Abstract
BACKGROUND:
Hospital-prepared tube feedings from three intensive care units of two hospitals in Isfahan, Iran were analyzed for microbial contamination.
METHODS:
A total number of 152 samples (76 samples each at the time of preparation and 18 hours following preparation) were collected. Standard plate count, coliform count and Staphylococcus aureus count for all samples were conducted. Samples were analyzed also for the presence of Salmonella spp. and Listeria spp.
RESULTS:
At the time of food preparation, out of 76 samples, 53 samples (70%) had coliform contamination and 87% of these contaminated samples had counts greater than 101 cfu/g. Also, 68 samples (90%) had S. aureus contamination greater than 101 cfu/g. In standard plate count, 74 samples (97%) had counts greater than 103 cfu/g, while 54 samples (71%) had counts greater than 104 cfu/g. In second sampling occasion, out of 76 samples, 68 samples (90%) had coliform contamination and 84% of these contaminated samples had counts greater than 101 cfu/g. Also, 72 samples (95%) had S. aureus contamination, 98.6% of these contaminated samples had counts greater than 102 cfu/g. In standard plate count, 74 samples (97%) had counts greater than 104 cfu/g. No Salmonella or Listeria was detected from samples.
CONCLUSIONS:
The results indicated that a majority of the blenderized enteral tube feedings in those hospitals are not safe. In comparison to the standard limits, these enteral tube feedings are highly contaminated and posed substantial risk for developing a foodborne disease or nosocomial infection.
Keywords: Enteral Feeding, Microbial Contamination, Nosocomial Infection, Standard Plate Count, Coliform
Enteral nutrition is essential in the care of patients who are unable to eat. Potential complication of enteral feeding is microbial contamination of the solution.1 Enteral feeding fluid is a good medium for exponential growth of most of the foodborne microorganisms.2,3 Contaminated feeding increases the risk of nosocomial infections such as diarrhea, pneumonia and septicaemia.4–6 Occurrence of various microorganisms in tube feeding formula, have been investigated in several countries.2,6–11 In the Philippines, 75% to 96% of blenderized tube feeding samples were reported to have standard plate counts greater than 101 cfu/g.12 Higher bacterial contamination of hospital-prepared tube feeding formulas have been reported in Saudi Arabia. Nearly all samples had aerobic plate counts greater than 104 cfu/g in this country.13 Although sterile ready-to-eat feeding is available in developed nations, blenderized feedings continue to be used in most parts of Iran, mostly due to economic and cultural reasons. Since there is no information on the level of contamination of hospital-prepared tube feeding systems in Iran, the present study aimed to evaluate the microbial quality of blenderized enteral tube feedings in three intensive care units of two university hospitals in Isfahan, Iran.
Methods
Three intensive care units selected from two university hospitals (A and B), were participated in this study. Feedings used in this study, were prepared in the main kitchen of each hospital by chef under nutritionist supervision. All tube feeding ingredients (egg, milk, meat, etc.) were cooked and mixed (blenderized) to provide appropriate calories. The feeds were prepared daily in quantities that would allow feeding patients for about 24 hours. The feedings were shipped to the wards every day (between 11-12 am) in closed containers and stored in refrigerator for 24 hours. In the period between October 2005 and September 2006, a total number of 152 samples were collected (46, 46 and 60 samples from hospital A-Central ICU, hospital A-Trauma ICU and hospital B-Neurosurgery ICU, respectively). Feeding samples were marked and 50mL of feeds were collected in two occasions, immediately and 18 hours following preparation, for microbial analysis. All samples were transported to the Food Microbiology Laboratory in School of Public Health of Isfahan University of Medical Sciences in an icebox for microbiological analysis. Standard plate count, coliform count and Staphylococcus aureus count for all samples were conducted. Samples were analyzed also for the presence of Salmonella spp. and Listeria spp.
The number of aerobic bacteria, coliforms and S. aureus were determined using pure plate technique.14 For total count, coliform count and S. aureus quantification, ten-fold serial dilutions were prepared in 0.1% sterile Buffered Peptone Water (Oxoid). From each dilution a 1mL aliquot was added to Nutrient Agar (NA, Merck, Germany), Violet Red Bile Agar (VRBA, Merck) and Baird-Parker Agar (BPA, Merck). Colony counts per mL of feed were done after incubation at 37°C for 24-48 hours. Typical colonies on VRBA and BPA were also examined using suitable biochemical tests.12 Results are expressed as colony forming units (cfu)/ mL of food.
