Abstract
BACKGROUND:
To investigate the effects of Baicalin and Octreotide on inflammatory mediators and pancreatic acinar cells apoptosis of rats with severe acute pancreatitis (SAP).
METHODS:
SD rats were randomly divided into sham operated group (I group), model control group (II group), Baicalin treated group (III group) and Octreotide treated group (IV group). Each group was also divided into subgroup of 3, 6 and 12 h (n = 15). The mortality rate, ascites/body weight ratio as well as the level of endotoxin, NO and ET-1 in blood were measured. The pathological severity score of pancreas, apoptotic indexes, and expression levels of Bax and Bcl-2 proteins in each group were investigated.
RESULTS:
The survival rate of III and IV group has a significant difference compared with II group (P12 h < 0.05). The ascites volume, contents of inflammatory mediators in blood and pathological severity score of pancreas of III and IV group declined at different degrees compared to II group (P < 0.05, P < 0.01 or P < 0.001). Apoptotic index in III group was significantly higher than that in II group at 3 and 6 h (P3, 6 h < 0.05). Apoptotic index in IV group was significantly higher than that in II group at pancreatic tail at 6 h (P6 h < 0.05). Expression level of Bax in III group was significantly higher than that in II group (pancreatic head P3 h,6 h < 0.01, pancreatic tail P3 h < 0.001).
CONCLUSIONS:
Compared with Octreotide in the treatment of SAP, the protective mechanisms of Baicalin include reducing the excessive inflammatory mediators’ release, inducing the pancreatic acinar cells apoptosis.
Keywords: Severe acute pancreatitis, baicalin, octreotide, inflammatory mediators, apoptosis, tissue microarrays
Severe acute pancreatitis (SAP) is a systemic disease, which results in systemic inflammatory response syndrome (SIRS) and even leads to multiple organ dysfunction syndrome (MODS).1,2
Despite so many progresses achieved in the comprehensive management of the SAP in the past several decades,3,4 it is still considered as a relatively high mortality rate disease with multiple complications.5 The systemic manifestation of SAP is believed to be related to the actions of specific inflammatory mediators responsible for the majority of pancreatitis associated morbidity and mortality rate. ET-1 is an important type of inflammatory media-tors, which participates in the development of SAP. ET-1 is mostly produced under the condition of SAP by inflammatory cells and organ epithelial cells such as macrophage and pancreatic acinar cells, which mediate the injury of pancreatic acinar cells and remote organ tissue of SAP, and the level of inflammatory mediators are closely related to the severity and the prognosis of SAP. Besides that, Endotoxin produced by Gram-negative bacterium especially under the condition of destroyed intestinal membrane barrier plays a central role in the pathophysiology of sepsis syndrome and stimulate the inflammatory cells to release the cytokines.6 Endotoxin is also linked to the pathogenesis of acute pancreatitis and the degree of endotoxemia is an indication of severity of acute pancreatitis.7,8 In vivo and in vitro experiment of lipopolysaccharide (LPS) on pancreatic acinar cells demonstrated that LPS induced pancreatic damage by directly affecting the pancreatic acinar cells.9 Therefore, eliminating endotoxin may be a selective strategy in the treatment of SAP.10 Nitro oxygen (NO) is a complicated chemical-biological activity and has a dual character in regulating internal environment (homeostasis).11 On the one hand, Excessive amount of NO generated in early stage of SAP causes remote organs tissue injury and aggravate pancreas severity. On the other hand, low concentration of NO in serum plays a protective role in SAP, which can improve microcirculation disturbance and protect endothelial cells.12
Somatostatin and its analogue Octreotide have become a kind of therapeutic drugs in the management of SAP.13–15 For years, clinical practice of Octreotide treatment of SAP has proved that Octreotide is beneficial for the SAP patients because of its confirmed therapeutic effects.16,17 As an analogue of Somatostatin, inhibiting inflammatory mediators release and inducing pancreatic acinar cells apoptosis are main mechanisms of Octreotide in the treatment of SAP. Compared with the pharmacologic effects of Octreotide,18 Baicalin treatment in SAP rats showed a similar action on inflammatory mediators and pancreatic acinar cells in our experiment. Furthermore, anti-bacteria suppresses tumor cells proliferation and eliminates radical free oxygen and its immunological regulation activities have been proved in other related studies. But in traditional Chinese medicine, “Qingyitang” is a representative prescription for SAP treatment19 and Scutellaria baicalensis georgi as the most effective component in the pharmacological efficacy is the main constituent of “Qingyi-tang” and its monomer Baicalin.20,21 It has been demonstrated that Baicalin has pharmacological functions in anti-inflammation, eliminating oxygen free radicals and alleviating the endotoxemia induced DIC.22,23
