Abstract
Tissue factor (TF), is a cellular receptor that binds the ligand factor VII/VIIa to initiate the blood coagulation cascade. In addition to its role as the initiator of the hemostatic cascade, TF is known to be involved in angiogenesis via an interaction with factor VIIa and protease-activated receptor-2 (PAR-2). In this article we review previous studies from our laboratory demonstrating that the pattern and level of TF expression is altered in multiple cell types derived from eutopic and ectopic endometrium from women with endometriosis compared with normal endometrium. We posit that the inflammatory environment that occurs in ectopic and eutopic endometrium from patients with disease results in high TF expression that in turn, signals via PAR-2 to further produce inflammatory cytokine or chemokine production and macrophage recruitment. Thus, our studies suggest that TF might be an ideal target for therapeutic intervention in endometriosis.
Keywords: endometriosis, endometrium, tissue factor
Tissue Factor
Tissue factor (TF; also known as coagulation factor III, tissue thromboplastin, or CD142) is a cell membrane–bound glycoprotein (MW 46 kDa) comprising a hydrophilic extracellular domain, a membrane-spanning hydrophobic domain, and a cytoplasmic tail of 21 residues.1–4 TF is a member of the human class II cytokine receptor family, displaying high homology in secondary and tertiary structure with the interferon-γ receptor.5,6 Although TF is expressed in the mesenchymal and epithelial cells of diverse tissues,7,8 endothelial cells and other cells in contact with the circulation do not normally express TF. However, following vascular disruption, perivascular cell-bound TF binds to circulating factor VIIa to mediate the activation of both factor IX and X and ultimately to generate thrombin.1,9–14 The FVI-IIa:FIXa complex of the intrinsic pathway provides an alternative route to generate FXa, which participates in the prothrombinase complex (FVa:FXa). This complex converts prothrombin to thrombin, which plays a central role in the coagulation protease cascade. In particular, thrombin activates FXI, which is an alternative way to generate FIXa. Thrombin also activates FXIII, as well as various cofactors, cleaves fibrinogen, and stimulates platelets via cleavage of protease-activated receptors (PARs). Platelets accelerate the activation of the coagulation cascade by binding FXI and by providing a thrombogenic surface for the assembly of the prothrombinase complex (FVa:FXa).15,16
The full TF molecule exists on the cell surface as either a cryptic form that is inert, a coagulant form that rapidly binds factor VIIa to initiate coagulation, and a signaling form that binds FVIIa and cleaves PAR-2, which functions in inflammation, tumor progression, and angiogenesis.17–19 Thus, detection of immunoreactive TF does not necessarily correspond to TF clotting activity.
The angiogenic function of TF is now known to be mediated through a complex series of intracellular signaling pathways,20–22 and its absolute requirement has been demonstrated by the embryonic lethality observed in knockout mice.23–25 Thus, TF−/− null embryos die at embryonic day E10.5 and display disorganization of the yolk sac vasculature.23–25
TF in Endometriosis
Endometriosis is a gynecological disorder characterized by the presence of endometrial tissue outside of the uterus.26 The disease affects up to 10% of all reproductive-aged women and the prevalence rises to 20–50% in infertile women.27,28 Despite its frequency and its impact on quality of life, our understanding of the pathogenesis of endometriosis remains incomplete and its treatment remains controversial.29 Endometrial lesions are primarily located on the pelvic peritoneum and ovarie but can also be found in the pericardium, pleura, lung parenchyma, and even the brain.30,31 Implants can result in substantial morbidity, including pelvic adhesions and pain, allergies, fatigue, bowel problems, and infertility, often requiring extensive medical and surgical treatments.32–39 Hence, this disease is costly and both physically and psychologically debilitating.