Samples were analyzed for the presence of Listeria spp. and in particular for Listeria monocytogenes using selective enrichment and isolation protocol, recommended by United States Department of Agriculture (USDA).15 Twenty–five grams of a sample was aseptically taken, homogenized for 2 minutes in 225mL of UVM Listeria enrichment broth (UVM I) (Difco, America) and incubated at 30°C for 24 hours. One mL of primary enrichments were transferred to 9mL of UVM II (Fraser broth) (Amyl Media, Australia) and incubated at 35°C for 48 hours. Secondary enrichments were streaked on Oxford Agar (Merck) and Palcam Agar (Merck) and incubated at 37°C for 48 hours. The plates then examined for typical Listeria colonies (black colonies with black sunken) and at least 3 suspected colonies were sub cultured on Trypton Soy Agar supplemented with 0.6% of yeast extract (TSAYE) and incubated at 37°C for 24 hours. All isolates were subjected to standard biochemical tests such as Gram’s stain, catalase test, motility at 25°C and 37°C, acid production from glucose, manitol, rhamnose, xylose, α- methyl- D- manoside, and nitrate reduction, hydrolysis of esculin and MR/VP test. For further confirmations of Listeria spp., other biochemical reactions, ß-haemolytic activity, and CAMP test were performed according to the Bergey’s Manual of Systematic Bacteriology.16
Samples were also examined for the presence of Salmonella spp. by the reference method (No 1810) recommended by the Iranian Standard Organization for the isolation of Salmonella.17 Briefly a 25g portion of each sample was weighed aseptically in a sterile stomacher bag containing 225mL sterile Buffered Peptone Water (BPW) and shaken for 2 minutes. BPW was used for pre-enrichment at 37°C for 18-24 hours. One mL of the pre-enriched sample was then inoculated into 10mL of Modified Rappaport-Vassiliadis (RV, Merck) broth and 9mL of Selenite Cystine (SC, Merck) broth and was incubated at 42°C and 37°C respectively for 24 hours. Xylose Lysine Deoxycholate (XLD, Merck) medium was used as selective isolation media and incubated at 37°C for 24 hours. At least three characteristic colonies were picked from each plate and purified by streaking on Tryptone Soy Agar (TSA, Merck). Cultures were further subjected to analysis for Gram’s stain, motility, ONPG, urease, lysine decarboxylase and reaction on Triple Sugar Iron Agar. Results were expressed as presence or absence of Salmonella or Listeria.
Statistical Analysis
Statistical analyses were conducted after computing log cfu/g and by using SPSS package, version 13.0.18 Wilcoxon Signed Ranks Test was used to determine a statistically significant difference between onset and 18 hours after food preparation. Differences were considered significant when p < 0.05.
Results
At the time of food preparation, there were the range of total viable counts, coliform count and S. aureus count among 23 samples of food in hospital A-Trauma ICU, 23 samples in hospital A-Central ICU and 30 samples in hospital B-Neurosurgery ICU. There were significant increases in counts 18 hours after food preparation (p value < 0.001). The results have been shown in table 1.
Table 1.
