Abstract
Background:
The emergence of resistance to antimicrobials by pathogens has reached crisis levels, calling for identification of alternative means to combat diseases.
Objective:
To determine antimicrobial activity of crude methanolic extract of Aloe secundiflora Engl. from Lake Victoria region of Kenya.
Materials and Methods:
Extract was tested against four strains of mycobacteria (Mycobacterium tuberculosis, M. kansasii, M. fortuitum and M. smegmatis), Salmonella typhi, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and a fungus Candida albicans. activity of the extract was determined using BACTEC™ MGIT™ 960 system. General antibacterial and antifungal activity was determined using standard procedures: zones of inhibition, Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal/Fungicidal Concentrations (MBCs/MFCs).
Results:
The extract was potent against M. fortuitum, M. smegmatis and M. kansasii where it completely inhibited growth (Zero growth units (GUs)) in all the extract concentrations used. It gave strong antimycobacterial activity (157 GUs) against M. tuberculosis. It showed strong antimicrobial activity (P≤0.05), giving inhibition zones ≥9.00 mm against most microorganisms, such as P. aeruginosa (MIC 9.375 mg mL-1 and MBC of 18.75 mg mL-1), E. coli (both MIC and MBC of 18.75 mg mL-1), S. aureus and S. typhi (both with MIC and MBC of 37.5 mg mL-1). Preliminary phytochemistry revealed presence of terpenoids, flavonoids and tannins.
Conclusion:
The data suggests that Aloe secundiflora could be a rich source of antimicrobial agents. The result gives scientific backing to its use by the local people of Lake Victoria region of Kenya, in the management of conditions associated with the tested microorganisms.
Keywords: Aloe secundiflora Engl., BACTEC™ MGIT™ 960, growth units, Lake Victoria region, tuberculosis
INTRODUCTION
Antimicrobials of plant origin have enormous therapeutic potential for various ailments.[1,2] They are effective in the treatment of infectious diseases and mitigate many of the side-effects that are often associated with synthetic antimicrobials. Natural products are evolutionary shaped molecules with a profound impact on human health.[3] The World Health Organization (WHO) estimates that more than 80% of the world population is dependent (wholly or partially) on plant-based drugs.[4] In East Africa, 90% of the population relies on traditional medicines (TM) and traditional health practitioners (THPs) as the primary source of healthcare.
Nature's biosynthetic engine produces innumerable secondary metabolites with distinct biological properties that make them valuable as health products or as structural templates for drug discovery. Aloes are reputed to have been used therapeutically since Roman times.[5] The genus Aloe is common in Kenya, with about 60 taxa recognized.[6] Because of unsustainable harvesting, trade in all species except Aloe vera is regulated by the Convention on the International Trade in Endangered Species of Wild Fauna and Flora (CITES).[7] The latest review of some Aloe species shows that they have antibacterial, antifungal, anticancer, antiviral and immunomodulatory properties.[8] From the survey, herbalists from the Lake Victoria region have traditionally used Aloe secundiflora to treat ailments including chest problems, polio, malaria and stomachache but with no knowledge of the scientific base of their activities.[9]
The increasing prevalence of antibiotic resistance is a major health problem worldwide.[10] The WHO and the European Commission (EC) have recognized the importance of studying the emergence and determinants of antimicrobial resistance and the need for strategies to control drug resistance.[10,11] Kenya has 2 million people living with HIV infections who may succumb to AIDS, which renders them susceptible to opportunistic infections.[11] An increasing incidence of deaths due to tuberculosis (TB) and the known drawbacks of the current existing drugs including the emergence of multidrug-resistant strains have led to a renewed interest in the search for new anti-tubercular agents with novel modes of actions. Tuberculosis, which is a deadly infectious disease that annually kills about 3 million people worldwide,[12] is now a highly associated infection of persons suffering from human immunodeficiency syndrome (HIS).
