Abstract
BACKGROUND:
Occult hepatitis B is defined as presence of HBV DNA in tissue or serum without hepatitis B surface antigen. The aim of this study is to determine frequency of occult hepatitis B among hepatitis C patients in Tehran and compare the route of transmission and liver enzymes between positive and negative HBV DNA patients.
METHODS:
In a cross sectional study, serum of 103 hepatitis C cases (79.6% men and 20.4% women) were analyzed for s, x and core genes via a nested polymerase chain reaction technique.
RESULTS:
HBV DNA was detectable in serum of 20 patients (19.4%). No significant difference in age, sex and route of transmission were seen in HBV DNA positive and negative patients. In HBV DNA positive and negative groups, mean of AST was 73, 47 (p < 0.05) and mean of ALT was 76 and 36 respectively (p < 0.05).
CONCLUSION:
Occult hepatitis B was observed in a considerable number of hepatitis C patients in Tehran. It was associated with elevation in liver enzyme but was not related to route of transmission.
Keywords: Occult hepatitis B, hepatitis C, cirrhosis
Hepatitis B surface antigen (HBs Ag) is the principal diagnostic biomarker of hepatitis B infection. Serological evidence of the presence of HBs Ag may remain undetectable in some patients.1–3 This is known as occult hepatitis B (OHB), which is characterized by the presence of hepatitis B virus (HBV) DNA in serum or blood.4,5 However, in 20% of patients with OHB no serological biomarker is found for hepatitis B.6
OHB has been investigated and demonstrated in different groups, including healthy blood donors, patients with hepatocellular carcinoma (HCC),7 dialysis patients,8 children,9 hemophiliacs,10 hepatitis C patients,11 and even animals.
Various mechanisms have been proposed to explain OHB; these include S locus mutation,12 immune complex formation between HBV DNA and HBs Ag,13 impaired immune response, especially impairment of TNF-α,14 and the inhibitory effect of hepatitis C on HBs Ag.15 Besides, hepatitis B is the most common cause of chronic hepatitis and cirrhosis in Iran. Between 1.8 and 5 percent of serum samples in Iran are reported positive for HBs Ag.16 Although the significance of OHB in these patients has yet to be assessed, some studies have implicated OHB in rapid progression of cirrhosis,17 weaker response to interferon therapy18 and increased risk of HCC.19
The prevalence of OHB in patients with chronic hepatitis varies in the world, e.g. a study in Brazil20 and another in Japan21 reported 0 and 95 percent, respectively.
A study on liver tissue samples of 35 patients with chronic liver disease revealed a 22% rate of OHB; most of these patients (77.1%) had hepatitis C. No serological assessment was performed in this study.22
In another study, 1.9% of 104 patients with chronic liver disease were found to have OHB; however, liver tissue samples were not studied.16
The aim of the present study was to investigate and compare the prevalence of OHB in patients with hepatitis C by examining serum samples.
Methods
One hundred and three chronic hepatitis C patients with a mean age of 42.5 ± 12.87 years, referred to Thaleghani hospital from 2002 to 2004 were studied. Diagnosis was based on positive HCV Ab and HCV RNA for at least six months.
Informed written consent was obtained from the patients prior to the study. The patients’ age and sex were recorded using questionnaires and routine laboratory tests were conducted. The exclusion criteria were as follows:
- Detecting Anti-HBs in serum
- Previous history of treatment for hepatitis C
- Undetectable HCV RNA
- History of consuming more than 40g alcohol in a week
- Other chronic liver diseases
- Using immunosuppressants
- Acquired Immunodeficiency Syndrome (AIDS)
Assessment for HBV DNA
Viral DNA was isolated from serum using the phenolchloroform method. OHB was confirmed by using nest-type polymerase chain reaction method and two pairs of nucleotide primers for each of the S, C and X regions. Anticontamination procedures were done according to protocol23 Primers of the type mentioned in the Kao study were used (Gene-Fanavaran, Tehran, Iran).24 Polymerase chain reaction for each region was performed using corresponding nucleotide primers 1 and 2.