Bacterial contamination of hospital-prepared tube feeding samples in three intensive care units, at the time of food preparation and 18 hrs after preparation
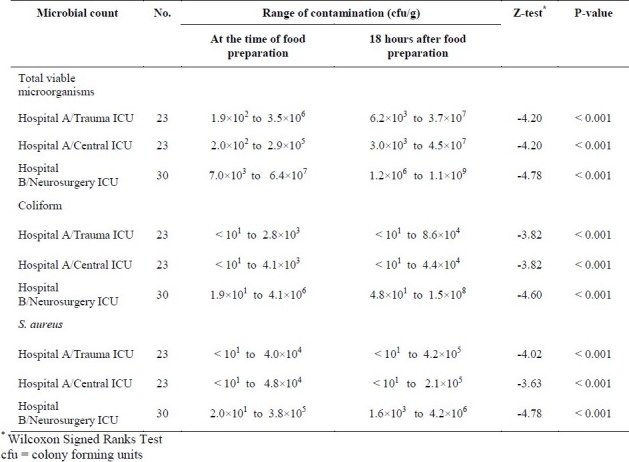
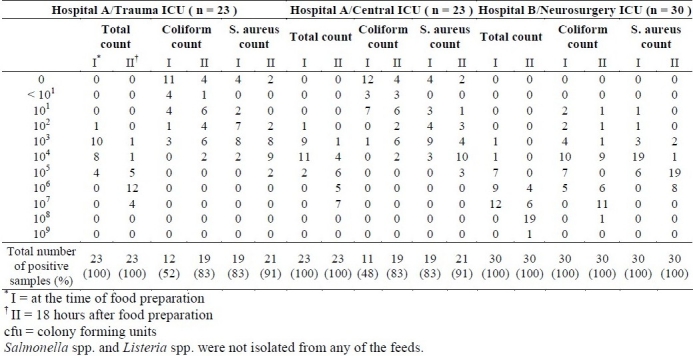
As indicated in table 2, in first sampling, out of total 76 samples taken from three wards of two hospitals at the time of food preparation, 53 samples (70%) had coliform contamination, 87% of the contaminated samples had counts greater than 101 cfu/g. Out of samples collected immediately after preparation, 68 samples (90%) had S. aureus contamination greater than 102 cfu/g. In standard plate count, 74 samples (97%) had counts greater than 103 cfu/g, while 54 samples (71%) had counts greater than 104 cfu/g. In a second sampling occasion, 18 hours after preparation, out of total 76 samples, 68 samples (90%) had coliform contamination, 84% of these contaminated samples had counts greater than 101 cfu/g. Also, 72 samples (95%) had S. aureus contamination, 98.6% of these contaminated samples had counts greater than 102 cfu/g. In standard plate count, 74 samples (97%) had counts greater than 104 cfu/g. No Salmonella spp. and L. monocytogenes were detected from any samples at the time of preparation and 18 hours after storage in the wards.
Table 2.
Number of contaminated hospital-prepared tube feeding samples in three intensive care units, at the time of food preparation and 18 hrs after preparation
Discussion
In recent years, there has been a significant shift in the use of enteral nutrition over parenteral nutrition.19–22 Using enteral nutrition can greatly impact the functional and structural integrity of the gastrointestinal tract.20,23 One potential complication of enteral feeding, is microbial contamination of the solution. Enteral feeding solutions support the growth of a wide variety of microorganisms, creating a risk factor for many patients. Many studies have associated nosocomial infections, namely diarrhea, bacteremia and pneumonia to contamination of enteral feeding. Due to the increasing amounts of nosocomial infections occurring as a result of contaminated tube feeding formulas, regulatory agencies are requiring improved control procedures during preparation and administration of enteral feeding. Recent guidelines of Food and Drug Administration (2006) regarding microbial quality of medical foods, including tube feeding formulas, stated that action must be taken if any such products contain more than 104 cfu/g or if three or more samples exceeded 103 cfu/g. Also they limit the acceptable level of coliforms to 3 organisms/gram.24 According to the Parenteral and Enteral Nutrition Group of British Dietetic Association, the critical limit for total microbial counts of tube feeding samples is 101 cfu/g at the start of administration and less than 103 cfu/g at the end.25 The Spanish legislation limits the S. aureus count to 101 organisms/gram.6 Using a definition of “unacceptable” contamination as a standard plate count greater than 103 cfu/g, the results of present study show that 98.6% of the foods were unacceptably contaminated at the time of preparation and 18 hours after preparation. Also, 72% and 92% of all samples had unacceptable counts for coliforms and S. aureus, respectively (Table 2). Similar study on bacterial contamination of hospital-prepared tube feeding formulas in Saudi Arabia indicated that nearly all samples had aerobic plate counts greater than 104 cfu/g. It also reported maximum coliform counts of about 50 cfu/g.13 The results of the present study show much higher maximum level of coliform contamination of about 4.1×106 cfu/g at the time of administration (Table 1). This figure demonstrates poor hygienic condition in preparation of tube feeding in the kitchen. Poor hand hygiene and handling procedures are identified as the main source of microbial contamination.26 The occurrence of coliforms in tube feeding samples receiving heat treatment indicates poor hygienic condition.14,27 The presence of high S. aureus counts (maximum count of 4.2×106 cfu/g) in sample may also indicates poor hygienic condition of the personnel. In the Philippines, 75% to 96% of blenderized tube feeding samples were reported to have standard plate counts greater than 101 cfu/g.12,28 These results are also similar to our finding. The results of this study show significant increase in bacterial counts in the period of storing at wards (Table 1). The maximum level of standard plate counts, coliform and S. aureus counts significantly increased 18 hours after preparation and reached to 1.1×109 cfu/g, 1.5×108 cfu/g and 4.2×106 cfu/g, respectively, which indicates the poor hygienic conditions or more contamination of feeds during storage in the wards. Temperature abuse of feeds during storage in the wards is the other possibility which may allow exponential growth of bacteria in feeds. Regular measurement of refrigerator temperature (results are not shown) in three wards indicates that average temperature was above 9.9°C. Note that 0 to 7°C has been suggested as a proper temperature of domestic refrigerator.14 Moreover, in some occasions, the feeds kept outside refrigerator for a long time. Similarly, other studies have also demonstrated substantial increase in bacterial counts over time (baseline of 101 -102 cfu/ml, increasing to 105 -108 cfu/ml over 8 hrs).3,29
