Abstract
Introduction:
Ayurvedic and herbal medicinal products contain a combination of botanicals; each of these contains a number of chemical compounds that may give the anticipated activity in combination. Therefore, it is very important to analyze and evaluate the compatibility of various active constituents and markers from different medicinal plants for their possible chemical interactions with various excipients at different storage conditions during the development of a stable polyherbal formulation.
Objective:
To study chemical stability of kalmegh (Andrographis paniculata) and kutki (Picrorhiza kurroa) extract for their active markers andrographolide, kutkoside and picroside-I and to develop stable polyherbal formulation based on the incompatibility studies.
Materials and Methods:
The compatibility study was carried out on individual ethanolic extracts of these two plants along with the commonly used excipients in the ratio of 1:1 at 40 ± 2°C and 75 ± 5% relative humidity and at a refrigeration temperature of 5 ± 1°C for initial, 7-, 15- and 30-day intervals. The analysis was carried out using the validated reverse phase–high-performance liquid chromatography methods. A stable tablet dosage form was developed based on the results of these studies.
Result:
The study suggested that the active markers of kutki (kutkoside and picroside-I) were found to be degraded in the presence of the kalmegh extract. However, the active marker of the kalmegh extract (andrographolide) was found to be stable. Both the extracts showed excellent compatibility with all the excipients used in making this formulation. No significant decrease in the kutkoside and picroside-I content from the formulation was observed.
Conclusion:
By separate granulation process the exposure of both the extracts can be minimized thus avoiding the degradation of active markers.
Keywords: Compatibility, andrographolide, kutkoside, picroside-I
INTRODUCTION
The increasing interest in the use of plant-based formulations is leading to a fast growing market for Ayurvedic, nutraceutical and polyherbal formulations. Unfortunately, the quality of a majority of them remains uncontrolled. The development of a stable polyherbal formulation is a challenging task because of the large number of varied chemical compounds present in the different medicinal plants. Hence, the entire herbal drug or herbal drug preparation is regarded as active drug substance, regardless of whether constituents with defined therapeutic activity are known. This difficulty has been acknowledged in the draft of the Strategic Plan for Regional Traditional Medicine of the World Health Organization.[1]
The chemical incompatibility leads to changes in the chemical nature, solubility, absorption and therapeutic response of these drugs. Therefore, during the formulation of new drugs or the reformulation of existing products, the interaction study between active markers of various plant extracts and commonly used excipients should be carried out thoroughly. However, no universally accepted protocol is available for evaluating the compatibility of drugs with different excipients. Assessment of possible compatibility between an active component and different excipients along with the evaluation of thermal stability are crucial parts of a normal study prior to the final formulation.[2]
In compatibility studies, temperature variation is one of the most important parameter to induce rapid chemical and physical alterations in formulations, which is determined by quantification of the active constituents over the time. Unlike a single chemical entity that forms the basis of conventional medicine, traditional Ayurvedic medicine views the polyherbal preparations as they induce combined therapeutic activity.[3–6] This creates a challenge in the development of a stable polyherbal formulation.
Andrographis paniculata (Burm.f) Nees (Acanthaceae), commonly known as Kalmegh, is used in various herbal medicines for the treatment of hepatic disorders. The plant mainly contains bitter diterpenoid lactone andrographolide (AGD) and related compounds like deoxy andrographolide, 11, 12-didehydro-14-deoxy-andrographolide, neoandrographolide and andropanoside.[7] The plant is reported to possess a protective activity against various liver disorders.[8] Kalmegh is one of the most important constituents of a variety of Ayurvedic formulations like “Bhunimbadi kwath,” which is commonly employed in liver disorders, fever etc.[9] AGD as a major component of kalmegh has potent hepatoprotective activity against galactosamine, carbon tetrachloride and paracetamol intoxication in rats.[10] AGD and related compounds were investigated for their pharmacological properties, and all showed varying degrees of anti-pyretic, anti-malarial and anti-inflammatory activity. The plant is also reported to be useful in upper respiratory tract infections.[11,12]
Picrorhiza kurroa Royle ex Benth. (Scrophulariaceae), commonly known as Kutki, is a perennial herb, growing primarily in the north-west Himalayan mountains. The rhizomes and roots of this plant are widely used in the treatment of a range of liver diseases.[13,14] Picroliv, a mixture of the iridoid glycosides kutkoside (KUT) and picroside-I (PIC) from Picrorhiza kurroa is reported to possess hepatoprotective activity as well as in vitro scavengers of oxygen free radicals, choleretic activity and antiulcer activity.[15–17] The minor constituents include picroside II, veronicoside, phenol glycosides, a number of cucurbitacin glycosides and 4-hydroxy-3-methoxyacetophenone, etc.
