Abstract
BACKGROUND:
Recently, it is proposed that oxidant-antioxidant imbalance may have a role in the pathophysiology of schizophrenia. The present study was performed to assess differences in plasma levels of nitric oxide (as oxidant), caeruloplasmin (secondary antioxidant), and antioxidant trace metals (Zn, Se, Mn, Cu and Fe) in patients with schizophrenia compared with healthy controls. Our secondary aim was to further evaluate the impact of psychopharmacologic treatment on these parameters.
METHODS:
Plasma levels of nitric oxides (NO), caeruloplasmin, zinc (Zn), selenium (Se), manganese (Mn), copper (Cu) and iron (Fe) in patients with schizophrenia before (n = 15) and after antipsychotic drug treatment (n = 20) were compared with those of healthy controls (n = 20). Convenient sampling method was used for the selection of subjects. NO was estimated by the use of Griess method, caeruloplasmin was estimated by the use of immunodiffusion method and antioxidant trace metals was estimated by the use of atomic absorption spectrophotometer.
RESULTS:
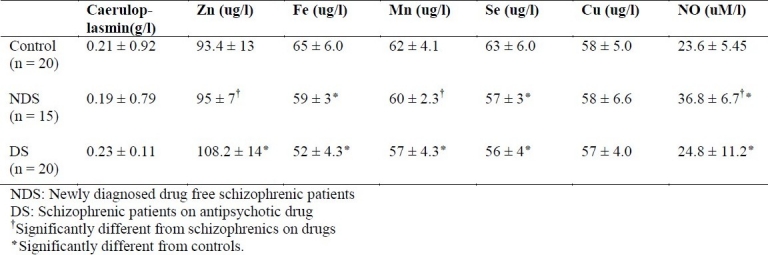
The levels of Cu and caeruloplasmin were not significantly different while Fe and Se were significantly reduced in both groups of schizophrenic patients compared with the controls. Zn was significantly elevated in medicated schizophrenics compared with drug-free patients or controls. NO was significantly elevated in drug free patients with schizophrenia compared with controls or treated patients.
CONCLUSIONS:
Our findings suggest the application of management strategies that will reduce NO but will increase antioxidant trace metals in patients with schizophrenia.
Keywords: Schizophrenia, antioxidant defense system, antioxidant status, oxidative Stress
Schizophrenia is a disabling brain disorder and the condition is one of the major contributors to the global burden of brain diseases.1 Free radicals and reactive oxygen species play a number of significant and diverse roles in neurodegenerative diseases, including schizophrenia.2 The brain and the nervous system are particularly prone to free radical damage.3 As the membrane lipids are very rich in polyunsaturated fatty acids and areas of the human brain are very rich in iron, these factors play an essential role in generating free radical species. The ability of a tissue or fluid to buffer the effects of reactive oxygen species is called total antioxidant capacity.4 Blood contains many antioxidant molecules that prevent and/or inhibit harmful free radical reactions.4
Karson et al5 showed that the nitric oxide synthase (NOS) concentration is increased in the cerebellar vermis of postmortem brains of those who had schizophrenia. By means of ex vivo experiments, Das et al6 also showed NOS activity to be significantly elevated in the platelets of drug-naïve patients with schizophrenia compared with controls, drugtreated patients with schizophrenia and patients with panic disorder. Herken et al7 reported a remarkable increase in nitrite plus nitrate levels in red blood cells of patients with schizophrenia compared with control subjects. This finding suggests that there is an excess NO production in the brains of individuals with schizo-phrenia. Excess NO is neurotoxic owing to the formation of peroxinitrite,8 which is formed from the reaction of NO with superoxide. Previous results indicated reduced levels of blood antioxidants such as albumin, uric acid and bilirubin in patients with schizophrenia.9 Low levels of total antioxidants capacity are also reported low in patients with schizophrenia.10 The potential toxicity of free radicals (such as excessive NO) is counteracted by a number of cytoprotective enzymes and antioxidants that limit the damage.11 This protective mechanism functions cooperatively in the form of a cascade in which various cytoprotective enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSHPx) and nutrients, as well as endogenous antioxidants act in combination.12 Nutritionally essential trace metals (Zn, Se, Mn, Cu and Fe) are either integral parts or catalysts to these cytoprotective enzymes.12
Given these lines of evidence, we hypothesized excess NO and reduced antioxidant status in the plasma of patients with schizophrenia. To support this working hypothesis, we determined the plasma levels of nitric oxides, caeruloplasmin, Zn, Se, Mn, Cu and Fe in schizophrenic patients and healthy controls.