The etiology of the disease likely reflects retrograde menstruation, coleomic metaplasia or both.27,40–43 However, other theories have been proposed, including a complex interplay of genetic, anatomic, environmental, and immunologic factors.31 Although reports concerning the origin of endometriosis are conflicting, there is general agreement that endometriosis is associated with a local inflammatory response, and that vascularization at the site of invasion plays a decisive role in the pathogenesis of the disease.27,40–47
Because TF has recently been shown to be angiogenic,48,49 initial studies from our laboratory were conducted to examine the expression of these molecules in eutopic endometrium from control women versus women with endometriosis as well as extrauterine endometriotic lesions.
Prior immunohistochemistry and in situ hybridization studies, as well as in vitro experiments from our laboratory have shown that, in normal endometrium, progesterone markedly enhances TF protein and mRNA expression in decidualized stromal cells during the luteal phase, whereas glandular epithelial cells display minimal TF expression throughout the menstrual cycle.50–52 By contrast, this pattern of TF expression is altered in eutopic and ectopic endometrium derived from women with endometriosis. Thus, as previously reported,53 marked elevation of TF expression was observed in glandular epithelial cells of eutopic or ectopic endometrium derived from women with this disease (Fig. 1). We have also demonstrated that PAR-2, the putative TF receptor believed to regulate intracellular signaling, is highly upregulated in the glandular epithelium of eutopic endometrium (Fig. 2). Hence, both TF and its putative receptor are strategically poised for angiogenic and inflammatory signaling in endometriotic lesions.
FIGURE 1.
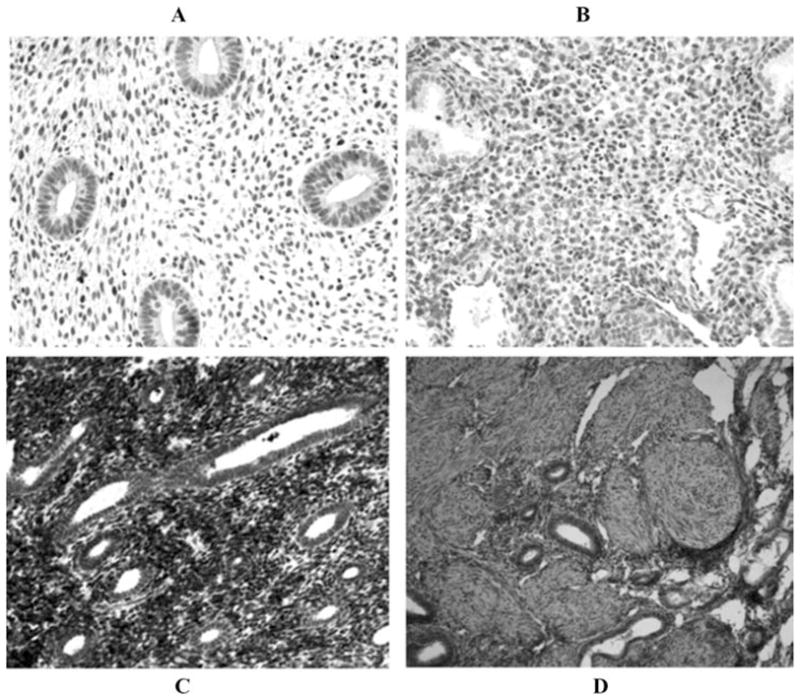
TF immunohistochemistry. (A) Normal proliferative endometrium showing low to no TF staining in glands or stromal cells. (B) Normal secretory endometrium showing decidualized stromal cell staining. (C) Ectopic endometriotic implant from proliferative phase endometrium with glandular staining. (D) Eutopic late proliferative phase endometrium from patients with endometriosis with glandular staining (×20).
FIGURE 2.