Phyllanthus amarus, commonly known as Bhuiamla, is widely used in the treatment of various liver ailments and has been shown to posses anti-hepatitis B virus surface antigen activity in both in vivo and in vitro studies.[18,19] It has been reported to have a strong hepatoprotective activity.[20] The major active constituents, namely phyllanthin (PLN) and hypophyllanthin (HPLN), have been shown to be anti-hepatotoxic against carbon tetrachloride- and galactosamine induced hepatotoxicity in primary cultured rat hepatocytes.[21] Several high-performance liquid chromatography (HPLC)[22] analytical procedures have been published for the quantitative determination of these active constituents from different Phyllanthus species. The four lignans, namely phyllanthin, hypophyllanthin, phyltetralin and niranthin, have been determined from Phyllanthus species by using the HPLC method.[23] Boerhaavia diffusa Linn. (Nyctanginaceae), is commonly known as punarnava. Pharmacological studies demonstrate that Boerhaavia diffusa possesses diuretic,[24] hepatoprotectant,[25,26] analgesic,[27] cardiotonic[28] and antioxidant[29] properties which makes it a very useful plant. Boerhaavia diffusa contains a large number of phytoconstituents, namely flavonoids, alkaloids, steroids, triterpenoids and lipids.
Investigations on the chemical constituents of the plant have indicated the occurrence of several rotenoids, namely boeravinone A-J.[30] The non-prenylated rotenoids boeravinone G and boeravinone H isolated from Boerhaavia diffusa are, which have been evaluated for their effect on intestinal motility.[31,32] Two rotenoids isolated from Boerhaavia diffusa, boeravinones G and H, have been found to be potently inhibit the drug efflux activity of breast cancer-resistant protein (BCRP/ABCG2), a multidrug transporter responsible for cancer cell resistance to chemotherapy.[33]
A literature search showed that both kalmegh and kutki are used in various polyherbal formulations for the treatment of a variety of liver disorders. Till date, no study has been performed on the chemical compatibility of their major active constituents (AGD, KUT and PIC). In the present study, an attempt has been made to study extensively the chemical compatibility of these active constituents with each other and with various excipients in the ratio of 1:1. The 1:1 (w:w) ratio was selected to maximize exposure of the active markers of these extracts with each other and with various excipients.[34]
The study was performed at 40 ± 2°C and 75 ± 5% relative humidity (RH) and at refrigeration temperature of 5 ± 1°C for initial, 7-, 15- and 30-day intervals. Based on these results, a tablet dosage form was developed and the stability study of the developed formulation was performed as per ICH guidelines at 30 ± 2°C and 65 ± 5% RH and 40 ± 2°C and 75 ± 5% RH for 1, 2 and 3 months.[35] The analysis was carried out using the validated reverse phase-HPLC methods.
MATERIALS AND METHODS
Standards and chemicals
Standard AGD (purity 98%, w/w) was purchased from Sigma-Aldrich,Mumbai, India. KUT (purity 97%, w/w) and PIC (purity 93%, w/w) were provided by Plantex, Vijayvada, India and were used without further purification. HPLC-grade solvents methanol, acetonitrile and ortho-phosphoric acid were obtained from Merck Ltd.,Mumbai, India. Microcrystalline cellulose RQ 101 (RanQ Remedies Pvt. Ltd., Nashik, India), methylparaben and propylparaben (Alta Lab Ltd., Mumbai, India), crospovidone XL (Boai NKY Pharmaceutical Ltd., Henan, China), croscarmellose sodium (Aditya Chemicals, Ahmedabad, India), colloidal silicon dioxide (Evonik Degussa Corp, Parsippany, USA) and magnesium stearate (Amithi Drugs, Ahmedabad, India) were also used in the study. Both the kalmegh extract (standardized for 10% AGD) and the kutki extract (standardized for 2.5 and 2.3 KUT and PIC, respectively) were purchased from Plantex.