In the present study, an attempt also was made to isolate two important foodborne pathogens. Listeria monocytogenes which is considered as a foodborne pathogen is able to cause meningitis, encephalitis, septicemia, endocarditis, abortion, premature birth, abscesses and local petulant lesions.30 Although listeriosis occurs infrequently, the mortality rate is high, up to 75% in high risk persons such as immunocompromised people suffering from cancer, AIDS, etc.31 Although, the presence of L. monocytogenes has been reported in a wide variety of foods in Iran,32 we were not able to isolate any Listeria from food samples. As far as our knowledge, similar to our findings, Listeria has not been yet detected from tube feeding formulas.
Salmonella spp. is also established as one of the leading causes of foodborne disease. Salmonella is a causative agent of foodborne diarrheal disease worldwide.33 Salmonellosis remains a major public health problem in many parts of the world34 including Iran.35 Salmonella enteritidis contamination of enteral tube feeding has been already reported.36 In contrast, we were not able to detect Salmonella from 152 tube feeding samples.
Tube feeding formulas become contaminated at several points. Anderton et al (1993) highlighted both the main sources of contamination and possible determinal effects of administrating contaminated feeds to patients. Main sources of food contamination include the feed ingredients, administration systems and their design, mishandling during assembly of systems, inadequate cleaned equipments, kitchen and ward environment, personnel and patients themselves.37 Recently, Mathus-Viegen et al (2006) also studied sites of bacterial contamination of enteral feeding system.38 In order to reduce microbial contamination of enteral tube feeding, various multidisciplinary approaches have been used.39,40 For example, Hazard Analysis Critical Control Points (HACCP) system is internationally recognized as the best method of assuring product safety by controlling foodborne safety hazards. In a study, HACCP system was implemented for improvement of microbial quality of enteral tube feeding in a hospital, and when the control measures applied and monitored, the bacterial counts in feeds reduced from 105 cfu/mL to < 101 cfu/mL.41
Conclusions
The results of present study indicate that the microbial quality of the majority of blenderized enteral tube feedings in both hospitals is not within published guidelines for safety. It is also important to note that there are no microbiological criteria or recommendation for tube feeding formula in Iran. The data we presented, demonstrate that it is urgent to assure strict hygienic methods including the development of protocols for clean techniques in the preparation, handling and storage of feeds and cleaning and sanitization of preparation equipments. In addition, recommendations for microbial quality of enteral tube feeding need to be made by authorities. In conclusion, these solutions must be handled in an aseptic manner during preparation and administration. The implementation of HACCP system will be required in a near future for better quality control of enteral nutrition formulas. The use of commercial products may also provide an additional margin of safety for hospitalized patients.
Authors’ Contributions
MJ carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. AMS provided assistance in the design of the study and participated in manuscript preparation. ShSB carried out all the experiments and participated in manuscript preparation. HAS participated in the design of the study and manuscript preparation. MRM carried out statistical analysis of the data. All authors have read and approved the content of the manuscript.
Acknowledgments
This work was financially supported by the research council of the Isfahan University of Medical Sciences (Research Project No. 184120).
Footnotes
Conflict of Interest:
Authors have no conflict of interests.