Other opportunistic infections, especially those associated with diarrhea are also increasingly fatal. The increasing resistance of Escherichia coli to ampicillin and trimethoprim sulfamethoxazole has been reported in many studies.[10] This worldwide emergence of E. coli, K. pneumoniae and many other ί-lactamase producers has become a major therapeutic problem. Multidrug-resistant strains of E. coli and K. pneumoniae are widely distributed in hospitals through patient contact and are increasingly being isolated from community-acquired infections.[13]
MATERIALS AND METHODS
Plant material collection, identification and extract preparation
Mature fresh leaves of Aloe secundiflora Engl. were collected from Kisii central district, Kenya, in June 2008 based on the ethnobotanical survey that was carried out earlier.[9] The plant was authenticated by a plant taxonomist (Mr. Karimi Lucas) from the Department of Pharmacy and Complementary Medicine, Kenyatta University, Nairobi, Kenya, in whose herbarium the voucher specimens are deposited. The leaves were chopped into small pieces, shade-dried and ground using a hammer-type milling machine (Meecan, CM/L-1364548, India). The powdered material was transferred into a 250 mL quick-fit round-bottomed flask and extracted in the soxhlet extractor using methanol for 72 h.[14] The extract was filtered through a Whatmann filter paper No. 42 (125 mm) and concentrated using a rotary evaporator (Laborota 4000, SN 090816862, Germany) with the water bath set at 40°C,[15] then dried under vacuum in a dessiccator over anhydrous CuSO4. The powdered residue was transferred into vials and stored at 4°C in airtight vials before analysis.
Test microorganisms
The four mycobacterial strains used for the assays were Mycobacterium tuberculosis, M. kansasii, M. smegmatis and M. fortuitum, which were all obtained from the Center for Respiratory Diseases Research (CRDR), Kenya Medical Research Institute (KEMRI), Nairobi, Kenya. The common bacterial and fungal strains included Salmonella typhi (clinical isolate), Klebsiella pneumoniae (clinical isolate), Pseudomonas aeruginosa (ATCC 25852), Escherichia coli (ATCC 25922) Staphylococcus aureus (ATCC 20591) and Candida albicans (ATCC EK138), which were obtained from the Public Health Laboratories, Kenyatta National Hospital in Nairobi, Kenya.
Anti-tubercular assay using rapid non-radioactive BACTEC MGIT™ 960 system
The non-radioactive respiratory technique using the BACTEC MGIT™ 960 system (Beckon Dickinson diagnostic equipment, Sparks, Maryland, USA) was used for susceptibility testing against the mycobacterial strains.[16] The extracts were dissolved in 0.01% dimethyl sulfoxide (DMSO) to final concentrations of 0.5, 1.0 and 2.0 mg mL-1. A stock solution of 2.0 mg mL-1 of the primary drug, isoniazid, was used as the positive control and 0.01% DMSO as the negative control, respectively. Into the 7 mL BBL™ MGIT™ tubes, 0.8 ml of the mixture containing OADC (supplement containing Oleic Acid, Bovine Albumin, Dextrose and Catalase added to provide essential substances for rapid growth of mycobacteria) and BBL™ MGIT™ PANTA (a mixture of antimicrobial agents that include Polymyxin B, Amphotericin B, Nalidixic Acid, Trimethoprim and Azlocillin) were added. Experiments were carried out in triplicate where BBL™ MGIT™ tubes with extract concentrations of 0.5, 1.0 and 2 mg mL-1 were inoculated with 500 μL of Mycobacterium sp. suspension. The strains included M. tuberculosis (Mtb), M. kansasii (Mk), M. fortuitum (Mf) and M. smegmatis (Ms). The BACTEC MGIT™ 960 system was loaded using manufacturer's instructions and incubated at 37°C. Culture vials which remained negative for a minimum of 42 days (maximum 56 days) were removed and recorded as negative, while growth units (GUs) for the positive ones were recorded appropriately.[17] The same was done for the controls. Results were provided as positive/negative using a non-radiometric evaluation technique and numerical GUs recorded.[17]
Evaluation of antibacterial and antifungal activity
The antibacterial and antifungal activities of the extract were assayed in vitro using agar disc diffusion (DD) method.[18] Mueller Hinton agar and Potato Dextrose Agar (PDA) were prepared using manufacturer's instructions for purposes of culturing the bacteria and fungi respectively. Normal saline solution was used to dilute a 24-h culture of the bacterial type culture or clinical isolate to attain a 0.5 McFarland standard. Spread plate method was used to culture 100 μl of the microbial suspension that was introduced into the Petri dishes.[19] Dry sterile discs (6 mm in diameter) were soaked in the plant extract (made by dissolving 300 mg of the extracts in 1000 μl of methanol, producing a homogenous orange solution), air-dried and placed on the spread plates at reasonable distances. Discs impregnated with methanol and air-dried were used as negative controls and various standard conventional antibiotics (Amoxicillin (Hangzhou Ruijian, China); Ciprofloxacin (Chengdu Ware Yuanheng, China); Fluconazole (Pfizer, UK)) as positive controls. The plates were then incubated at 35°C for 24 h. This was replicated three times for each pathogen.