Methods
Ethical Approval was obtained from Uselu Psychiatric Hospital's Management Ethical Committee before the commencement of the study and informed consent was obtained from all subjects, guardians and families of the subjects as circumstances demanded. A total of thirtyfive patients suffering from schizophrenia (20 males and 15 females between ages of 18 and 50 years) were recruited from Uselu Psychiatric Hospital, Nigeria. The patients with schizophrenia were divided into two groups consisting of 20 on chlorpromazine antipsychotic drugs for at least 12 weeks, and 15 newly diagnosed and not taking antipsychotic drugs. The patients were diagnosed by a Consultant Psychiatrist according to axis 1 of DSM –IV (the fourth edition of the diagnostic and statistical manual of mental disorders) criteria.13 Psychological evaluation of each subject was done using Positive and Negative Syndrome Scale (PANSS). None of the patients had significant psychiatric or somatic comorbidity.
Twenty healthy volunteers (12 males and 8 females) who were age and sex matched with the patients served as controls for this study. The control group had no previous individual or family history of any psychiatric disorders. A history was obtained and a clinical psychiatric examination was performed. The history included information regarding age, approximate duration of illness, length of medication, type of medication, family medical history of schizophrenia, economic status, level of education, location of abode (rural or urban dweller). All patients were evaluated clinically (history and clinical examination), searching for signs of immunological changes, e.g. recurrent viral infection and searching for any diseases that can affect immunity, e.g. sore throat, bronchitis, liver diseases, thyroid enlargement etc.
The following laboratory investigations were carried out:
Complete blood count to exclude anaemia, leucopenia, leucocytosis, eosinophilia or any other abnormal figures in blood count.
Thyroid function tests to exclude increased T3 and T4 serum levels or to exclude patients with low serum T3 and T4 levels.
Renal function tests (blood urea and serum creatinine) to exclude renal impairment.
Liver function tests to exclude liver affection, especially those with high liver enzymes or those with diminished albumin levels or high globulin levels.
Urine and stool analysis to exclude urinary tract infection or parasitic infestations. Other exclusion criteria were subjects with suicide attempt in past years, serious medical conditions, severe head injury, seizure disorder, rheumatic fever, rheumatoid arthritis, subjects who received oral contraceptives, nonsteroidal antiinflammatory drugs, corticosteroids, anticonvulsants and antidepressants.
About 5ml of venous blood was collected from each subject into a bottle containing lithium heparin. The plasma was separated by centrifugation at 5000rpm for 20 minutes. Caeruloplasmin was quantified by the single radial immunodiffusion method as previously described.14 It is based on the principle of anti-gen-antibody precipitation reaction in agarose gel. Nitric oxide was estimated using Griess method15 while the plasma essential minerals were analysed using atomic absorption spectrophotometer.16
Statistical analysis: The data were presented as mean ± SDs and were analysed with 1-way analysis (ANOVA). Post-hoc analysis was performed with Wilcoxon-Rank sum test.
Results
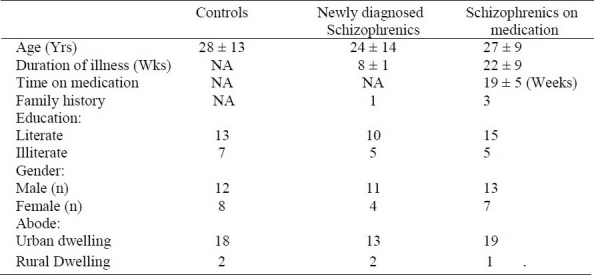
The demographic characteristics of patients and controls are presented in Table 1. The ages of all subjects were similar. There were more males, urban dwellers, and literate subjects in the studied population. The duration of illness was significantly different between schizophrenic patients on medication compared with newly diagnosed patients.