Validation of the andrographolide high-performance liquid chromatography method
Preparation of the standard and test solutions
Standard AGD was weighed accurately and dissolved in methanol to get 50 μg/mL solutions. The test solution was prepared by weighing 50 mg equivalent weight of the kalmegh extract and was transferred into a 100 mL volumetric flask. About 80 mL methanol was added and sonicated for 20 min. The volume was made up to the mark and the resulting solution was used as the test solution.
Chromatographic conditions
HPLC was performed with a Waters 2695 Alliance system (Waters, Milford, MA, USA) with a 2998 photodiode array detector (PDA). AGD was eluted on a reverse-phase 250 mm × 4.6 mm, 5μ, symmetry C8 column (Waters). The mobile phase was prepared from 0.1% OPA (component A) and acetonitrile (component B). The mobile phase was degassed and filtered through a 0.45-μm filter before use. The gradient program was used as follows. Initially, the flow was constant as A–B (75:25 v/v) from 0 to 22 min, linear change from A–B (75:25 v/v) to A–B (5:95v/v) from 22 to 23 min, linear change from A–B (05:95 v/v) to A–B (05:95 v/v) from 23 to 30 min, linear change from A–B (5:95 v/v) to A–B (75:25 v/v) from 30 to 31 min and constant from A-B (75:25 v/v) from 31 to 35 min. The mobile phase flow rate was 1 mL/min. Before the first injection, the column was saturated for 30 min with the initial mobile phase. The column temperature was maintained at 40°C. The injection volume was kept 20 μL. The PDA was set at 230 nm to acquire the chromatogram. A UV spectrum was acquired in the range of 200–400 nm. AGD was identified by comparing the retention times and spectra obtained from the sample and standard solutions. The present work was performed in an air-conditioned room maintained at 25°C.
Preparation of the calibration graph
The stock solutions of AGD in different concentrations were injected and chromatographed and the peak area was measured. A calibration plot was constructed by plotting the concentration against the peak area for AGD.
Method validation
Precision
The system precision was carried out by six replicate injections from the same vial of the standard and was expressed in terms of percent relative standard deviation (%RSD). Six different samples of the same extract were analyzed for method precision. The percent assay of each analyte was calculated and the %RSD is mentioned in Table 1. The intermediate precision was performed on a different system for six different samples by a different analyst. The analyte content was calculated. The %RSD is mentioned in Table 1.
Table 1.
Validation parameter of andrographolide, kutkoside and picroside-I
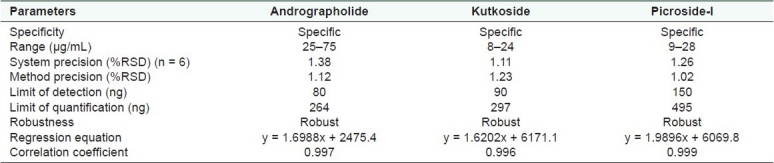
Specificity
The specificity of the method was studied by assessment of the peak purity of AGD using Waters empower software and a diode array detector [Figures 1 and 2].
Figure 1.
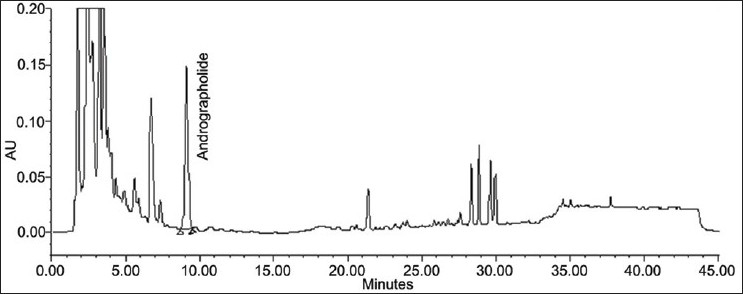
Chromatogram of andrographolide from test solution
Figure 2.