Candida albicans was cultured by taking 100 μl from the broth and spreading on PDA. The culture was incubated at 25°C for 72 h. The cork boarer was used to pick a section of the young mycelium which was placed at the centre of the PDA plate and the dry discs which were impregnated with 100 μl of the plant extracts placed at a distance around the mycelium inoculum. The inoculum was incubated at 25°C for 72 h. Fluconazole and dry discs treated with methanol were also used as positive and negative controls respectively. All tests were performed in triplicate. Microbial growth inhibition was determined by measuring the zones of inhibition using a transparent ruler.
Minimum inhibitory concentration and minimum bactericidal/fungicidal concentration
This was done only where the plant extract showed strong antibacterial activity by the DD method (≥9-15 mm).[20] The wells were filled with 50 μl of the nutrient broth for bacterial strains and potato dextrose broth for C. albicans. The extract was then prepared by taking 300 mg of the plant extract and mixing with 1000 μl of DMF (0.01% Dimethyl formamide) for complete dissolution of the extract. Then 50 μl of the plant extract was dispensed into the first well before serial dilutions were done by transferring 50 μl of nutrient or potato dextrose broth containing the extract from the first well to the second well, and from the second well to the third well through the fourth well. Fifty microlitres (50 μl) of the test isolate were then dispensed into each well. One well (without extract or drug) was used as negative control whereas another well with 50 μl of the antibiotic (Amoxicillin /Ciprofloxacin/fluconazole) was used as positive control. Incubation was done at 37°C for 24 h.
The minimum inhibitory concentration (MIC) values were determined as the lowest concentrations of the extract that completely inhibited microbial growth. For the determination of minimum bactericidal/fungicidal concentration (MBC/MFC), wells where there was no growth were subcultured on nutrient agar and PDA. The lowest concentration of the plant extracts that did not yield any colony on the solid medium (Nutrient or PDA agar) after subculturing and incubating at 37°C for 24 h for bacterial strains and 25°C for 72 h for C. albicans was taken as the MFC/MBC.
Preliminary phytochemistry
The Aloe secundiflora Engl. extracts were subjected to preliminary phytochemical screening to determine the major chemical groups corresponding to active compounds responsible for the observed activity. For detection of tannins, saponins, alkaloids, cardiac glycosides, flavonoids and terpenoids, phytochemical screening was performed using standard procedures as described in Omwenga et al.[16]
Data analysis
Data was analyzed using the Minitab Statistical Software 13.20, © 2000 Minitab Inc. PA 16801-9928, USA. Among the groups, significance test was performed using the one-way ANOVA at 95% significance level.
RESULTS
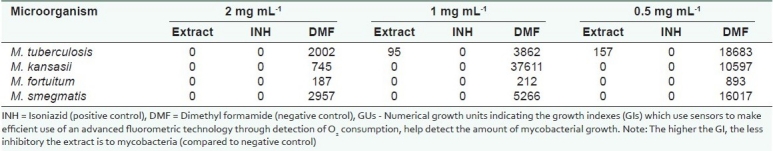
Table 1 shows the anti-tubercular (antimycobacterial) activity in varying concentrations of the methanol extract of Aloe secundiflora Engl. by BACTEC MGIT™ 960 system. The extract was found to be effective even at the lowest concentration (Zero GUs against all the mycobacteria strains, except M. tuberculosis which had 95 GUs). This was comparable to isoniazid (positive control) which gave Zero GUs at all concentrations. There was varied growth in DMF.