This reaction was performed in a volume of 25 μl, including 1x buffer, dNTP (10 mM), MgC12 (1.5 mM), 100 ng of each of the aforementioned nucleotide primers, 5 μl of extracted DNA, and 0.5 units of SuperTaq Polymerase.
Heat cycles of the polymerase reaction consisted of 30 cycles at 95°C, 55°C, 72°C, each for one minute, and a 7-minute cycle at 72°C.
1 μl of the product of stage I was used in the polymerase chain reaction of stage II. The reaction in stage II was performed under conditions similar to stage I, the only difference being the use of nucleotide primers 3 and 4, and a total reaction volume of 50 μl.
The end results were studied on 2% agarose gel. Patients with two positive polymerase chain reactions for the three mentioned regions were considered OHB-positive. The PCR test had a sensitivity of 50 copies/ml and there was no difference between the PCR procedures for regions C, S and X.
In 10 positive controls, PCR test was positive for all three genes, while neither of the tests was positive for any of our 12 negative controls. Data were analyzed with Student's t-test and chi-square test in SPSS 11.
Results
We studied 103 patients with chronic hepatitis C. Out of this number, 82 (79.56%) were male and 21 (20.4%) were female. The patients had a mean age of 42.5 ± 12.87 years. OHB was detected in 20 patients (19.4%); 5 of who were female (25%) and 15 were male (75%). Out of the non-infected subjects, 16 (19.3%) were female and 67 (80.7%) were male.
The two groups were not significantly different in respect of sex and/or age.
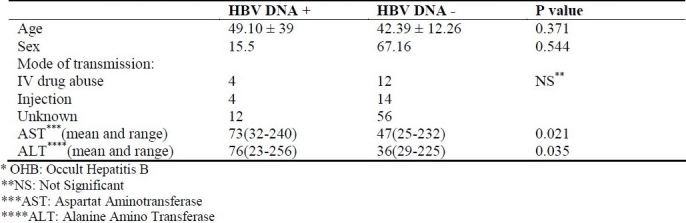
Among 103 patients included in the study, 43 were Anti Hbc positive, and among these 43 patients, 13 were positive for HBV DNA. But from among 60 anti Hbc negatives, only 7 patients were positive for HBV DNA (p < 0.05).The patients with chronic hepatitis C and OHB, and those without OHB had mean ages of 39.9 ± 10.49 years and 42.39 ± 12.26 years, respectively, and there was no significant difference between the age of the two groups. AST (Aspartat Aminotransferase), ALT (Alanine Amino Transferase) level and route of transmission were compared and there was no statistically significant difference between the two groups (Table 1).
Table 1.
Characteristics of hepatitis C patients with and without OHB*.
Discussion
Our study showed that OHB is not uncommon in hepatitis C patients. As stated earlier, the prevalence of OHB in the world is highly varied (e.g. 0% reported in Britain, 11% in Italy).25 However, OHB seems highly prevalent in Asia.6
Although the prevalence of occult hepatitis B between chronic liver disease patients is variable in many reports, highest prevalence is among hepatitis C patients.26 In a study Minuk et al found HBV DNA in 8% of healthy individuals without any biomarkers for Hepatitis B.27
The difference in the reported prevalence may be due to a multitude of reasons. Notably, risk factors for hepatitis B infection and other biomarkers of hepatitis B infection are different in the studied populations. There are also methodological differences in studying HBV DNA. Immune response to hepatitis B infection varies between communities,14 this difference may be due to the fluctuating presence of HBV DNA.25
We tested the serum of hepatitis C patients for evaluation of HBV DNA. As it is reported by earlier studies, histological evaluation is of greater sensitivity than serological evaluation in detecting OHB28; hence it is probable that real incidence of OHB is more than our estima-tion. some studies, however, have reported the opposite,29 warranting further investigations.