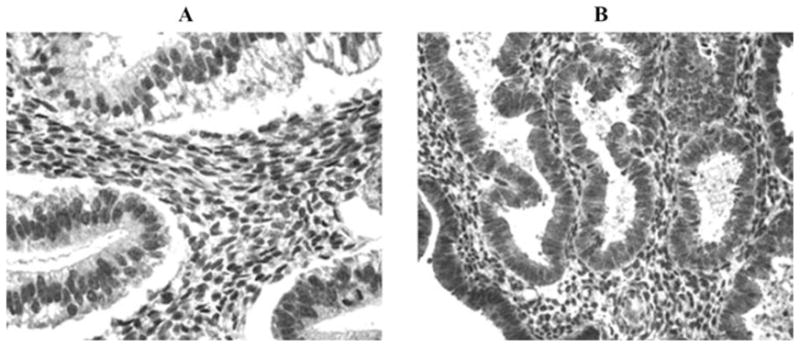
Expression of PAR-2 by normal versus eutopic endometrium of women with endometriosis. Endometria were immunostained as previously described.51 (A) Normal early secretory eutopic endometrium. (B) Eutopic mid-secretory endometrium from a patient with endometriosis.
Summary
The increased expression of TF in eutopic and ectopic endometrium from patients with endometriosis compared with controls is a novel finding. It may reflect the known association of endometriosis with increased eutopic and ectopic inflammatory cytokine production.54–58 It is well established that interleukin-1β and tumor necrosis factor-α acting via the NFκB transcription factor increased TF gene expression in multiple cell types.59–61
Our findings complement those from previous studies demonstrating an increased activity of the fibrinolytic system in the endometrium and peritoneal fluid of women with endometriosis.62–64 Hence, the peritoneum possesses an inherent fibrinolytic activity that is responsible for the degradation of the fibrin deposits originated after an injury.62–64 It is logical, therefore, to expect an upregulation of TF within this milieu of injury. However, increased TF expression in endometrial tissues may also reflect genetic polymorphisms in the promoter region of genes known to regulate TF expression.65–67
It is interesting that, in addition to altered localization and expression of TF, we also demonstrated an induction of the putative TF signaling receptor PAR-2 in endometriotic lesions (Fig. 2). It is now known that the interaction of TF with PAR-2 regulates gene transcription, protein translation, cell proliferation, cell motility, and integrin activation.21,48,59–61,68 We propose that the induction of TF and PAR-2 in endometriotic tissues likely initiates intracellular signaling mechanisms that lead to overexpression of inflammatory cytokines, including Mφ-chemotactants, macrophage metalloproteases (MMPs), vascular endothelial growth factor, and TF. As a result, a pathological feedback cycle of TF expression and intracellular signaling is established, ensuring successful endometriotic nidation and angiogenesis.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Bach R, et al. Purification and characterization of bovine tissue factor. J Biol Chem. 1981;256:8324–8331. [PubMed] [Google Scholar]
- 2.Kirchhofer D, Nemerson Y. Initiation of blood coagulation: the tissue factor/factor VIIa complex. Curr Opin Biotechnol. 1996;7:386–391. doi: 10.1016/s0958-1669(96)80112-1. [DOI] [PubMed] [Google Scholar]
- 3.Nemerson Y. Tissue factor and hemostasis. Blood. 1988;71:1–8. [PubMed] [Google Scholar]
- 4.Nemerson Y. Tissue factor: then and now. Thromb Haemost. 1995;74:180–184. [PubMed] [Google Scholar]
- 5.Szotowski B, et al. Alternatively spliced tissue factor: a previously unknown piece in the puzzle of hemostasis. Trends Cardiovasc Med. 2006;16:177–182. doi: 10.1016/j.tcm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Muller YA, et al. Structure of the extracellular domain of human tissue factor: location of the factor VIIa binding site. Biochemistry. 1994;33:10864–10870. doi: 10.1021/bi00202a003. [DOI] [PubMed] [Google Scholar]
- 7.Mackman N. Regulation of the tissue factor gene. Thromb Haemost. 1997;78:747–754. [PubMed] [Google Scholar]
- 8.Edgington TS, et al. The structural biology of expression and function of tissue factor. Thromb Haemost. 1991;66:67–79. [PubMed] [Google Scholar]