Studies have found that Baicalin (5, 6, 7-trihydroxyflavone-7-O-D-glucuronic acid) have multiple functions including acting as anti-bacteria and anti-inflammation, inhibiting the aggregation of blood platelets, clearing the free oxygent radical, decreasing the production of endotoxin, improving the microcirculation, and ultimately alleviating the reperfusion injury in ischemic tissues. In addition, the metabolites in vito of Baicalin has strong antisecretion effect on pancreas and can inhibit pancreatin secretion effectively.26–31 There are so many similarities between pharmacological effects of Baicalin and Octreotide, so it is feasible to be applied on SAP.29–31 In our experi-ment, we investigated the effects of Baicalin on inflammatory mediators and apoptosis of pancreatic acinar cells of SAP rats, and compared the effects of Octreotide and Baicalin.
Methods
1. Experimental animals
One hundred and eighty healthy male Sprague-Dawley rats (average weight 250-300g) were adopted in this study. All rats were housed in wire bottom cages and fed with standard rat chow and water. Environment adaptation was carried out before the experiment. All protocols received prior approval by the Laboratory of Animal Care and Use Ethics Committee of ZheJiang University, China.
2. Study design
The rats were anesthetized by intraperitoneal injection of 2% sodium pentobarbital (0.25ml/100g). First, the right external jugular vein transfusion passage was set up and the microinfusion pump was used for continuous transfusion (1ml/h). SAP rats were established by retrograde injection of 3.5% sodium taurocholate to the pancreatic duct through epidural catheter and duodenal papilla. 180 rats were prepared and randomly divided into four groups of I, II, III and IV (n = 45 in each group). Each group was also divided into subgroups of 3, 6 and 12h (n = 15). The pancreas and duodenum were flipped and finally abdomen closed in group I.
3. Chemicals and reagents used
Sodium taurocholate and sodium phentobarbital were obtained from Sigma Company (USA). Octreotide was obtained from Novartis pharmaceutical company (Swiss). 5% Baicalin injection was prepared by the first author (China national invention patent number ZL200310122673.6). Plasma endotoxin tachypleus amebocyte lysate kit was obtained from Shanghai Yihua Medical Science and Technology Corporation (China), and the calculation unit for content was EU/ml. The serum nitrogen monoxidum (NO) was obtained from Nanjing Jiancheng Bioengineering Research Institute, and the calculation units for content was μmol/L. The serum Endothelin-1 ELA kit (ET-1) was obtained from Cayman chemical company (Catalog Number: 583151, USA) and the calculation unit for content was ng/L (pg/ml). The Bax and Bcl-2 antibody were obtained from Santa Cruz Company (USA). The main reagents for DNA nick in situ endlabeling (TUNEL) staining was obtained from TaKaRa Biotechnology Co., Ltd (Japan).
4. Experimental protocol
Source of lab animals: the rats were obtained from the Experimental Animal Center of Medical School, Zhejiang University (China).
Handling of the animals: the rats were randomly divided into four groups, each 5 rats were placed in one cage. The temperature was set up between 22-24 °C by air conditioning and the light and dark cycles were 15h and 9h, respectively. The lab was clean, ventilate and dry.
Drug dosage: ten minutes after successful modeling, group III rats were injected with 5% Baicalin injection at a dose of 10mg/100g via an external jugular vein followed by continuous intravenous administration (10mg/ h/100g) by microinfusion pump. Group IV rats were first injected with Octreotide 0.2ug/100g via an external jugular vein followed by continuous intravenous transfusion by microinfusion pump at a transfusion speed of 0.2ug/h/100g. Group I and group II were injected with saline of equivalent volume at the corresponding time after operation.26–31
Methods of sample collection and processing: The mortality rates of each group at 3, 6, 12h were calculated, and the rats were anesthetized by sodium pentobarbital once again and second laparotomy were performed. The ascite volumes in abdominal cavity were measured and ascites/body weight ratio was calculated. Finally, the pancreatic tissues were fixed in 3.5% formalin for histological observation. The whole blood samples were obtained by heart puncture and then centrifugated at 2000rpm for 2 min. The supernatant was collected as serum and plasma for determination.
