, optimal doses improved working memory performance in monkeys, while higher doses impaired performance. The enhancing effects were blocked by co-administration of either an NE alpha-2 or DA D1 antagonist (Gamo et al., 2010). Interestingly, the optimal dose varied widely, which may explain variability of drug response in patients (Castellanos and Kelly, 2010). An inverted U dose-response was also observed at the neuronal level when atomoxetine was iontophoretically applied onto PFC neurons in monkeys performing a working memory task. Lower “optimal” amounts of atomoxetine enhanced “signals” (neuronal firing during the delay period for the neuron’s preferred direction) via indirectly increasing NE stimulation of alpha-2A receptors, and/or decreased “noise” (neuronal firing for nonpreferred directions) via indirectly increasing DA stimulation of D1 receptors (Gamo et al., 2010). In humans, both normal subjects (Chamberlain et al., 2006), and patients with ADHD (Chamberlain et al., 2007) show improved PFC inhibition of behavior following atomoxetine, consistent with the animal data.
Guanfacine mimics NE at postsynaptic PFC α2A-receptors
Guanfacine is the most selective α2A-adrenoceptor agonist currently available, with higher affinity for the α2A- than for the α2B- or α2C-adrenoceptor subtypes (Uhlén et al., 1995; Uhlen and Wikberg, 1991), and little affinity for the brainstem imidazoline I1 receptors that contribute to the potent hypotensive actions of clonidine (van Zwieten and Chalmers, 1994). In addition, guanfacine has weaker actions at presynaptic α2-receptors, where its activity is only one-tenth that of clonidine (Engberg and Eriksson, 1991). Not only does guanfacine mimic noradrenergic actions at α2A-receptors, its activity at postsynaptic α2-receptors may restore PFC function in patients with ADHD (Biederman et al., 2008; Scahill et al., 2001). Conversely, yohimbine blockade of α2-receptors in the PFC recreates the symptoms of ADHD: inducing locomotor hyperactivity (Ma et al., 2005), poor impulse control (Ma et al., 2003), and weaker working memory (Li and Mei, 1994).
Guanfacine enhances various PFC functions in animals, including improvements in working memory (Arnsten et al., 1988), behavioral inhibition (Steere and Arnsten, 1997), and reduced distractibility (Arnsten and Contant, 1992). These improvements are observed immediately when guanfacine is infused directly into the PFC (Mao et al., 1999) and when administered systemically (Arnsten et al., 1988; O'Neill et al., 2000; Rama et al., 1996). Dose-response studies in monkeys suggest that guanfacine-induced enhancement of PFC function can be completely dissociated from the sedative effects of guanfacine (Arnsten et al., 1988). The sedative effects of guanfacine may arise from a variety of actions, including stimulation of presynaptic α2A-receptors on noradrenergic cell bodies and terminals as well as postsynaptic α2B-receptors in the thalamus (Berridge et al., 2003; McCormick et al., 1991).
The improvements in PFC function observed in animal studies of guanfacine treatment appear to extend to human subjects; double-blind placebo-controlled studies have demonstrated that guanfacine is effective in treating ADHD symptoms. In 2 short-term, placebo-controlled, double-blind pivotal trials, an extended-release formulation of guanfacine (Intuniv™, Shire US Inc.) has shown efficacy as monotherapy for reducing symptoms of ADHD based on clinician-rated ADHD Rating Scale IV (ADHD-RS-IV) total score as well as the hyperactivity/impulsivity and inattentiveness subscale scores (Biederman et al., 2008; Sallee et al., 2009). Immediate-release guanfacine has also demonstrated positive effects in other disorders characterized by impaired PFC function, such as children with ADHD and tic disorders (Scahill et al., 2001). In that study, immediate-release guanfacine was superior to placebo on teacher ratings of inattention and hyperactivity on the ADHD-RS-IV. Finally, there are 2 trials in children with pervasive developmental disorder accompanied by hyperactivity and impulsiveness in which immediate-release guanfacine shows promise, though more study is needed in this population (Handen et al., 2008; Scahill et al., 2006).