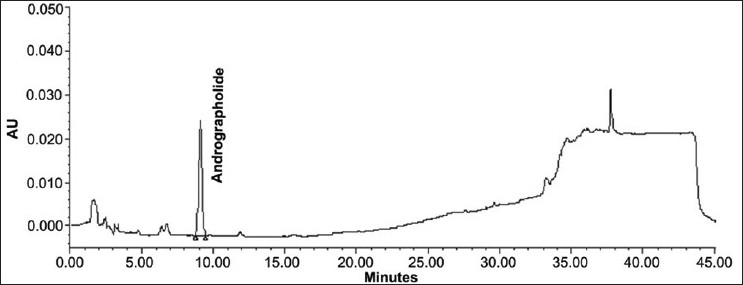
Chromatogram of standard andrographolide
Recovery studies
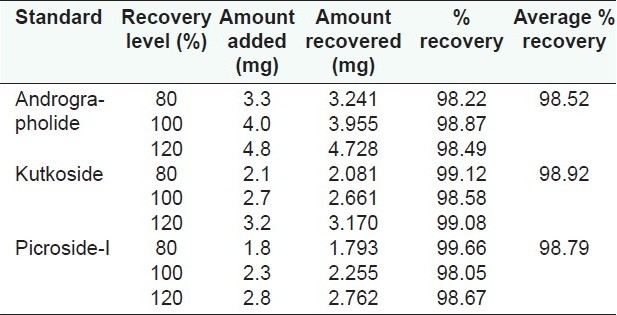
The accuracy of the method was determined from recovery studies by adding known amounts of standards at the 80, 100 and 120% levels to the pre-analyzed sample followed by replicate quantitative analyses by the proposed method [Table 2].
Table 2.
Recovery study of andrographolide, kutkoside and picroside-I
Validation of the kutkoside and picroside-I high-performance liquid chromatography method
Preparation of the standard and test solutions
Standard KUT and PIC were weighed accurately and dissolved in methanol to get 50 μg/mL solutions. The test solution was prepared by weighing 70 mg equivalent weight of the Kutki extract and was transferred into a 100 mL volumetric flask. About 80 mL methanol was added and sonicated for 20 min. The volume was made up to the mark and the resulting solution was used as the test solution.
Chromatographic conditions
HPLC was performed with a Waters 2695 Alliance system with a 2998 PDA detector. KUT and PIC were separated on a reverse-phase 250 mm × 4.6 mm, 5μ, Inertsil ODS column (GL sciences Inc, Tokyo, Japan). The mobile phase was prepared from water (component A) and acetonitrile (component B). The mobile phase was degassed and filtered through a 0.45-μm filter before use. The gradient program was used as follows: initially, the flow was constant at 0–12 min, from A–B (75-25 v/v); at 12–15 min, linear change from A–B (75:25 v/v) to A–B (10:90 v/v); at 15–20 min, linear change from A–B (10:90 v/v) to A–B (10:90 v/v); at 20–21 min, linear change from A–B (10:90 v/v) to A–B (75:25 v/v); at 21–25 min, constant changes from A-B (75:25 v/v). The mobile phase flow rate was 1 mL/min. Before the first injection, the column was saturated for 30 min with the initial mobile phase. The column temperature was maintained at 30°C. The injection volume was kept at 20 μL. The PDA was set at 265 nm to acquire the chromatogram. A UV spectrum was acquired in the range of 200–400 nm. KUT and PIC were identified by comparing the retention times and spectra obtained from the sample and standard solutions. The present work was performed in an air-conditioned room maintained at 25°C
Preparation of the calibration graph
The stock solutions of KUT and PIC in different concentrations were injected and chromatographed and the peak area was measured. A calibration plot was constructed by plotting concentration against peak area for KUT and PIC.
Method validation
Precision
The system precision was carried out by six replicate injections from the same vial of standard and was expressed in terms of %RSD. Six different samples of the same extract were analyzed for method precision. The percent assay of each analyte was calculated and the %RSD is mentioned in Table 1. The intermediate precision was performed on different systems for six different samples by a different analyst. The analyte content was calculated. The %RSD is mentioned in Table 1.
Specificity
The specificity of the method was studied by assessment of the peak purity of KUT and PIC, using Waters empower software and diode array detector [Figures 3 and 4].
Figure 3.
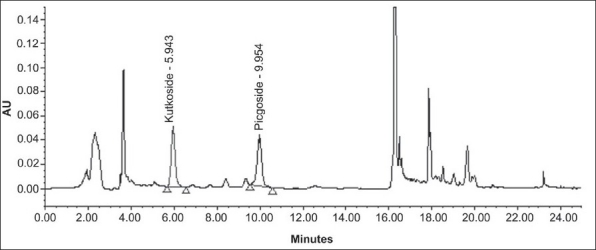
Chromatogram of kutkoside and picroside-I from test solution
Figure 4.