Principles and procedures for biochemical assays: all samples were assayed according to the reagent's explanation or introduction.
Histopathological methods: the pancreas tissues were fixed by 3.5% formaldehyde. The slides (4¶m) were made with paraffin embedding and the pancreatic tissue microarray were prepared according to the Kononen's method.32 The slides were stained by hematoxylin and eosin and slides were observed with a light microscope. Two pathologists in doubled-blind control condition performed the evaluation of pathological severity score using a modified Schmidt's score system.33 Bax and Bcl-2 proteins expression were detected on the pancreatic tissue microarray and the strepta-vidin-peroxidase (SP) method was adopted for immunochemistry staining. Bax and Bcl-2 protein expression were observed under light-microscope and the comprehensive assessment were carried out according to the positive cell percentage: positive cell count < 10% means (-); positive cell count 10-20% means (+); positive cell count 20-50% means (++); positive cell count > 50% means (+++). Apoptotic acinar cells were observed under light microscope and apoptotic index was calculated by TUNEL technology. The TUNEL staining was performed according to the instruction of manufacturer. Apoptotic indexes = apoptotic cell counts/ total cell counts×100%.
How was ascitis volume measured? How ascitis/bw ratio was determined: the dry gauze was used to wipe intraabdominal hydrops, then, a scale was used to weigh the weights of gauze before and after its soaking. The difference between weights (g) was converted into ascites volume (ml), and ascites/body weight ratio was thus obtained.
5. Data analysis
The values were presented as mean and standard deviation for normal distribution variables or median and quartile range for highly skewed variables. The significance of differences among the four groups was tested using the Kruskal–Wallis test for highly skewed data and variance analysis (ANOVA) was used for normal distribution data. Mutiple comparisons were subjected to Bonfferoni correction test. Correlations were tested using the Spearman rank correlation coefficients. P value less than 0.05 was considered statistically significant, and all statistical analyses were conducted using SPSS version 11.5 for windows.
Results
1. Mortality rate and ascites/body weight ratio
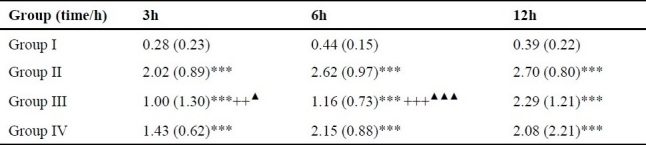
The mortality rates of group II were respectively 0% (0/15), 13.33% (2/15) and 33.33% (5/15) at 3, 6 and 12h, all the mortality rates of groups III and IV were 0% at different time points. The whole group I survived at different time points. The survival rates of groups III and IV at 12h were significantly higher than that in group II (P12h < 0.05). Comparison between groups III and IV showed no marked difference (P > 0.05). There was no death in group I at 3, 6, 12 h. The ascites volumes were significantly higher in group II and groups III and IV than those in group I, and group III were significantly lower than those in group II (P3, 6, 12h < 0.001). At 3 h, group IV were significantly lower than group II (P3h < 0.01), and group III was significantly lower than group IV (P3h < 0.01). At 6 h, group IV was significantly lower than group II (P6h < 0.001). There was no marked difference between group III and group IV at 12 h (P12 h > 0.05). See table 1.
Table 1.
Comparison of ascites/body weight ratio in each group (M(QR)).
2. Plasma endotoxin, serum ET-1 and NO level
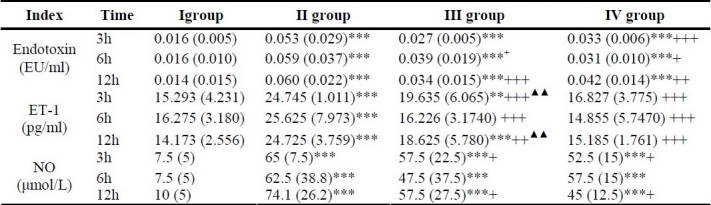
The concentration of plasma endotoxin in groups II, III and IV were significantly higher than that in group I (P3, 6, 12h < 0.001), there were no marked difference between groups III and IV at 6 and 12h (P6, 12h > 0.05). The endotoxin level in groups III and IV were significantly lower than that in group II (P3h < 0.001, P6,h < 0.05, P12h < 0.01).