Table 1.
Antimycobacterial activity (GUs) of Aloe secundiflora Engl. using BACTEC MGIT™ 960 system
The plant extract generally had strong antimicrobial activity [Figure 1]. The zones of inhibition from the extracts were statistically significant (P ≤ 0.05). The zones of inhibition from the three treatments were statistically different except against P. aeruginosa where the activity of the plant extracts was not significantly different from the activity of the positive control (Amoxicillin) [Figure 1]. The extract was more active (18.0 mm) than the positive control (amoxicillin) (17.33 mm) against P. aeruginosa [Table 2, Figure 1]. The extract was found be active against S. typhi, S. aureus, E. coli and P. aeruginosa, with zones of inhibition being ≥9.00 mm.
Figure 1.
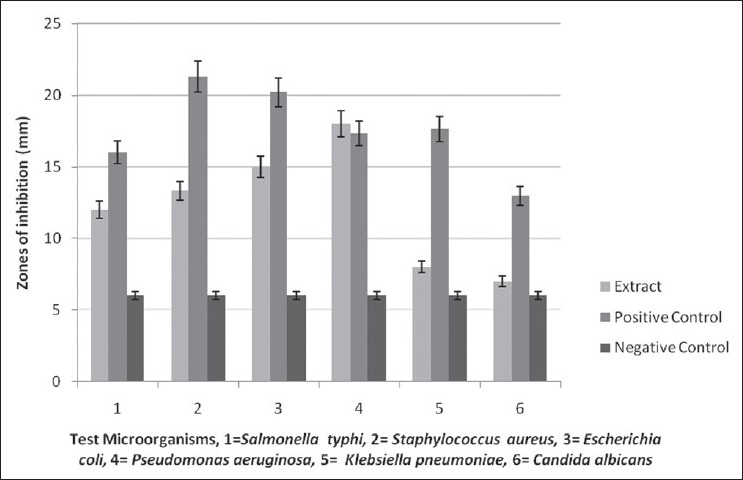
Activity of Aloe secundiflora Engl. extract against various test microorganisms (P=0.05) Positive controls-Fluconazole for C. albicans, Zeftazidime for S. typhi, Ciprofloxacin for K. pneumoniae and Amoxicillin for S. aureus, E. coli and P. aeruginosa. Negative control-dried methanol discs
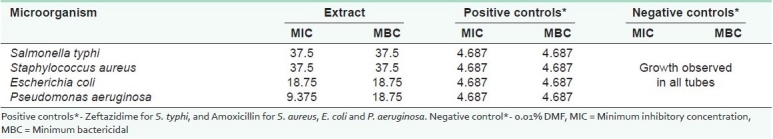
Table 2.
Minimum inhibitory/ bactericidal concentrations (mg mL-1) of leaf extracts of Aloe secundiflora Engl
DISCUSSION
In the study, the activity of Aloe secundiflora Engl. extracts against mycobacterial strains showed that the plants contain pharmacologically active substances [Table 1]. The extract was active against all the mycobacterial strains used (Zero GUs against M. kansasii, M. fortuitum and M. smegmatis and 157 GUs against M. tuberculosis at the lowest concentration of 0.5 mgmL-1). For anti-ttuberculosis susceptibility tests, the MIC is considered as the lowest concentration inhibiting more than 99% of the initial bacterial concentration.[1] The extract inhibited M. tuberculosis at 0.5 mg mL-1 by 99.159%. This means that Aloe secundiflora has compounds or groups of compounds that could prevent the growth and proliferation of mycobacteria.