- 9.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 10.Eilertsen KE, Osterud B. Tissue factor: (patho)physiology and cellular biology. Blood Coagul Fibrinolysis. 2004;15:521–538. doi: 10.1097/00001721-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Price GC, et al. Tissue factor and tissue factor pathway inhibitor. Anaesthesia. 2004;59:483–492. doi: 10.1111/j.1365-2044.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- 12.Nemerson Y. My life with tissue factor. J Thromb Haemost. 2007;5:221–223. doi: 10.1111/j.1538-7836.2007.02256.x. [DOI] [PubMed] [Google Scholar]
- 13.Guha A, et al. Affinity purification of human tissue factor: interaction of factor VII and tissue factor in detergent micelles. Proc Natl Acad Sci USA. 1986;83:299–302. doi: 10.1073/pnas.83.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spicer EK, et al. Isolation of cDNA clones coding for human tissue factor: primary structure of the protein and cDNA. Proc Natl Acad Sci USA. 1987;84:5148–5152. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–107. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 17.Chen VM, et al. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 18.Chen VM, Hogg PJ. Allosteric disulfide bonds in thrombosis and thrombolysis. J Thromb Haemost. 2006;4:2533–2541. doi: 10.1111/j.1538-7836.2006.02236.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolberg AS, et al. Tissue factor de-encryption: ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul Fibrinolysis. 1999;10:201–210. [PubMed] [Google Scholar]
- 20.Versteeg HH, et al. Tissue factor signal transduction in angiogenesis. Carcinogenesis. 2003;24:1009–1013. doi: 10.1093/carcin/bgg039. [DOI] [PubMed] [Google Scholar]
- 21.Versteeg HH, Ruf W. Emerging insights in tissue factor-dependent signaling events. Semin Thromb Hemost. 2006;32:24–32. doi: 10.1055/s-2006-933337. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Pedrera C, et al. Tissue factor as an effector of angiogenesis and tumor progression in hematological malignancies. Leukemia. 2006;20:1331–1340. doi: 10.1038/sj.leu.2404264. [DOI] [PubMed] [Google Scholar]
- 23.Bugge TH, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toomey JR, et al. Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 25.Carmeliet P, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 26.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 27.Taylor RN, et al. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100. doi: 10.1111/j.1749-6632.2002.tb02769.x. discussion 118:396–406. [DOI] [PubMed] [Google Scholar]
- 28.Rapkin A, et al. Peritoneal fluid interleukin-6 in women with chronic pelvic pain. Fertil Steril. 2000;74:325–328. doi: 10.1016/s0015-0282(00)00653-1. [DOI] [PubMed] [Google Scholar]
- 29.Sharpe-Timms KL, Young SL. Understanding endometriosis is the key to successful therapeutic management. Fertil Steril. 2004;81:1201–1203. doi: 10.1016/j.fertnstert.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 30.Giudice LC, et al. Status of current research on endometriosis. J Reprod Med. 1998;43:252–262. [PubMed] [Google Scholar]
- 31.Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update. 2007;13:331–342. doi: 10.1093/humupd/dmm006. [DOI] [PubMed] [Google Scholar]
- 32.Behera M, et al. Laparoscopic findings, histopathologic evaluation, and clinical outcomes in women with chronic pelvic pain after hysterectomy and bilateral salpingo-oophorectomy. J Minim Invasive Gynecol. 2006;13:431–435. doi: 10.1016/j.jmig.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Del Frate C, et al. Deep retroperitoneal pelvic endometriosis: MR imaging appearance with laparoscopic correlation. Radiographics. 2006;26:1705–1718. doi: 10.1148/rg.266065048. [DOI] [PubMed] [Google Scholar]