The NO serum level in groups II, III and IV were significantly higher than that in group I at 3, 6, 12 h (P3, 6, 12h < 0.001). The NO level in group III was significantly lower than group II at 3h and 12h (P3, 12h < 0.05), and NO level in group IV was significantly lower than that in group II at 3, 6, 12h (P3, 6, 12h < 0.01), and there was no marked difference between groups III and IV (P3, 6, 12h > 0.05).
The ET-1 serum level in group II was significantly higher than that in group I at 3, 6 and 12h (P3,6,12h < 0.001), and significantly lower than that in group III and group IV (P3,6,12h < 0.001). In group IV, it was significantly lower than that in group III at 3h and 12h (P3,12h < 0.01). See table 2.
Table 2.
Comparison of different indexes level in blood (M(QR)).
3. Pathological severity score of pancreas
The pancreatic severity score in groups II, III and IV were significantly exceeded that in group I (P3,6,12h < 0.001). The pancreatic severity score of group III was significantly lower than that of group II at 12h (P12h < 0.01), and that of group IV was significantly lower than that in group II at 6 and 12h (P6,12h < 0.01). There was no marked difference of pancreatic severity score between groups III and IV at 3, 6 and 12 h (P3, 6, 12h > 0.05). See table 3.
Table 3.
Comparison between different pathological indexes in each group (M(QR)).

4. Bcl-2/Bax expression in pancreatic head and tail of each group
The positive staining station of Bax and Bcl-2 protein mostly located in the cytoplasm of pancreatic acinar cells, and partly in the cytoplasm of pancreatic islet cells. There was no marked difference of Bax protein positive staining rate between group II and group I at 3, 6, 12 h in pancreatic head and tail (P3,6, 12h > 0.05). The Bax protein positive rate of group III was significantly higher than those in group II, group I at 3, 6h in pancreatic head (P3,6h < 0.01) and group II at 3h in pancreatic tail (P3h < 0.001). The Bax positive staining rate of group IV was significantly higher than group II at 6h in pan creatic head (P6h < 0.01) and tail (P6h < 0.05). The Bax positive staining rate of group IV was significantly lower than that in group III at 3h in pancreatic head (P3h < 0.01) and higher than that in group III at 3, 12h in pancreatic tail (P3,12h < 0.05).
There was no marked difference of Bcl-2 positive staining rate between group IV and group I at each time points (P3, 6, 12h > 0.05). The positive rates of Bcl-2 in II group and group III were higher than group I and group IV in pancreatic head (P3,6,12h <0.05). The Bcl-2 positive staining rate in the group III was higher than group II at 6, 12h in pancreatic head (P6,12h < 0.01) and lower than group II at 6 h in pancreatic tail (P6h < 0.001). The Bcl-2 protein positive rate of group III in pancreatic tail was significantly higher than groups I and IV (P3, 6, 12h < 0.01). See table 3 (Figure 1, 2).
Figure 1.
Group I at 6h (negative expression) TUNEL×400.
Figure 2.
Group II at 12h (negative expression) TUNEL×400
5. Apoptotic indexes in pancreas head and tail of each group
Most apoptotic cells were observed on pancreatic acinar cells and some lymphocytes in pancreatic tissue. There was no marked difference between group II and group I at each time points (P3,6,12h > 0.05). There was no marked difference among each groups at 12h (P12h > 0.05). Apoptotic index in group III was significantly higher than group I (P3, 6, 12h < 0.01) and II group at 3, 6 h (P3, 6h < 0.05). Apoptotic index in group IV was significantly higher than group I at pancreatic head and group II at 6h (P6h < 0.05). There was no marked difference between groups III and IV in pancreatic tail at 3, 6, 12h (P3,6,12h < 0.05). But the apoptotic index at 12h of pancreatic head and tail showed no marked difference among groups II, III and IV (P12h > 0.05). See table 3 (Figure 1, 2, 3).