References
- 1.White WT, 3rd, Acuff TE, Jr, Sykes TR, Dobbie RP. Bacterial contamination of enteral nutrition solutions: a preliminary report. J Parenter Enteral Nutr. 1979;3(6):459–61. doi: 10.1177/014860717900300611. [DOI] [PubMed] [Google Scholar]
- 2.Arias ML, Monge R, Chavez C. Microbial contamination of enteral feeding solutions used in Costa Rican hospitals. Arch Latinoam Nutr. 2003;53(3):277–81. [PubMed] [Google Scholar]
- 3.Anderton A. The potential of Escherichia coli in enteral feeds to cause food poisoning: a study under simulated ward conditions. J Hosp Infect. 1984;5(2):155–63. doi: 10.1016/0195-6701(84)90119-1. [DOI] [PubMed] [Google Scholar]
- 4.Anderton A. Bacterial contamination of enteral feeds and feeding systems. Clin Nutr. 1993;12(1 Suppl):S16–32. [Google Scholar]
- 5.Casewell MW. Bacteriological hazards of contaminated enteral feeds. J Hosp Infect. 1982;3(4):329–31. doi: 10.1016/0195-6701(82)90063-9. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Crehuet Navajas M, Jurado Chacon D, Guillen Solvas JF, Galvez Vargas R. Bacterial contamination of enteral feeds as a possible risk of nosocomial infection. J Hosp Infect. 1992;21(2):111–20. doi: 10.1016/0195-6701(92)90030-p. [DOI] [PubMed] [Google Scholar]
- 7.Casewell M, Phillips I. Food as a source of Klebsiella species for colonization and infection of intensive care patients. J Clin Pathol. 1978;31(9):845–9. doi: 10.1136/jcp.31.9.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurn J, Crossley K, Gerdts A, Maki M, Johnson J. Enteral hyperalimentation as a source of nosocomial infection. J Hosp Infect. 1990;15(3):203–17. doi: 10.1016/0195-6701(90)90028-m. [DOI] [PubMed] [Google Scholar]
- 9.Arias ML, Monge R, Rodriguez J. Presence of total coliforms, Escherichia coli, and Listeria sp. in enteral formulas. Arch Latinoam Nutr. 1998;48(1):68–70. [PubMed] [Google Scholar]
- 10.Roberts CA, Lyman E. Microbial contamination of enteral feeding sets used in the home of pediatric patients. Nutr Clin Pract. 2008;23(1):85–9. doi: 10.1177/011542650802300185. [DOI] [PubMed] [Google Scholar]
- 11.Roy S, Rigal M, Doit C, Fontan JE, Machinot S, Bingen E, et al. Bacterial contamination of enteral nutrition in a paediatric hospital. J Hosp Infect. 2005;59(4):311–6. doi: 10.1016/j.jhin.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan MM, Sorreda-Esguerra P, Santos EE, Platon BG, Castro CG, Idrisalman ER, et al. Bacterial contamination of blenderized whole food and commercial enteral tube feedings in the Philippines. J Hosp Infect. 2001;49(4):268–73. doi: 10.1053/jhin.2001.1093. [DOI] [PubMed] [Google Scholar]
- 13.Mokhalalati JK, Druyan ME, Shott SB, Comer GM. Microbial, nutritional and physical quality of commercial and hospital prepared tube feedings in Saudi Arabia. Saudi Med J. 2004;25(3):331–41. [PubMed] [Google Scholar]
- 14.Jay JM. Modern food microbiology. New Delhi: CBS Publishers & Distributors; 2003. [Google Scholar]
- 15.McClain D, Lee WH. Development of USDA-FSIS method for isolation of Listeria monocytogenes from raw meat and poultry. J Assoc Off Anal Chem. 1988;71(3):660–4. [PubMed] [Google Scholar]
- 16.Sneath PHA. Bergey’s manual of systematic bacteriology. 4th ed. Philadelphia: Williams & Wilkins; 1986. pp. 1235–45. [Google Scholar]
- 17.Iranian Standard Organization. No 1810: Method recommended for the isolation of Salmonella from food. 1995 [Google Scholar]
- 18.UCit Instructional and Research Computing, Software Distribution Office, 303B Zimmer, Cincinnati, OH [Google Scholar]
- 19.Lin MT, Saito H, Fukushima R, Inaba T, Fukatsu K, Inoue T, et al. Route of nutritional supply influences local, systemic, and remote organ responses to intraperitoneal bacterial challenge. Ann Surg. 1996;223(1):84–93. doi: 10.1097/00000658-199601000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudsk KA. Importance of enteral feeding in maintaining gut integrity. Tech Gastrointest Endosc. 2001;3(1):2–8. [Google Scholar]