Table 1.
Demographic profile of the subjects.
As shown in Table 2, the levels of Cu and caeruloplasmin were not significantly different while Fe and Se were significantly reduced in patients with schizophrenia compared with the controls. Zn was significantly elevated in patients with schizophrenia compared with drug-free patients or control. NO was significantly raised in drug free schizophrenic patients compared with controls or treated patients.
Table 2.
The levels (mean ± SD) of non-enzymatic antioxidants and nitric oxide in newly diagnosed drug free schizophrenic patients (NDS), schizophrenic patients on anti-psychotic drug (DS) and control.
Discussion
As evidence supporting the relation between NO and central nervous system/function continues to accumulate,5 relation between plasma NO and schizophrenia could also be conjec-tured. We found significant increase in plasma NO levels in drug free schizophrenic patients compared with controls or schizophrenics on antipsychotic medication. This supports previous studies that suggest NO production is increased in brains of individuals with schizophrenia.5 Glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) are antioxidant enzymes which protects against the potential toxicity of free radicals. In conditions of increased production of free radicals, an imbalance towards a prooxidant is formed, which is fundamentally important in the pathogenesis of schizophrenia.2,17,18 Decreased GSH-Px, decreased SOD activity and increased lipid peroxidation products in RBC of drug free patients with schizophrenia was reported.17 Glen et al18 also suggested that drug free patients with schizophrenia have high level of long chain unsaturated fatty acids in RBC membranes. High lipid peroxidation of poly unsaturated fatty acid and GSH-Px might result in accumulation of H2O2 and other per-oxides, thus explaining elevated level of NO in drug free patients with schizophrenia. It was earlier reported that accumulated NO may comprises ATP synthesis and organelle's ability to sequester excess cellular Ca2+.19 These could contribute to neuronal death.
CuZnSOD is utilized for neutralising super-oxide ions to H2O2 and O2. Se dependent-GSH-Px and Fedependent catalase reduces H2O2 to H2O and oxygen. Thus reduction of Fe,Mn, Cu and Se in patients with schizophrenia may lead to disturbed functions of antioxidant enzymes. The disturbance of the oxidant-antioxidant equilibrium owing to abnormal activity of SOD and GSHPx was reported.20,21 Pavlovic et al22 showed depleted antioxidant status due to increased utilization with increasing oxidative stress in patients with schizo-phrenia. Schizophrenia has also been shown to disturb the glutathione homeostasis, which is one of the factors responsible for weakening the antioxidant defense provided by enzymatic antioxidants.
Selenium is an important constituent of glutathione peroxidase enzyme. Also, manganese, copper, and zinc are important components of super oxide dismutase (SOD) and iron in catalase.23 Due to high lipid peroxidation and oxidative stress as shown by raised NO in patients with schizophrenia, deficiencies of these nutritionally essential metals are expected in patients with schizophrenia. As shown in the present study Fe, Mn and Se were reduced while Zn was elevated. Depleted antioxidant status due to increased utilization with increasing oxidative stress has been reported.20,21,23–25 However, few studies have advocated strengthening of antioxidant status in schizophrenia so as to counteract oxidative stress.
Fe was significantly decreased in newly diagnosed drug-free patients with schizophrenia compared with controls. Fe level has been shown to be deposited in the brain of patients with schizophrenia, this may account for our observed low plasma iron in patients with schizophrenia. Since low circulating Fe has been found to alter dopaminergic pathway21, low plasma Fe found in our patients with schizophrenia might have predispose them to the development of schizophrenia.
Se level was significantly reduced in patients with schizophrenia compared with control. Se deficiency has been reported in untreated patients with schizophrenia.21,24 Se is an important component of GSH-Px. Se deficiency is associated with altered GSH-Px enzyme and consequently altered glutathione redox state.15 Therefore reduced selenium in patients with schizophrenia is one of the causes of oxidative stress in these patients. Also, a low soil Se level has been associated with increased incidence of schizophrenia.24 Soils in most of the urban areas have low level of Se due to increased pollution which increases soil acidity. This may be responsible for higher prevalence of schizophrenia in urban areas. Moreover, modern stressful living contributes to free radical generation25 and may also lead to schizophrenia.