Figure 3.
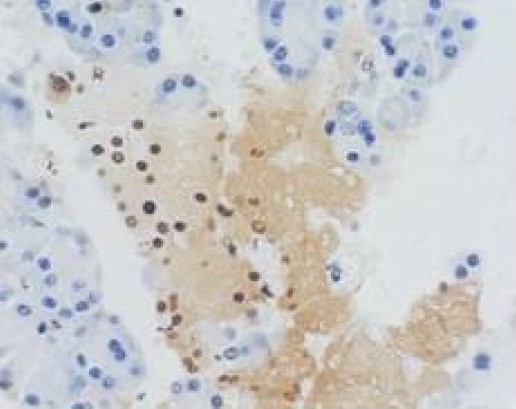
Group IV at 6h (acinar epithelial cell apoptosis) TUNEL×400
Figure 4.
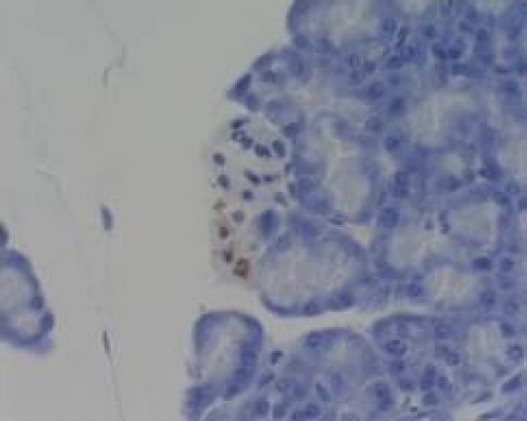
Group III at 3h (acinar epithelial cell apoptosis) TUNEL×400
Figure 5.
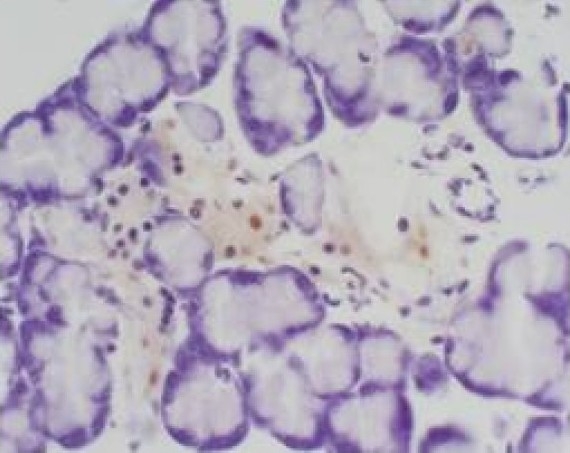
Group III at 6h (acinar epithelial cell apoptosis) TUNEL×400
6. Correlation analysis results
At 6 and 12h after operation in group III, a positive correlation between the apoptotic indexes and Bax in pancreas head was noted (P < 0.01). At 3h after operation in group III, a positive correlation between the apoptotic indexes and Bax in pancreas tail was seen (P < 0.05).
Discussion
Many studies have proved that inflammatory mediators and pancreatic acinar cells apoptosis are involved in the process of acute pancreatitis, and overwhelming inflammatory mediators releasing is closely correlated with the development of AP from mild type to severe type, and even leads to remote organ failure and multiple organ dysfunction syndrome (MODS).1,2 But on the contrary, it has also been demonstrated that pancreatic acinar cells apoptosis is an important self-protection mechanism of acute pancreatitis and it can alleviate the severity of pancreas via limiting the deterioration of pancreatic acinar cells.34 Octreotide is widely used in the clinical comprehensive treatment of SAP, and its effective impacts on regulating the inflammatory mediators releasing and inducing the pancreatic acinar cells apoptosis in experimental SAP rats have been proved.35
In our experiment, it was shown that the concentration of ET-1 in serum of III and IV groups were significantly lower than that in II group, and there was no marked difference between Baicalin and Octreotide on the impacts on these inflammatory mediators. The endotoxin concentration in SAP rats was significantly decreased by the Baicalin or Octreotide treatments. The correlation analysis indicated that pancreatic severity scores, ascites/body weight ratio and survival rate were positively correlated with decrease of inflammatory mediators and endotoxin in the Baicalin and IV group. So we think that downregulating the inflammatory mediator release and eliminating the endotoxin are two important phamcological mechanism of Baicalin or Octreotide treatments in SAP. Serum NO concentration was dropped significantly after the treatment of Baicalin or Octreotide, and there was a negative correlation between the pancreatic severity score and NO concentration, so we believe that Baicalin or Octreotide can downregulate the serum NO concentration to alleviate the severity of pancreas in SAP.