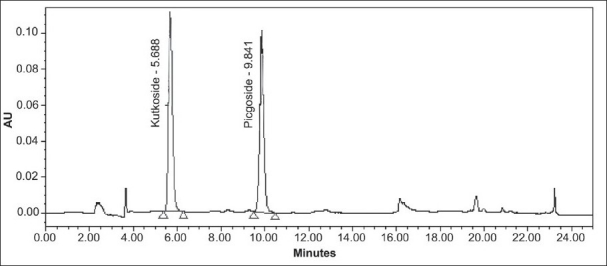
Chromatogram of standard kutkoside and picroside-I
Recovery studies
The accuracy of the method was determined from recovery studies by adding known amounts of standards at the 80, 100 and 120% levels to the pre-analyzed sample followed by replicate quantitative analyses by the proposed method [Table 2].
Compatibility studies
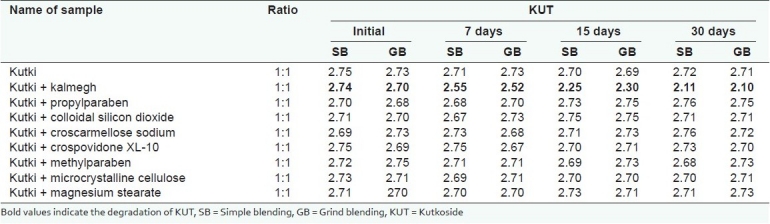
The study was performed by mixing the kalmegh and kutki extracts in the ratio of 1:1 with each other and different commonly used excipients [Tables 3–5].
Table 3.
Compatibility studies of kalmegh extract with kutki extract and other excipients at 40 ± 2°C and 75 ± 5% RH
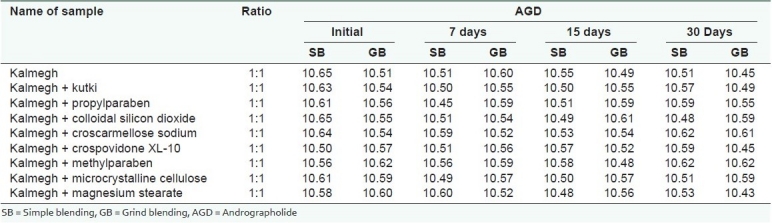
Table 5.
Compatibility studies of kutki extract for picroside-I with kalmegh extract and excipients at 40 ± 2°C and 75 ± 5% RH
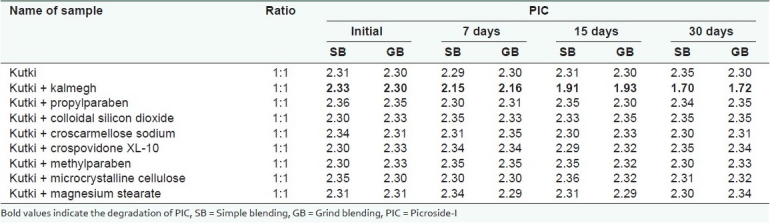
Table 4.
Compatibility studies of kutki extract for kutkoside with kalmegh extract and excipients at 40 ± 2°C and 75 ± 5% RH
Formulation development
The batches of tablet formulation using the kalmegh extract (Plantex) and the kutki extract (Plantex) were developed using microcrystalline cellulose RQ 101 as diluent, methyl paraben and propyl paraben as antimicrobial preservative, crospovidone XL-10 as disintegrant, croscarmellose sodium as disintegrant, colloidal silicon dioxide as glidant and magnesium stearate as lubricant.
Strategy I
By this strategy, the tablet formulation was developed using a combined granulation method (simultaneously using both the extracts intragranularly). Physical parameters (such as tablet weight, weight variation, disintegration time, hardness, etc.) of the core formulation were optimized. The core tablets were then analyzed chemically by the HPLC method for the content of KUT, PIC and AGD.