- 21.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–13. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216(2):172–83. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deitch EA, Dazhong XU, Naruhn MB, Deitch DC, Lu Q, Marino AA. Elemental diet and IV-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg. 1995;221(3):299–307. doi: 10.1097/00000658-199503000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Drug Administration. Compliance program guidance manual CPGM 7321.002; chapter 21. Rockville, MD, USA: 2006 [Google Scholar]
- 25.Anderton A, Howard JP, Scott DW. Microbiological control in enteral feeding. Summary of a guidance document prepared on behalf of The Parenteral and Enteral Nutrition Group of the British Dietetic Association. Hum Nutr Appl Nutr. 1986;40(3):163–7. [PubMed] [Google Scholar]
- 26.Best C. Enteral tube feeding and infection control: how safe is our practice? Br J Nurs. 2008;17(16):1036, 1038–41. doi: 10.12968/bjon.2008.17.16.31069. [DOI] [PubMed] [Google Scholar]
- 27.Arnold G, Jenson I, Sutherland P, Hocking AD, Newton KG. Foodborne microorganisms of public health significance. North Sydney: Australian Institute of Food Science and Technology Ltd; 1997. [Google Scholar]
- 28.Tanchoco CC, Florentino RF, Flores EG, Castro MaCA, Portugal TR. Survey of blenderized diets prepared by some hospitals in metro Manila: Phase II. Nutrient composition of blenderized diets. Hospital Journal. 1990;22:17–26. [Google Scholar]
- 29.Bastow MD, Allison SP, Greaves P. Microbial contamination of naso-gastric feeds. Hum Nutr Appl Nutr. 1982;36A:213–7. [PubMed] [Google Scholar]
- 30.Farber JM, Peterkin PI. Listeria monocytogenes, a foodborne pathogen. Microbiol Rev. 1991;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouquette C, Berche P. The pathogenesis of infection by Listeria monocytogenes. Microbiologia. 1996;12(2):245–58. [PubMed] [Google Scholar]
- 32.Jalali M, Abedi D. Prevalence of Listeria species in food products in Isfahan, Iran. Int J Food Microbiol. 2008;122(3):336–40. doi: 10.1016/j.ijfoodmicro.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 33.Tauxe RV. An update on Salmonella. Health & Environ Digest. 1996;10(1):1–4. [Google Scholar]
- 34.Taylor JL, Dwyer DM, Groves C, Bailowitz A, Tilghman D, Kim V, et al. Simultaneous outbreak of Salmonella enteritidis and Salmonella schwarzengrund in a nursing home: association of Salmonella enteritidis with bacteremia and hospitalization. J Infect Dis. 1993;167(3):781–2. doi: 10.1093/infdis/167.3.781. [DOI] [PubMed] [Google Scholar]
- 35.Jalali M, Abedi D, Pourbakhsh SA, Ghoukasin K. Prevalence of Salmonella spp. in raw and cooked foods in Isfahan-Iran. Journal of Food Safety. 2008;28(3):442–52. [Google Scholar]
- 36.Gill KJ, Gill P. Contaminated enteral feeds. Br Med J. 1981;282(6280):1971. doi: 10.1136/bmj.282.6280.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderton A, Nwoguh CE, McKune I, Morrison L, Greig M, Clark B. A comparative study of the numbers of bacteria present in enteral feeds prepared and administered in hospital and the home. J Hosp Infect. 1993;23(1):43–9. doi: 10.1016/0195-6701(93)90129-n. [DOI] [PubMed] [Google Scholar]
- 38.Mathus-Vliegen EMH, Bredius MWJ, Binnekade JM. Analysis of sites of bacterial contamination in an enteral feeding system. J Parenter Enteral Nutr. 2006;30(6):519–25. doi: 10.1177/0148607106030006519. [DOI] [PubMed] [Google Scholar]
- 39.Howard P, Bowen N. The challenges of innovation in the organization of home enteral tube feeding. J Hum Nutr Dietet. 2001;14(1):3–11. doi: 10.1046/j.1365-277x.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 40.McNamara EP, Flood P, Kennedy NP. Home tube feeding: an integrated multidisciplinary approach. J Hum Nutr Dietet. 2001;14(1):13–9. doi: 10.1046/j.1365-277x.2001.00266.x. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira M, Batista CRV, Aidoo KE. Application of Hazard Analysis Critical Control Points system to enteral tube feeding in hospital. J Hum Nutr Diet. 2001;14(5):397–403. doi: 10.1046/j.1365-277x.2001.00305.x. [DOI] [PubMed] [Google Scholar]