Apoptosis is an active and genetically controlled process that removes unwanted or damaged cells, and it is often synonymously used with the term of programmed cell death (PCD). The recent observation in the experimental acute pancreatitis demonstrated that the severity of pancreatitis was inversely related to the extent of apoptosis, and suggested that pancreatic acinar cells apoptosis could protect against pancreatic injury in pancreatitis.36 Many of the genetic players orchestrating the phenomenon of apoptosis have been identified in SAP, and the Bcl-2 family genes have been recognized as participating in the phenomenon of apoptosis in AP. Bcl-2 gene can enhance the pancreas autodigestion and leads to necrosis by inhibiting pancreatic acinar cells apoptosis. But the encode protein of Bax gene, another member of Bcl-2 family, is a promoter to accelerate the pancreatic acinar cells apoptosis. Bax and Bcl-2 are two important components of apoptosis regulating system. When Bax forms dimer, it will induce apoptosis. As Bcl-2 expression increases, the apoptosis promoting effect of Bax dimmers will be inhibited. After analysing the expression of Bax/Bcl-2 protein and apoptosis index in pancreatic head and tail of SAP rats, we found that the apoptosis of the pancreatic acinar cells were promoted effectively in Baicalin and IV group while the pancreatic severity scores were dropped. So we believe that promoting the pancreatic acinar cells apoptosis is also one of the important protective mechanism of Baicalin or Octreotide treatments in SAP rats.
We theoretically believe that Baicalin is a promising monomer of traditional Chinese medicine in the treatment of SAP, and it is possible for Baicalin to be an option for clinical treatment of SAP. In addition, in our experiment, we adopted pancreatic tissue microarray technique and because of its high throughput, multiple samples, economical and time saving and effective error control characters,37,38 we greatly reduced the study cost and improved the efficiency of pathohistological study, and could combine many biological detective techniques at the same tissue microarray. We recommend this advanced technique to be used in great samples study of SAP or other experiments.
Conclusions
Baicalin can improve the prognosis of SAP rats and it is maybe a promising new drug of traditional Chinese medicine in the treatment of SAP. Compared with Octreotide in the treatment of SAP, the protective mechanisms of Baicalin include reducing the excessive inflammatory mediators release, inducing the pancreatic acinar cells apoptosis and regulating apoptotic related proteins Bax/Bcl-2 expression. The application of pancreatic tissue microarrays in the study of SAP is economical and efficient, and it is worthy to be further popularized.
Authors’ Contributions
ZXP carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript.
TH and CL provide assistance in the design of the study, coordinated and carried out all the experiments and participated in manuscript preparation.
CHQ, YBY and MJ provide assistance for preparing the manuscript and analyzing dates.
All authors have read and approved the content of the manuscript.
Footnotes
Conflict of interest: Authors have no conflict of interest.