Our result showed that Zn was significantly increased in schizophrenic patients on medication. Zn is important in the normal functioning of Zn-dependent CuZnSOD.23 The consequence of raised Zn in patients with schizophrenia on treatment is reduction of oxidative stress. It is known that Zn and Cu are antagonists both competing for sites on metallothionein.26 Therefore, uncontrolled elevation of Zn level in patients with schizophrenia may lead to further reduction in Cu level. The implication of further reduction in Cu level may be detrimental in patients with schizophrenia since Cu increases the activity of tyrosinase and beta-hydroxylase enzymes which are involved in dopaminergic activity.
Mn was observed to be significantly decreased with medications. It has been reported that antipsychotic drugs chelate body manganese,26 thus explaining the decrease in Mn level in patients with schizophrenia on antipsychotic medications as reported in the present study. Mn is necessary for brain function and component of SOD (an antioxidant enzyme). Reduced level of Mn may be due to low antioxidant capacity of MnSOD resulting in increased oxidative stress or NO as found in this study.
Schizophrenia is a severe psychiatric disorder whose etiology still remains elusive. Converging evidences indicate that oxidative mechanisms may play a role in schizophrenia.2 Plasma free radicals were found to be elevated while SOD, GSH-Px, albumin, uric acid and bilirubin were reported to be reduced in patients with schizophrenia.9,10 However, supplementation of antioxidant vitamin C with atypical antipsychotics was shown to reduce oxidative stress and improves the outcome of schizophrenia.28 Trace elements play a vital role in brain development and/or functions. Prenatal and neonatal exposure to toxic metal (arsenic, lead, cadmium) exposure had been linked with the development of schizophrenia.23 Deficiency of selenium and iron had been shown to play a role in the aetiopathogenesis of schizophrenia.23
The major limitation of the present study is the decline of consent by most patients, thus reducing the sample size. Thus, further studies on larger number of patients, subgroup analysis according to age, symptom and duration of illness should be carried out.
Conclusion
Our results showed that NO was elevated in untreated patients with schizophrenia but it was normal in treated patients and had an inconsistent relationship with trace metals. This may suggest the potential role of antioxidant trace metals in the therapeutic strategy and their implication in preventive approaches of populations at risk for schizophrenia.
Authors’ Contributions
AOG designed, coordinated and supervised the study, and also prepared the manuscript.
IOB participated in patient's selection and carried out all the experiments.
Both authors have read and approved the contents of the manuscript.
Acknowledgments
The authors are grateful to Mr Oyewumi Titiloye, Department Of Chemical Pathology, University of Ibadan, Ibadan, Nigeria for his technical assistance, Dr Ihenyen O (Chief Medical Director) and all Consultants, Uselu Psychiatric Hospital, Benin, Nigeria for permission and selection of the patients.
Footnotes
Conflict of interest: Authors have no conflicts of interest.
References
- 1.McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akyol O, Zoroglu SS, Armutcu F, Sahin S, Gurel A. Nitric oxide as a physiopathological factor in neuropsychiatric disorders. In Vivo. 2004;18(3):377–90. [PubMed] [Google Scholar]
- 3.Smythies J. Recent advances in the neurobiology of schizophrenia. German J Psychiatry. 1998;1(2):24–40. [Google Scholar]
- 4.Lorrain DS, Hull EM. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport. 1993;5(1):87–9. doi: 10.1097/00001756-199310000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Karson CN, Griffin WS, Mrak RE, Husain M, Dawson TM, Snyder SH, et al. Nitric oxide synthase (NOS) in schizophrenia: increases in cerebellar vermis. Mol Chem Neuropathol. 1996;27(3):275–84. doi: 10.1007/BF02815109. [DOI] [PubMed] [Google Scholar]