Serum AST and ALT were higher in patients with OHB and this finding may have clinical implication. In some of previous stud-ies, there was an elevation in transaminase in OHB patients, and it was associated with progression to cirrhosis.30,31 Also in some studies on hepatitis C patients with OHB, no elevation in ALT and ALT was found, and surprisingly in these studies histological changes and cirrhosis in OHB group were the same as hepatitis C only group.32,33
There was no difference between OHB patients with others in route of transmission, this finding is in agreement with study of Kazemi et al.33
In view of the high prevalence of OHB in hepatitis C patients, it seems advisable to decrease the screening threshold for detecting OHB in Iranian patients. We may also need to be more vigilant to findings such as inexplicably elevated ALT levels following treatment of hepatitis C.6
We did not avail of complete information relating to Child score of the patients to perform comparisons. The fluctuating nature of hepatitis B viremia in OHB patients has been demonstrated,34 i.e. the prevalence of OHB may be higher in hepatitis C patients.
In hepatitis C patients, the likelihood of a positive HBV DNA test increases with elevated AST levels. Hence, screening for HBV DNA at a time of elevated ALT may be a good focal point for future studies.35
We recommend cost-effectiveness studies to determine the indication for HBV DNA screening in hepatitis C patients unresponsive to treatment, or with elevated ALT levels.
Authors’ Contributions
ASh CAD carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript
BN & FE & MS & MKh & MM & MRZ carried out the design and coordinated the study MH S coordinated the study, participated in most of the experiments and prepared the manuscript.
All authors have read and approved the content of the manuscript.
Footnotes
Conflict of interest: Authors have no conflicts of interest.
References
- 1.Brechot C, Degos F, Lugassy C, Thiers V, Zafrani S, Franco D, et al. Hepatitis B virus DNA in patients with chronic liver disease and negative tests for hepatitis B surface antigen. N Engl J Med. 1985;312(5):270–6. doi: 10.1056/NEJM198501313120503. [DOI] [PubMed] [Google Scholar]
- 2.Lai MY, Chen PJ, Yang PM, Sheu JC, Sung JL, Chen DS. Identification and characterization of intrahepatic hepatitis B virus DNA in HBsAg-seronegative patients with chronic liver disease and hepatocellular carcinoma in Taiwan. Hepatology. 1990;12(3 Pt 1):575–81. doi: 10.1002/hep.1840120321. [DOI] [PubMed] [Google Scholar]
- 3.Kao JH, Chen DS. Overview of hepatitis B and C viruses. In: Goedert JJ, editor. Infectious causes of cancer: Targets for intervention. Totowa(NJ): Humana Press; 2000. pp. 313–30. [Google Scholar]
- 4.Liang TJ, Baruch Y, Ben Porath E, Enat R, Bassan L, Brown NV, et al. Hepatitis B virus infection in patients with idiopathic liver disease. Hepatology. 1991;13(6):1044–51. [PubMed] [Google Scholar]
- 5.Zhang YY, Hansson BG, Kuo LS, Widell A, Nordenfelt E. Hepatitis B virus DNA in serum and liver is commonly found in Chinese patients with chronic liver disease despite the presence of antibodies to HBsAg. Hepatology. 1993;17(4):538–44. doi: 10.1002/hep.1840170403. [DOI] [PubMed] [Google Scholar]
- 6.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2(8):479–86. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 7.Paterlini P, Gerken G, Nakajima E, Terre S, D’Errico A, Grigioni W, et al. Polymerase chain reaction to detect hepatitis B virus DNA and RNA sequences in primary liver cancers from patients negative for hepatitis B surface antigen. N Engl J Med. 1990;323(2):80–5. doi: 10.1056/NEJM199007123230202. [DOI] [PubMed] [Google Scholar]