References
- 1.Shi C, Andersson R, Zhao X, Wang X. Potential role of reactive oxygen species in pancreatitis associated multiple organ dysfunction. Pancreatology. 2005;5(4-5):492–500. doi: 10.1159/000087063. [DOI] [PubMed] [Google Scholar]
- 2.Koussoulas V, Tzivras M, Karagianni V, Spyridaki E, Plachouras D, Giamarellou H, et al. Monocytes in systematic inflammatory response syndrome: differences between sepsis and acute pancreatitis. World J Gastroenterol. 2006;12(41):6711–4. doi: 10.3748/wjg.v12.i41.6711. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Zidan FM, Windsor JA. Lexipafant and acute pancreatitis: a critical appraisal of the clinical trials. Eur J Surg. 2002;168(4):215–9. doi: 10.1080/11024150260102816. [DOI] [PubMed] [Google Scholar]
- 4.Yousaf M, McCallion K, Diamond T. Management of severe acute pancreatitis. Br J Surg. 2003;90(4):407–20. doi: 10.1002/bjs.4179. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y, Liu Y, Jia L, Jiang H, Ji M, Lv N, et al. Severe acute pancreatitis in China: etiology and mortality in 1976 patients. Pancreas. 2007;35(3):232–7. doi: 10.1097/MPA.0b013e3180654d20. [DOI] [PubMed] [Google Scholar]
- 6.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7(3):167–202. [PubMed] [Google Scholar]
- 7.Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110(2):639–42. doi: 10.1053/gast.1996.v110.agast960639. [DOI] [PubMed] [Google Scholar]
- 8.Exley AR, Leese T, Holliday MP, Swann RA, Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992;33(8):1126–8. doi: 10.1136/gut.33.8.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaccaro MI, Calvo EL, Suburo AM, Sordelli DO, Lanosa G, Iovanna JL. Lipopolysaccharide directly affects pancreatic acinar cells: implications on acute pancreatitis pathophysiology. Dig Dis Sci. 2000;45(5):915–26. doi: 10.1023/a:1005521007609. [DOI] [PubMed] [Google Scholar]
- 10.Wig JD, Kochhar R, Ray JD, Krishna Rao DV, Gupta NM, Ganguly NK. Endotoxemia predicts outcome in acute pancreatitis. J Clin Gastroenterol. 1998;26(2):121–4. doi: 10.1097/00004836-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25(4-5):434–56. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen CC, Wang SS, Lee FY, Chang FY, Lee SD. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am J Gastroenterol. 1999;94(1):213–8. doi: 10.1111/j.1572-0241.1999.00709.x. [DOI] [PubMed] [Google Scholar]
- 13.Planas M, Perez A, Iglesia R, Porta I, Masclans JR, Bermejo B. Severe acute pancreatitis: treatment with somatostatin. Intensive Care Med. 1998;24(1):37–9. doi: 10.1007/s001340050512. [DOI] [PubMed] [Google Scholar]
- 14.Shor NA, Levina VP, Ioffe IV, Andreeva IV, Chumak I, Zhadanov VI, et al. Application of octreotide in patients with acute pancreatitis. Klin Khir. 2004;(2):15–17. [PubMed] [Google Scholar]
- 15.Wenger FA, Kilian M, Heukamp I, Foitzik T, Jacobi CA, Guski H, et al. Effects of octreotide in acute hemorrhagic necrotizing pancreatitis in rats. J Gastroenterol Hepatol. 2007;22(11):1872–76. doi: 10.1111/j.1440-1746.2006.04627.x. [DOI] [PubMed] [Google Scholar]
- 16.Salem MZ, Cunha JE, Coelho AM, Sampietri SN, Machado MC, Penteado S, et al. Effects of octreotide pretreatment in experimental acute pancreatitis. Pancreatology. 2003;3(2):164–8. doi: 10.1159/000070086. [DOI] [PubMed] [Google Scholar]
- 17.Yeh DC, Wu CC, Liu TJ, P’eng FK. Management of acute pancreatitis: results of a 15-year experience in Taiwan. J Hepatobiliary Pancreat Surg. 2001;8(3):204–10. doi: 10.1007/s005340170017. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Y, Gong Z, Lou K, Tu S, Di Z, Xu J. Effects and mechanisms of somatostatin analogs on apoptosis of pancreatic acinar cells in acute pancreatitis in mice. J Gastroenterol Hepatol. 2001;16(6):683–8. doi: 10.1046/j.1440-1746.2001.02499.x. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Li YY, Li SG, Song BG, Zhao GF. Effect of Qingyitang on activity of intracellular Ca2+-Mg2+-ATPase in rats with acute pancreatitis. World J Gastroenterol. 2004;10(1):100–4. doi: 10.3748/wjg.v10.i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo KJ, Lim JH, Suh SI, Kwon YK, Shin SW, Kim SC, et al. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA-binding activity. Immunobiology. 