Some flavonoids like catechins are well known for their antimicrobial properties against fungi, Gram-positive and Gram-negative bacteria.[1,21] The activity against tuberculosis strains could be due to the ability of flavonoids to form complexes with the bacterial cell wall, affecting cell-linked processes thereby inhibiting microbial growth.[1,22] They also inhibit several enzyme activities.[22] The activity of Aloe secundiflora is in agreement with the report by Chakraborty and Chakraborti[21] where catechins were said to have antibacterial activity by inhibiting the action of DNA polymerase.
Except for K. pneumoniae and C. albicans, general antibacterial results were also noted [Figure 1]. The activity of extracts against various test microorganisms was statistically significant at P ≤ 0.05 [Figure 1]. The activity of the test plant extracts against P. aeruginosa was comparable to that of amoxicillin (positive control). There was no significant difference in their activity. A zone of inhibition ≥9.00 mm is an indication of strong antimicrobial activity.[20] Minimum inhibitory concentrations and MBCs produced by the extract against various bacterial test cultures showed strong antimicrobial activity [Table 2]. Good antimicrobial activity was shown against E. coli (MIC and MBC of 18.75 mg mL-1) and P. aeruginosa (MIC of 9.375 and MBC of 18.75 mg mL-1). The extract also gave good activity against S. typhi and S. aureus (with MICs and MBCs of 37.5 mg mL-1).
The extracts tested positive for tannins, terpenoids and flavonoids, and negative for saponins, cardiac glycosides and alkaloids. Activity against S. aureus and E. coli could be due to the presence of tannins. Tannins have been reported to be bacteriostatic or bactericidal against S. aureus and E. coli.[23] The tannins could be disrupting the cell membranes of the microorganisms, hence their inhibitory activities. A study carried out by Waihenya et al.,[8] revealed that Aloe secundiflora extract has been found to have a role in the control of fowl typhoid, indicating the possibility of the efficacy of Aloe secundiflora in other bacterial infections.
CONCLUSIONS
The crude extracts of Aloe secundiflora Engl. could be useful in the development of new antimicrobial drugs, especially against infections caused by the four mycobacterial strains and against P. aeruginosa. Isolation of active principles, pharmacological and toxicological studies are currently underway and will be reported later. The study also supports the use of the plant by the local communities on the treatment of infections associated with the studied microorganisms, the results of which are promising.
Acknowledgments
The authors are grateful to the traditional health practitioners in the Lake Victoria region of Kenya for their cooperation and to the Lake Victoria Research (VicRes) Initiative, who financed the project. We are also grateful to the Lake Victoria Development Programme funded by SIDA/SAREC, who support VicRes. Many thanks to the Kenya Medical Research Institute's (KEMRI) Centre for Respiratory Diseases′ Research (CRDR) for allowing the work to be done from their Level III TB laboratory.
Footnotes
Source of Support: VicRes
Conflict of Interest: None.
REFERENCES
- 1.Kuete V, Ngameni B, Simo CC, Tankeu RK, Ngadjui BT, Meyer JJ, et al. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae) J Ethnopharmacol. 2008;120:17–24. doi: 10.1016/j.jep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Manisha V, Neha S, Satish S. Antimicrobial Activity of Stem Bark Extracts of Nyctanthes arbortristis Linn.(Oleaceae) Int J Pharmacogn Phytochem Res. 2009;1:12–4. [Google Scholar]
- 3.Kishore N, Mishra BB, Tripathi V, Tiwari VK. Alkaloids as potential anti-tubercular agents. Fitoterapia. 2009;80:149–63. doi: 10.1016/j.fitote.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Orwa JA, Jondiko IJ, Minja RJ, Bekunda M. The use of Toddalia asiatica (L) Lam.(Rutaceae) in traditional medicine practice in East Africa. J Ethnopharmacol. 2008;115:257–62. doi: 10.1016/j.jep.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey KL, Jäger AK, Viljoen AM. Cyclooxygenase inhibitory activity of Aloe species. S Afr J Bot. 2002;68:47–50. [Google Scholar]