Strategy II
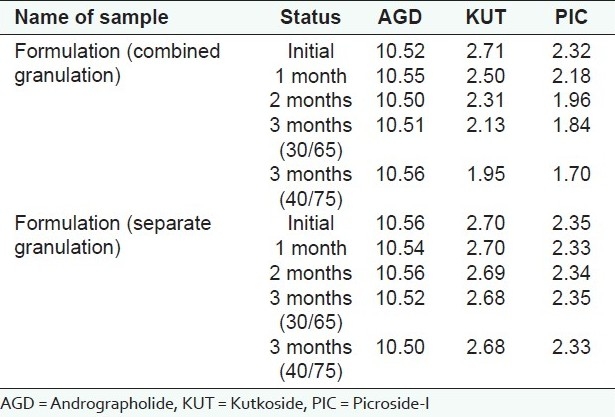
By this strategy, the tablet formulation was developed using a separate granulation method (granulating both the extracts separately) to determine its effect on the stability of KUT and PIC in the formulation. Both the extracts were granulated separately, mixed with extragranular excipients (disintegrant, glidant and lubricant) and then compressed. Physical parameters (as mentioned above) of the core formulation were optimized and the core tablets were then analyzed chemically by the HPLC method for the content of KUT and PIC. The tablet formulations were analyzed at regular intervals (initial, 1, 2 and 3 months) and the results were compared against the samples stored at 5°C.
The compatibility study of the individual extract of kalmegh and kutki with excipients (1:1) used in making this formulation was also studied at (40 ± 2°C and 75 ± 5% RH).
In order to examine the effect of accelerated conditions on active markers, mixed samples for compatibility studies were prepared in two different ways, such as simple blending and grind blending.
Data and statistical analyses
All three active markers were considered stable if the assay remained within 90–110%.
RESULTS AND DISCUSSION
Validation of andrographolide
The composition of the solvent system for HPLC analysis was optimized by testing different solvent systems of varying polarities and the best results were obtained with 0.1% OPA (component A) and acetonitrile (component B) in the proposed gradient mode. The retention time of AGD was recorded at 10 min [Figure 2]. The UV detection at 230 nm showed maximum sensitivity for AGD. The method does not require any sample purification steps. The specificity of the developed method was confirmed by HPLC-PDA analysis. The stability of the sample solution was up to 24 h at room temperature. Intermediate precision was investigated on a different instrument on a different day by a different analyst. The method was found to be linear with correlation coefficient (R2)for AGD 0.9986. The known amount of standard at three different levels (80, 100 and 150%) was spiked with the pre-analyzed sample and the experiment was performed as per the proposed method. The percent recovery of each standard was determined [Table 2].
Validation of Kutkoside and Picroside-I
The composition of the solvent system for the HPLC analysis was optimized by testing different solvent systems of varying polarity and the best results were obtained with water (component A) and acetonitrile (component B) in the proposed gradient mode. The retention time of KUT and PIC was recorded at 5.8 and 9.9 min, respectively [Figure 4]. The resolution between KUT and PIC was found to be more than 2. The UV detection at 265 nm showed maximum sensitivity for both the components. The method does not require any sample purification steps. The specificity of the developed method was confirmed by HPLC-PDA analysis. The sample solution was stable up to 24 h at room temperature. Intermediate precision was investigated on a different instrument on a different day by a different analyst. The method was found to be linear with correlation coefficient (R2)for KUT and PIC at 0.9982 and 0.9995, respectively. The known amount of each standard at three different levels (80, 100 and 150%) was spiked with the pre-analyzed sample and the experiment was performed as per the proposed method. The percent recovery of each standard was determined [Table 2].
Compatibility study
The studysuggested that the active markers of kutki (KUT and PIC) were found to be degraded in the presence of the kalmegh extract. However, the active marker of the kalmegh extract (AGD) was found to be stable. Both the extracts showed excellent compatibility with all the excipients used in making this formulation [Tables 3–5]. A decrease in the KUT and PIC content from the formulation was observed, as confirmed in the pre-formulation study. No significant decrease in the KUT and PIC content from the formulation was observed [Table 6].
Table 6.