2006;211(5):359–68. doi: 10.1016/j.imbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang GF, Wu ZF, Wan L, Wang QT, Chen FM. Influence of baicalin on the expression of receptor activator of nuclear factor-kappaB ligand in cultured human periodontal ligament cells. Pharmacology. 2006;77(2):71–7. doi: 10.1159/000092853. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Li H, Gao Z, Gong Y, Xu H. Effects of flavonoids extracted from Scutellaria baicalensis Georgi on heminnitrite-H2O2 induced liver injury. Eur J Pharmacol. 2006;536(1-2):192–9. doi: 10.1016/j.ejphar.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 23.Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472(3):643–50. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XP, Tian H, Cheng QH. The current situation in pharmacological study on baicalin. CHINESE PHARMA-COLOGICAL BULLETIN. 2003;19(11):1212–15. [Google Scholar]
- 25.Liu IX, Durham DG, Richards RM. Baicalin synergy with betalactam antibiotics against methicillin resistant Staphylococcus aureus and other betalactam-resistant strains of S. aureus. J Pharm Pharmacol. 2000;52(3):361–6. doi: 10.1211/0022357001773922. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XP, Zhang L, He JX, Zhang RP, Cheng QH, Zhou YF, et al. Experimental study of therapeutic efficacy of Baicalin in rats with severe acute pancreatitis. World J Gastroenterol. 2007;13(5):717–24. doi: 10.3748/wjg.v13.i5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiping Z, Jie Z, Qin X, Guanghua F, Yang C, Tongfa J, et al. Influence of Baicalin and Octerotide on NF-kappa B and P-selectin expression in liver and kidney of rats with severe acute pancreatitis. Inflammation. 2009;32(1):1–11. doi: 10.1007/s10753-008-9096-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XP, Zhang J, Ren Z, Feng GH, Zhu W, Cai Y, et al. study on protecting effects of Baicalin and Octreotide on hepatic injury in rats with severe acute pancreatitis. World J Gastroenterol. 2008;14:6551–9. doi: 10.3748/wjg.14.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XP, Zhang L, Yang P, Zhang RP, Cheng QH. Protective effects of baicalin and octreotide on multiple organ injury in severe acute pancreatitis. Dig Dis Sci. 2008;53(2):581–91. doi: 10.1007/s10620-007-9868-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XP, Tian H, Lai YH, Chen L, Zhang L, Cheng QH, et al. Protective effects and mechanisms of Baicalin and octreotide on renal injury of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13(38):5079–89. doi: 10.3748/wjg.v13.i38.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiping Z, Hua T, Hanqing C, Li C, Zhiwei W, Keyi W, et al. The protecting effects and mechanisms of Baicalin and Octreotide on heart injury in rats with SAP. Mediators Inflamm. 2007;2007:19469. doi: 10.1155/2007/19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XP, Zhang L, He JX, Zhang RP, Cheng QH, Zhou YF, et al. Experimental study of therapeutic efficacy of Baicalin in rats with severe acute pancreatitis. World J Gastroenterol. 2007;13(5):717–24. doi: 10.3748/wjg.v13.i5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser AM, Saluja AK, Lu L, Yamanaka K, Yamaguchi Y, Steer ML. Effects of cycloheximide on pancreatic endonuclease activity, apoptosis, and severity of acute pancreatitis. Am J Physiol. 1996;271(3 Pt 1):C982–C93. doi: 10.1152/ajpcell.1996.271.3.C982. [DOI] [PubMed] [Google Scholar]
- 35.Uhl W, Anghelacopoulos SE, Friess H, Buchler MW. The role of octreotide and somatostatin in acute and chronic pancreatitis. Digestion. 1999;60(2):23–31. doi: 10.1159/000051477. [DOI] [PubMed] [Google Scholar]
- 36.Saluja A, Hofbauer B, Yamaguchi Y, Yamanaka K, Steer M. Induction of apoptosis reduces the severity of caerulein-induced pancreatitis in mice. Biochem Biophys Res Commun. 1996;220(3):875–8. doi: 10.1006/bbrc.1996.0498. [DOI] [PubMed] [Google Scholar]
- 37.Moch H, Kononen T, Kallioniemi OP, Sauter G. Tissue microarrays: what will they bring to molecular and anatomic pathology? Adv Anat Pathol. 2001;8(1):14–20. doi: 10.1097/00125480-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Richter J, Wagner U, Kononen J, Fijan A, Bruderer J, Schmid U, et al. High-throughput tissue microarray analysis of cyclin E gene amplification and overexpression in urinary bladder cancer. Am J Pathol. 2000;157(3):787–94. doi: 10.1016/s0002-9440(10)64592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