- 6.Wabuyele E, Bjora CS, Nordal I, Newton LE. Distribution, diversity and conservation of the genus Aloe in Kenya. J East Afr Nat Hist. 2006;95:213–25. [Google Scholar]
- 7.Grace OM, Simmonds MS, Smith GF, van Wyk AE. Therapeutic uses of Aloe L. (Asphodelaceae) in southern Africa. J Ethnopharmacol. 2008;119:604–14. doi: 10.1016/j.jep.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Waihenya RK, Mtambo MM, Nkwengulila G, Minga UM. Efficacy of crude extract of Aloe secundiflora against Salmonella gallinarum in experimentally infected free-range chickens in Tanzania. J Ethnopharmacol. 2002;79:317–23. doi: 10.1016/s0378-8741(01)00397-x. [DOI] [PubMed] [Google Scholar]
- 9.Mariita MR, Okemo OP, Orodho JA, Kirimuhuzya C, Otieno JN, Magadula JJ. Efficacy of 13 medicinal plants used by indigenous communities around Lake Victoria, Kenya, against tuberculosis, diarrhoea causing bacteria and Candida albicans. Int J Pharm Technol. 2010:771–971. [Google Scholar]
- 10.Eryilmaz M, Bozkurt ME, Yildiz MM, Akin A. Antimicrobial Resistance of Urinary Escherichia coli Isolates. Trop J Pharm Res. 2010;9:205–9. [Google Scholar]
- 11.Emacar J, Okemo P, Gatheri G, Kariuki S. Antibiotic resistance patterns of Escherichia coli isolated from HIV-sero positive adults at Mbagathi District Hospital, Nairobi, Kenya. J Appl Biosci. 2010;27:1705–14. [Google Scholar]
- 12.Camacho-Corona Mdel R, Ramírez-Cabrera MA, Santiago OG, Garza-González E, Palacios Ide P, Luna-Herrera J. Activity against Drug Resistant-Tuberculosis Strains of Plants used in Mexican Traditional Medicine to treat Tuberculosis and Other Respiratory Diseases. Phytother Res. 2008;22:82–5. doi: 10.1002/ptr.2269. [DOI] [PubMed] [Google Scholar]
- 13.Khan R, Islam B, Akram M, Shakil S, Ahmad A, Ali SM, et al. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical Origin. molecules. 2009;14:586–97. doi: 10.3390/molecules14020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiyelaagbe OO, Osamudiamen PM. Phytochemical screening for active compounds in Mangifera indica leaves from Ibadan, Oyo State. Plant Sci Res. 2009;2:11–3. [Google Scholar]
- 15.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]
- 16.Omwenga EO, Okemo PO, Mbugua PK, Ogol CK. Ethnobotanical survey and antimicrobial evaluation of medicinal plants used by the Samburu Community (Kenya) for treatment of diarrhorea. Pharmacogn Mag. 2009;4:165–76. [Google Scholar]
- 17.BBL MGIT Mycobacteria growth indicator manual. Maryland, USA: 2007. Becton, Dickinson company; pp. 1–23. [Google Scholar]
- 18.Parekh J, Chanda SV. In vitro Antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk J Biol. 2007;31:53–8. [Google Scholar]
- 19.Meite S, N′guessan JD, Bahi C, Yapi HF, Djaman AJ, Guina FG. Antidiarrheal activity of the ethyl acetate extract of Morinda morindoides in rats. Trop J Pharm Res. 2009;8:201–7. [Google Scholar]
- 20.Rani P, Khullar N. Antimicrobial Evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytother Res. 2004;18:670–3. doi: 10.1002/ptr.1522. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty D, Chakraborti S. Bioassay-guided isolation and identification of antibacterial and antifungal component from methanolic extract of green tea leaves (Camellia sinensis) Res J Med Plant. 2010;4:78–86. [Google Scholar]
- 22.Cohen MF, Sakihama Y, Yamasaki H. Roles of plant flavonoids in interactions with microbes: From protection against pathogens to the mediation of mutualism. Recent Res Dev Plant Physiol. 2001;2:157–73. [Google Scholar]
- 23.Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial activity of several tannins against Staphylococcus aureus. J Antimicrob Chemother. 2001;48:487–91. doi: 10.1093/jac/48.4.487. [DOI] [PubMed] [Google Scholar]