Summary and future directions
In summary, improved understanding of catecholamine actions in the PFC has helped to lead to new treatments for ADHD. Important directions for the future include better understanding of PFC subregions, and deeper understanding of second messenger actions that regulate PFC circuits. As altered “top-down” PFC control of behavior, thought and emotion is central to most neuropsychiatric disorders, these studies are relevant to many cognitive disorders. We must respect that cognition is not a unitary phenomenon, and that different brain circuits and cognitive operations may have distinct neurochemical needs.
Acknowledgments
Dr. Arnsten’s research has been supported by PHS grants PO1 AG030004, MERIT Award AG06036, and 1RL1AA017536 within U54RR024350. This article was written by the authors, with editorial assistance from Jennifer Steeber, PhD (Health Learning Systems, part of CommonHealth, Parsippany, NJ, supported by Shire Development, Wayne, PA), who helped with artistic rendering of the figures. All ideas conveyed in this article represent those of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures:
Amy F. T. Arnsten, PhD, and Yale University receive royalties from Shire Pharmaceuticals from the sales of guanfacine (Intuniv™) for the treatment of ADHD and related disorders. Dr. Arnsten also performs research, consults, and has given seminars with Shire.
Steven R. Pliszka, MD, has received research grants from Ortho-McNeil-Janssen and Shire, is an expert witness for Eli Lilly, and has received speaker honoraria from Janssen KK (Japan).
References
- Aoki C, Venkatesan C, Go C-G, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cerebral Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of "representational knowledge": a rational bridge between genetics and the symptoms of mental illness. Cerebral Cortex. 2007;17 Suppl 1:i6–i15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Through the looking glass: Differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Contant TA. Alpha-2 adrenergic agonists decrease distractability in aged monkeys performing a delayed response task. Psychopharmacology. 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through a2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behavioral and Brain Functions (Biomed Central) 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Paspalas CD, Gamo NJ, Y Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cog Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine’s influence on prefrontal cortical cognition: Actions and circuits in behaving primates. In: Bjorklund A, Dunnett S, Iversen L, Iversen S, editors. Dopamine Handbook. Oxford, UK: Oxford University Press; 2009. pp. 230–249. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Valentino RJ, Van Bockstaele EJ, Meyerson AT. Locus coeruleus, stress, and PTSD: Neurobiological and clinical parallels. In: Murburg MM, editor. Catecholamine function in post-traumatic stress disorder: emerging concepts. Washington, D.C.: APA Press; 1994. pp. 17–62. [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Isaac S, España RA. Additive wake-promoting actions of medial basal forebrain noradrenergic alpha1- and beta-receptor stimulation. Behavioral Neuroscience. 2003;117:350–359. doi: 10.1037/0735-7044.117.2.350. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci U S A. 2002;99:8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N, Group SS. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- Birnbaum SB, Yuan P, Wang M, Vijayraghavan S, Bloom A, Davis D, Gobeske K, Sweatt D, Manji HK, Arnsten AFT. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of Vnorepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Cai JX, Ma Y, Xu L, Hu X. Reserpine impairs spatial working memory performance in monkeys: Reversal by the alpha-2 adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, Garrett A, Hinshaw SP, Greenhill LL, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Kelly C. Catecholamine modulators: lessons from nonhuman primates. J Am Acad Child Adolesc Psychiatry. 2010;49:977–979. doi: 10.1016/j.jaac.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Müller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine Improved Response Inhibition in Adults with Attention Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog. in Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Dyme IZ, Sahakian BJ, Golinko BE, Rabe EF. Perseveration induced by methylphenidate in children: preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:269–273. doi: 10.1016/s0278-5846(82)80177-2. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology. 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- Engberg G, Eriksson E. Effects of alpha-2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in EEDQ-treated rats. Naunyn-Schmiedebergs Arch. Pharmacol. 1991;343:472–477. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. PNAS. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Wang M, Arnsten AFT. Methylphenidate and atomoxetine improve prefrontal cortical function via noradrenergic alpha-2 and dopaminergic D1 receptor stimulation. J Amer Acad Child Adol Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Greene CM, Bellgrove MA, Gill M, Robertson IH. Noradrenergic genotype predicts lapses in sustained attention. Neuropsychologia. 2009;47:591–594. doi: 10.1016/j.neuropsychologia.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29:303–308. doi: 10.1097/DBP.0b013e3181739b9d. [DOI] [PubMed] [Google Scholar]