Compatibility studies of kutki extract and kalmegh extract in formulation as per ICH guidelines
CONCLUSION
The active markers of the kutki extract (KUT and PIC) were found to be unstable when formulated with the kalmegh extract. However, there was no degradation in the active markers of the kutki extract (KUT and PIC) and the kalmegh extract (AGD) when mixed with the excipients. On the other hand, there is no degradation of AGD in the presence of iridoid glycosides. By granulating separately, the exposure of both the extracts can be minimized thus avoiding the degradation of active markers.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.WPR/RC52/7, WHO Regional Committee, 52nd Session Brunei Darussalam. 2001. World Health Organization (WHO) [Google Scholar]
- 2.Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Anal. 2005;37:949–52. doi: 10.1016/j.jpba.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Guaratini T, Gianeti MD. Stability of cosmetic formulations containing esters of Vitamins E and A: Chemical and physical aspects. Int J Pharm. 2006;327:12–6. doi: 10.1016/j.ijpharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Heigl D, Franz G. Stability testing on typical flavonoid containing herbal drugs. Pharmazie. 2003;58:881–5. [PubMed] [Google Scholar]
- 5.Mennickent S, Pino L, Vega M, Dieg MD. Chemical stability of haloperidol injection by high performance thin-layer chromatography. J Sep Sci. 2008;31:201–6. doi: 10.1002/jssc.200700404. [DOI] [PubMed] [Google Scholar]
- 6.Tashtoush BM, Quasen J, Williams JD, Dewald TP, Jacobson EL, Jacobson MK. Analysis and stability study of myristyl nicotinate in dermatological preparations by high-performance liquid chromatography. J Pharm Biomed Anal. 2007;43:893–9. doi: 10.1016/j.jpba.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Mishra SK, Sangwan NS, Sangwan RS. Andrographis paniculata (Kalmegh): A review. Pharmacogn Rev. 2007;2:283–98. [Google Scholar]
- 8.Singha PK, Roy S, Dey S. Protective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees. against ethanol-induced toxicity in mice. J Ethnopharmacol. 2007;1:13–21. doi: 10.1016/j.jep.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Murti KR. India: Chaukhamba Orientalia Publication; 2007. Shrangadhar Samhita. [Google Scholar]
- 10.Visen PK, Shukla B, Patnaik GK, Dhawan BN. Andrographolide protects rat hepatocytes against paracetamol-induced damage. J Ethnopharmacol. 1993;40:131–6. doi: 10.1016/0378-8741(93)90058-d. [DOI] [PubMed] [Google Scholar]
- 11.Dua VK. Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J Ethnopharmacol. 2004;95:247–51. doi: 10.1016/j.jep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Habtemariam S. Andrographolide inhibits the tumour necrosis factor-α-induced upregulation of ICAM-1 expression and endothelial-monocyte adhesion. Phytother Res. 1998;12:37–40. [Google Scholar]
- 13.Ansari RA, Tripathi SC, Patnaik GK, Dhawan BN. Antihepatotoxic properties of picroliv: An active fraction from rhizomes of Picrorhiza kurroa. J Ethnopharmacol. 1991;34:61–8. doi: 10.1016/0378-8741(91)90189-k. [DOI] [PubMed] [Google Scholar]
- 14.Anandan R, Prabhakaran M, Devaki T. Biochemical studies on the hepatoprotective effect of Picrorhiza kurroa on changes in liver mitochondrial respiration and oxidative phosphorylation in D-galactosamine-induced hepatitis in rats. Fitoterapia. 1999;70:548–51. [Google Scholar]
- 15.Shukla B, Visen PK, Patnaik GK, Dhawan BN. Choleretic Effect of Picroliv, the Hepatoprotective Principle of Picrorhiza kurroa. Planta Med. 1991;57:29–33. doi: 10.1055/s-2006-960010. [DOI] [PubMed] [Google Scholar]
- 16.Chander R, Kapoor NK, Dhawan BN. Picroliv, picroside-I and kutkoside from Picrorhiza kurrooa are scavengers of superoxide anions. Biochem Pharmacol. 1992;44:180–3. doi: 10.1016/0006-2952(92)90054-m. [DOI] [PubMed] [Google Scholar]
- 17.Anadan N, Rekha RD, Saravanan N, Devaki T. Protective effects of Picrorhiza kurroa against HCl/ethanol-induced ulceration in rats. Fitoterapia. 1999;70:498–501. [Google Scholar]