- 8.Besisik F, Karaca C, Akyuz F, Horosanli S, Onel D, Badur S, et al. Occult HBV infection and YMDD variants in hemodialysis patients with chronic HCV infection. J Hepatol. 2003;38(4):506–10. doi: 10.1016/s0168-8278(02)00457-9. [DOI] [PubMed] [Google Scholar]
- 9.Bortolotti F, Wirth S, Crivellaro C, Alberti A, Martine U, de Moliner L. Long-term persistence of hepatitis B virus DNA in the serum of children with chronic hepatitis B after hepatitis B e antigen to antibody seroconversion. J Pediatr Gastroenterol Nutr. 1996;22(3):270–4. doi: 10.1097/00005176-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Toyoda H, Hayashi K, Murakami Y, Honda T, Katano Y, Nakano I, et al. Prevalence and clinical implications of occult hepatitis B viral infection in hemophilia patients in Japan. J Med Virol. 2004;73(2):195–9. doi: 10.1002/jmv.20075. [DOI] [PubMed] [Google Scholar]
- 11.Coffin CS, Michalak TI. Persistence of infectious hepadnavirus in the offspring of woodchuck mothers recovered from viral hepatitis. J Clin Invest. 1999;104(2):203–12. doi: 10.1172/JCI5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger KM, Bauer T, Bohm S, Jilg W. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J Gen Virol. 2000;81(Pt 5):1165–74. doi: 10.1099/0022-1317-81-5-1165. [DOI] [PubMed] [Google Scholar]
- 13.Yotsuyanagi H, Yasuda K, Iino S, Moriya K, Shintani Y, Fujie H, et al. Persistent viremia after recovery from self-limited acute hepatitis B. Hepatology. 1998;27(5):1377–82. doi: 10.1002/hep.510270526. [DOI] [PubMed] [Google Scholar]
- 14.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284(5415):825–9. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 15.Uchida T, Kaneita Y, Gotoh K, Kanagawa H, Kouyama H, Kawanishi T, et al. Hepatitis C virus is frequently coinfected with serum marker-negative hepatitis B virus: probable replication promotion of the former by the latter as demonstrated by in vitro cotransfection. J Med Virol. 1997;52(4):399–405. doi: 10.1002/(sici)1096-9071(199708)52:4<399::aid-jmv10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Kaviani MJ, Behbahani B, Mosallaii MJ, Sari-Aslani F, Taghavi SA. Occult hepatitis B virus infection and cryptogenic chronic hepatitis in an area with intermediate prevalence of HBV infection. World J Gastroenterol. 2006;12(31):5048–50. doi: 10.3748/wjg.v12.i31.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341(1):22–6. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 18.Sagnelli E, Coppola N, Scolastico C, Mogavero AR, Stanzione M, Filippini P, et al. Isolated anti-HBc in chronic hepatitis C predicts a poor response to interferon treatment. J Med Virol. 2001;65(4):681–7. doi: 10.1002/jmv.2090. [DOI] [PubMed] [Google Scholar]
- 19.Donato F, Gelatti U, Limina RM, Fattovich G. Southern Europe as an example of interaction between various environmental factors: a systematic review of the epidemiologic evidence. Oncogene. 2006;25(27):3756–70. doi: 10.1038/sj.onc.1209557. [DOI] [PubMed] [Google Scholar]
- 20.Souza LO, Pinho JR, Carrilho FJ, da Silva LC. Absence of hepatitis B virus DNA in patients with hepatitis C and non-A-E hepatitis in the State of Sao Paulo, Brazil. Braz J Med Biol Res. 2004;37(11):1665–8. doi: 10.1590/s0100-879x2004001100011. [DOI] [PubMed] [Google Scholar]
- 21.Koike K, Kobayashi M, Gondo M, Hayashi I, Osuga T, Takada S. Hepatitis B virus DNA is frequently found in liver biopsy samples from hepatitis C virus-infected chronic hepatitis patients. J Med Virol. 1998;54(4):249–55. [PubMed] [Google Scholar]