- 34.Frishman GN, Salak JR. Conservative surgical management of endometriosis in women with pelvic pain. J Minim Invasive Gynecol. 2006;13:546–558. doi: 10.1016/j.jmig.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Exacoustos C, et al. Recurrence of endometriomas after laparoscopic removal: sonographic and clinical follow-up and indication for second surgery. J Minim Invasive Gynecol. 2006;13:281–288. doi: 10.1016/j.jmig.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Hammoud A, et al. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82:1483–1491. doi: 10.1016/j.fertnstert.2004.07.948. [DOI] [PubMed] [Google Scholar]
- 37.Milingos S, et al. Endometriosis in patients with chronic pelvic pain: is staging predictive of the efficacy of laparoscopic surgery in pain relief? Gynecol Obstet Invest. 2006;62:48–54. doi: 10.1159/000092023. [DOI] [PubMed] [Google Scholar]
- 38.Parker JD, et al. Adhesion formation after laparoscopic excision of endometriosis and lysis of adhesions. Fertil Steril. 2005;84:1457–1461. doi: 10.1016/j.fertnstert.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 39.Redwine DB. Dissecting and temporarily resuspending the adherent ovary. Fertil Steril. 2006;86:772. doi: 10.1016/j.fertnstert.2006.07.1464. author reply 772–773. [DOI] [PubMed] [Google Scholar]
- 40.Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123:217–226. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 41.Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet. 2002;76:117–126. doi: 10.1016/s0020-7292(01)00577-x. [DOI] [PubMed] [Google Scholar]
- 42.Slater M, et al. Endometriotic cells exhibit metaplastic change and oxidative DNA damage as well as decreased function, compared to normal endometrium. J Mol Histol. 2005;36:257–263. doi: 10.1007/s10735-005-3802-9. [DOI] [PubMed] [Google Scholar]
- 43.Starzinski-Powitz A, et al. In search of pathogenic mechanisms in endometriosis: the challenge for molecular cell biology. Curr Mol Med. 2001;1:655–664. doi: 10.2174/1566524013363168. [DOI] [PubMed] [Google Scholar]
- 44.Ulukus M, et al. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13:467–476. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Yang WC, et al. Serum and endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2004;18:305–318. doi: 10.1016/j.bpobgyn.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Wenzl R, et al. Endometriosis: a genetic disease. Drugs Today (Barc) 2003;39:961–972. doi: 10.1358/dot.2003.39.12.799414. [DOI] [PubMed] [Google Scholar]
- 47.Haskill S, et al. Normal human peritoneal macrophages are unable to cap and internalize class II antigens. Cell Immunol. 1988;115:100–111. doi: 10.1016/0008-8749(88)90165-7. [DOI] [PubMed] [Google Scholar]
- 48.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279:23038–23044. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 49.Belting M, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10:502–509. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 50.Lockwood CJ, et al. Steroid-modulated stromal cell tissue factor expression: a model for the regulation of endometrial hemostasis and menstruation. J Clin Endocrinol Metab. 1993;77:1014–1019. doi: 10.1210/jcem.77.4.8408448. [DOI] [PubMed] [Google Scholar]
- 51.Lockwood CJ, et al. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab. 1993;76:231–236. doi: 10.1210/jcem.76.1.8421090. [DOI] [PubMed] [Google Scholar]
- 52.Schatz F, et al. Progestin-regulated expression of tissue factor in decidual cells: implications in endometrial hemostasis, menstruation and angiogenesis. Steroids. 2003;68:849–860. doi: 10.1016/s0039-128x(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 53.Krikun G, Lockwood CJ. Factor Tisular, VEGF y PAR-2. Corpus Editorial y Distribuidora; Rosario, Argentina: 2007. [Google Scholar]