- Hess C, Reif A, Strobel A, Boreatti-Hümmer A, Heine M, Lesch KP, Jacob CP. A functional dopamine-beta-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J Neural Transm. 2009;116:121–130. doi: 10.1007/s00702-008-0138-0. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieling C, Genro JP, Hutz MH, Rohde LA. The −1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:485–490. doi: 10.1002/ajmg.b.30636. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural. Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone, and [3H]SCH 23390. Neurosci. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Ma C-L, Arnsten AFT, Li B-M. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha-2-adrenoceptors in monkeys. Biological Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ma C-L, Qi X-L, Peng J-Y, Li B-M. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18:1210. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Mao Z-M, Arnsten AFT, Li B-M. Local infusion of alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol. Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Progress Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neuroscience. 2000;20:RC651–RC656. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Levenson R, Huff R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berl) 2008;299:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Fitten LJ, Siembieda DW, Ortiz F, Halgren E. Effects of guanfacine on three forms of distraction in the aging macaque. Life Sciences. 2000;67:877–885. doi: 10.1016/s0024-3205(00)00681-0. [DOI] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem. Behav. 1996;54:1–7. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Ramos B, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AFT. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biological Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang XG, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET. Hypofrontality in Attention Deficit Hyperactivity Disorder during higher-order motor control: A study with functional MRI. Am. J. Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Sallee FR, McGough JJ, Wigal T, Donahue J, Lyn eA, Biederman J, GROUP SS. Guanfacine Extended Release in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder: A Placebo-Controlled Trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- Scahill L, Aman MG, McDougle CJ, McCracken JT, Tierney E, Dziura J, Arnold LE, Posey D, Young C, Shah B, Ghuman J, Ritz L, Vitiello B. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16:589–598. doi: 10.1089/cap.2006.16.589. [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AFT, Cohen DJ, Leckman JF. Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Amer. J. Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Advances in Pharmacology. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans AC, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans AC, Rapoport J, Giedd J. Cortical Development in Typically Developing Children With Symptoms of Hyperactivity and Impulsivity: Support for a Dimensional View of Attention Deficit Hyperactivity Disorder. Am J Psychiatry. 2010 Dec 15; doi: 10.1176/appi.ajp.2010.10030385. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere JC, Arnsten AFT. The alpha-2A noradrenergic agonist, guanfacine, improves visual object discrimination reversal performance in rhesus monkeys. Behav. Neurosci. 1997;111:1–9. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- U'Prichard DC, Bechtel WD, Rouot BM, Snyder SH. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol. Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]
- Uhlén S, Muceniece R, Rangel N, Tiger G, Wikberg JE. Comparison of the binding activities of some drugs on alpha 2A, alpha 2B and alpha 2C-adrenoceptors and non-adrenergic imidazoline sites in the guinea pig. Pharmacol Toxicol. 1995;76:353–364. doi: 10.1111/j.1600-0773.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Wikberg JES. Delineation of rat kidney alpha 2A and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modeling reveals that guanfacine is an alpha-2A-selective compound. Eur. J. Pharmacol. 1991;202:235–243. doi: 10.1016/0014-2999(91)90299-6. [DOI] [PubMed] [Google Scholar]
- van Zwieten PA, Chalmers JP. Different types of centrally acting antihypertensives and their targets in the central nervous system. Cardiovasc Drugs Ther. 1994;8:787–799. doi: 10.1007/BF00877397. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley AG, Nou E, Mazer JA, McCormick DA, Arnsten AFT. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neuroscience. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Gating of somatosensory input by human prefrontal cortex. Brain Res. 1990;521:281–288. doi: 10.1016/0006-8993(90)91553-s. [DOI] [PubMed] [Google Scholar]