- 18.Calixto JB, Santos AR, Filho VC, Yunes RA. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology, and therapeutic potential. Med Res Rev. 1998;18:225–8. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Khatoon S, Rai V, Rawat AK, Mehrotra S. Comparative pharmacognostic studies of three Phyllanthusspecies. J Ethnopharmacol. 2006;104:79–86. doi: 10.1016/j.jep.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 20.Shum TN, Farah J, Saleem AZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. Thonn. on aflatoxin B1-induced liver damage in mice. J Ethnopharmacol. 2007;113:503–9. doi: 10.1016/j.jep.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Syamsunder KV, Singh B, Thakur RS, Husain A, Kiso Y, Hikino H. Antihepatotoxic actions of gingerols and diarylheptanoids. J Ethnopharmacol. 1985;14:41–4. doi: 10.1016/0378-8741(85)90025-x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Singh RT, Handa SS. Estimation of phyllanthin and hypophyllanthin by high performance liquid chromatography in Phyllanthus amarus. Phytochem Anal. 1993;4:226–39. [Google Scholar]
- 23.Murugaiyah V, Chan KL. Determination of four lignans in Phyllanthus niruri L. by a simple high-performance liquid chromatography method with fluorescence detection. J Chromatogr A. 2007;1154:198–204. doi: 10.1016/j.chroma.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 24.Gaitonde BB, Kulkarni HJ, Nabar SD. Diuretic activity of punarnava (Boerhaavia diffusa) Bulletins of Haffkine Institute. 1974;2:24. [Google Scholar]
- 25.Melhrotra S, Mistra KP, Maurya R, Srimal RC, Singh VK. Immunomodulation by ethanolic extract of Boerhaavia diffusa roots. Int Immunopharmacol. 2002;2:987–96. doi: 10.1016/s1567-5769(02)00031-0. [DOI] [PubMed] [Google Scholar]
- 26.Hiruma-Lima CA, Gracioso JS, Bighetti EJ, Germonsén Robineou L, Souza Brito AR. The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J Ethnopharmacol. 2000;71:267–74. doi: 10.1016/s0378-8741(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 27.Sateesh MA, Pari L. Antioxidant effect of Boerhavia diffusa L. in tissues of alloxan induced diabetic rats. Indian J Exp Biol. 2004;42:989–92. [PubMed] [Google Scholar]
- 28.Kadota S, Lami N, Tezuka Y. Kikuchit boeravinone A and B, new rotenoid analogues from Boerhaavia diffusa Linn. Chem Pharm Bull. 1988;36:834–6. [Google Scholar]
- 29.Kadota S, Lami N, Tezuka Y, Kikuchi T. Constituents of the Roots of Boerhaavia diffusa L. I.: Examination of Sterols and Structures of New Rotenoids, Boeravinones A and B. Chem Pharm Bull. 1989;37:3214–20. [Google Scholar]
- 30.Lami N, Katoda S, Tezuka Y, Kikuchi T. Constituents of the Roots of Boerhaavia diffusa L. II.: Structure and Stereochemistry of a New Rotenoid, Boeravinone C. Chem Pharm Bull (Tokyo) 1990;38:1558–62. [Google Scholar]
- 31.Borrelli F, Milic N, Ascione V, Capasso R, Izzo AA, Capasso F. Isolation of New Rotenoids from Boerhaavia diffusa and Evaluation of their Effect on Intestinal Motility. Planta Med. 2005;71:928–32. doi: 10.1055/s-2005-871282. [DOI] [PubMed] [Google Scholar]
- 32.Borrelii F, Ascione V, Capasso R, Izzo AA, Fattorusso E, Taglialatela-Scafati O. Spasmolytic Effects of Nonprenylated Rotenoid Constituents of Boerhaavia diffusa Roots. J Nat Prod. 2006;69:903–6. doi: 10.1021/np060073h. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed-Belkacem A, Macalou S, Borrelli F, Capasso R, Fattorusso E, Taglialatela- Scafati O. Nonprenylated Rotenoids, a New Class of Potent Breast Cancer Resistance Protein Inhibitors. J Med Chem. 2007;50:1933–38. doi: 10.1021/jm061450q. [DOI] [PubMed] [Google Scholar]
- 34.Mura P, Faucci MT, Manderioli A, Bramanti G, Ceccarelli L. Compatibility study between ibuproxam and pharmaceutical excipients using differential scanning calorimetry, hot-stage microscopy and scanning electron microscopy. J Pharm Biomed Anal. 1998;18:151–63. doi: 10.1016/s0731-7085(98)00171-x. [DOI] [PubMed] [Google Scholar]
- 35.Harmonized tripartite guideline, validation of analytical procedures: Methodology, IFPMA. Geneva: Proceeding of the International Conference on Harmonization; 1996. ICH Q2B. [Google Scholar]