- 22.Honarkar Z, Alavian SM, Samiee S, Saeedfar K, Zali MR. Occult hepatitis B among chronic liver disease patients. Saudi Med J. 2005;26(4):601–6. [PubMed] [Google Scholar]
- 23.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339(6221):237–8. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 24.Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol. 2002;40(11):4068–71. doi: 10.1128/JCM.40.11.4068-4071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo YM, Lo ES, Mehal WZ, Sampietro M, Fiorelli G, Ronchi G, et al. Geographical variation in prevalence of hepatitis B virus DNA in HBsAg negative patients. J Clin Pathol. 1993;46(4):304–8. doi: 10.1136/jcp.46.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46(1):160–70. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Minuk GY, Sun DF, Uhanova J, Zhang M, Caouette S, Nicolle LE, et al. Occult hepatitis B virus infection in a North American community-based population. J Hepatol. 2005;42(4):480–5. doi: 10.1016/j.jhep.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Villari D, de Franchis R, et al. Quantification of intrahepatic hepatitis B virus (HBV) DNA in patients with chronic HBV infection. Hepatology. 2000;31(2):507–12. doi: 10.1002/hep.510310235. [DOI] [PubMed] [Google Scholar]
- 29.Loriot MA, Marcellin P, Bismuth E, Martinot-Peignoux M, Boyer N, Degott C, et al. Demonstration of hepatitis B virus DNA by polymerase chain reaction in the serum and the liver after spontaneous or therapeutically induced HBeAg to anti-HBe or HBsAg to anti-HBs seroconversion in patients with chronic hepatitis B. Hepatology. 1992;15(1):32–6. doi: 10.1002/hep.1840150107. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda R, Ishimura N, Niigaki M, Hamamoto S, Satoh S, Tanaka S, et al. Serologically silent hepatitis B virus coinfection in patients with hepatitis C virus-associated chronic liver disease: clinical and virological significance. J Med Virol. 1999;58(3):201–7. doi: 10.1002/(sici)1096-9071(199907)58:3<201::aid-jmv3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Uchida T, Kaneita Y, Gotoh K, Kanagawa H, Kouyama H, Kawanishi T, et al. Hepatitis C virus is frequently coinfected with serum marker-negative hepatitis B virus: probable replication promotion of the former by the latter as demonstrated by in vitro cotransfection. J Med Virol. 1997;52(4):399–405. doi: 10.1002/(sici)1096-9071(199708)52:4<399::aid-jmv10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.Hui CK, Lau E, Wu H, Monto A, Kim M, Luk JM, et al. Fibrosis progression in chronic hepatitis C patients with occult hepatitis B coinfection. J Clin Virol. 2006;35(2):185–92. doi: 10.1016/j.jcv.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Kazemi-Shirazi L, Petermann D, Muller C. Hepatitis B virus DNA in sera and liver tissue of HBsAg negative patients with chronic hepatitis C. J Hepatol. 2000;33(5):785–90. doi: 10.1016/s0168-8278(00)80311-6. [DOI] [PubMed] [Google Scholar]
- 34.Hofer M, Joller-Jemelka HI, Grob PJ, Luthy R, Opravil M. Frequent chronic hepatitis B virus infection in HIV-infected patients positive for antibody to hepatitis B core antigen only. Swiss HIV Cohort Study. Eur J Clin Micro-biol Infect Dis. 1998;17(1):6–13. doi: 10.1007/BF01584356. [DOI] [PubMed] [Google Scholar]
- 35.Zignego AL, Fontana R, Puliti S, Barbagli S, Monti M, Careccia G, et al. Relevance of inapparent coinfection by hepatitis B virus in alpha interferon-treated patients with hepatitis C virus chronic hepatitis. J Med Virol. 1997;51(4):313–8. [PubMed] [Google Scholar]