- 54.Akoum A, et al. Estradiol amplifies interleukin-1-induced monocyte chemotactic protein-1 expression by ectopic endometrial cells of women with endometriosis. J Clin Endocrinol Metab. 2000;85:896–904. doi: 10.1210/jcem.85.2.6348. [DOI] [PubMed] [Google Scholar]
- 55.Arici A, et al. Interleukin-8 induces proliferation of endometrial stromal cells: a potential autocrine growth factor. J Clin Endocrinol Metab. 1998;83:1201–1205. doi: 10.1210/jcem.83.4.4743. [DOI] [PubMed] [Google Scholar]
- 56.Arici A, et al. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996;2:40–45. doi: 10.1093/molehr/2.1.40. [DOI] [PubMed] [Google Scholar]
- 57.Taylor RN, et al. Angiogenesis and macrophage activation in endometriosis. Ann NY Acad Sci. 1997;828:194–207. doi: 10.1111/j.1749-6632.1997.tb48540.x. [DOI] [PubMed] [Google Scholar]
- 58.Hammond MG, et al. The effect of growth factors on the proliferation of human endometrial stromal cells in culture. Am J Obstet Gynecol. 1993;168:1131–1136. doi: 10.1016/0002-9378(93)90356-n. discussion 1136–1138. [DOI] [PubMed] [Google Scholar]
- 59.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:612–621. doi: 10.1161/01.atv.15.5.612. [DOI] [PubMed] [Google Scholar]
- 60.Syrovets T, et al. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood. 2001;97:3941–3950. doi: 10.1182/blood.v97.12.3941. [DOI] [PubMed] [Google Scholar]
- 61.Oeth P, et al. Regulation of the tissue factor gene in human monocytic cells. Role of AP-1, NF-kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide-induced expression. Arterioscler Thromb Vasc Biol. 1997;17:365–374. doi: 10.1161/01.atv.17.2.365. [DOI] [PubMed] [Google Scholar]
- 62.Bruse C, et al. Fibrinolytic factors in endometriotic tissue, endometrium, peritoneal fluid, and plasma from women with endometriosis and in endometrium and peritoneal fluid from healthy women. Fertil Steril. 1998;70:821–826. doi: 10.1016/s0015-0282(98)00285-4. [DOI] [PubMed] [Google Scholar]
- 63.Gilabert-Estelles J, et al. Plasminogen activators and plasminogen activator inhibitors in endometriosis. Front Biosci. 2005;10:1162–1176. doi: 10.2741/1609. [DOI] [PubMed] [Google Scholar]
- 64.Gilabert-Estelles J, et al. Expression of the fibrinolytic components in endometriosis. Pathophysiol Haemost Thromb. 2006;35:136–140. doi: 10.1159/000093556. [DOI] [PubMed] [Google Scholar]
- 65.Terry CM, et al. Polymorphisms in the 5′-UTR of the tissue factor gene are associated with altered expression in human endothelial cells. J Thromb Haemost. 2004;2:1351–1358. doi: 10.1111/j.1538-7836.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 66.Reny JL, et al. The TF-603A/G gene promoter polymorphism and circulating monocyte tissue factor gene expression in healthy volunteers. Thromb Haemost. 2004;91:248–254. doi: 10.1160/TH03-09-0566. [DOI] [PubMed] [Google Scholar]
- 67.Arnaud E, et al. Polymorphisms in the 5′regulatory region of the tissue factor gene and the risk of myocardial infarction and venous thromboembolism: the ECTIM and PATHROS studies. Etude Cas-Temoins de l’Infarctus du Myocarde Paris Thrombosis case-control Study. Arterioscler Thromb Vasc Biol. 2000;20:892–898. doi: 10.1161/01.atv.20.3.892. [DOI] [PubMed] [Google Scholar]
- 68.Fan L, et al. Tissue factor enhances protease-activated receptor-2-mediated factor VIIa cell proliferative properties. J Thromb Haemost. 2005;3:1056–1063. doi: 10.1111/j.1538-7836.2005.01250.x. [DOI] [PubMed] [Google Scholar]