- 6.Das I, Khan NS, Puri BK, Sooranna SR, de Belleroche J, Hirsch SR. Elevated platelet calcium mobilization and nitric oxide synthase activity may reflect abnormalities in schizophrenic brain. Biochem Biophys Res Commun. 1995;212(2):375–80. doi: 10.1006/bbrc.1995.1980. [DOI] [PubMed] [Google Scholar]
- 7.Herken H, Uz E, Ozyurt H, Akyol O. Red blood cell nitric oxide levels in patients with schizophrenia. Schizophr Res. 2001;52(3):289–90. doi: 10.1016/s0920-9964(00)00169-9. [DOI] [PubMed] [Google Scholar]
- 8.Nakaki T, Mishima A, Fujii T, Suzuki E, Shintani F. Nitric oxide and neurodegenerative diseases. Pharmacol Toxi-col. 1997;3:157–63. [Google Scholar]
- 9.Yao JK, Reddy R, van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998;80(1):29–39. doi: 10.1016/s0165-1781(98)00051-1. [DOI] [PubMed] [Google Scholar]
- 10.Yao JK, Reddy R, McElhinny LG, van Kammen DP. Reduced status of plasma total antioxidant capacity in schizo-phrenia. Schizophr Res. 1998;32(1):1–8. doi: 10.1016/s0920-9964(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 11.Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophr Res. 1996;19(1):1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 12.Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med. 1991;91(3C):2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 13.Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4th ed. New York: American Psychiatric Publishing Inc; 2000. American Psychiatric Association. [Google Scholar]
- 14.Arinola G, Arowojolu A, Bamgboye A, Akinwale A, Adeniyi A. Serum concentrations of immunoglobulins and acute phase proteins in Nigerian women with preeclampsia. Reprod Biol. 2006;6(3):265–74. [PubMed] [Google Scholar]
- 15.Laudanska E, Gwozdz A, Brudel G, Rajchert J. [Evaluation of Griess reagent and TTC tests in obstetrics] Ginekol Pol. 1970;41(8):857–62. [PubMed] [Google Scholar]
- 16.Kaneko JJ. 4 Sub ed. New York: Academic Pr; 1989. Clinical biochemistry of domestic animals; p. 932. [Google Scholar]
- 17.Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Srimal RC, et al. Nitrite content and antioxidant enzyme levels in the blood of schizophrenia patients. Psychopharmacology (Berl) 2001;158(2):140–5. doi: 10.1007/s002130100860. [DOI] [PubMed] [Google Scholar]
- 18.Glen AI, Glen EM, Horrobin DF, Vaddadi KS, Spellman M, Morse-Fisher N, et al. A red cell membrane abnormality in a subgroup of schizophrenic patients: evidence for two diseases. Schizophr Res. 1994;12(1):53–61. doi: 10.1016/0920-9964(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 19.Strunecka A, Ripova D. What can the investigation of phosphoinositide signaling system in platelets of schizophrenic patients tell us? Prostaglandins Leukot Essent Fatty Acids. 1999;61(1):1–5. doi: 10.1054/plef.1999.0063. [DOI] [PubMed] [Google Scholar]
- 20.Weiser M, Levkowitch Y, Neuman M, Yehuda S. Decrease of serum iron in acutely psychotic schizophrenic patients. Int J Neurosci. 1994;78(1,2):49–52. doi: 10.3109/00207459408986045. [DOI] [PubMed] [Google Scholar]
- 21.Yanik M, Kocyigit A, Tutkun H, Vural H, Herken H. Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol Trace Elem Res. 2004;98(2):109–17. doi: 10.1385/BTER:98:2:109. [DOI] [PubMed] [Google Scholar]
- 22.Pavlovic D, Tamburic V, Stojanovic I, Kocic G, Jevtovic T, Dordevic V. Oxidative stress as marker of positive symptoms in schizophrenia. Medicine and Biology. 2002;9(2):157–61. [Google Scholar]
- 23.Johnson S. Micronutrient accumulation and depletion in schizophrenia, epilepsy, autism and Parkinson's disease? Med Hypotheses. 2001;56(5):641–5. doi: 10.1054/mehy.2000.1302. [DOI] [PubMed] [Google Scholar]
- 24.Brown JS., Jr Role of selenium and other trace elements in the geography of schizophrenia. Schizophr Bull. 1994;20(2):387–98. doi: 10.1093/schbul/20.2.387. [DOI] [PubMed] [Google Scholar]
- 25.Dadheech G, Mishra S, Gautam SH, Sharma P. Evaluation of antioxidant deficit in schizophrenia. Indian J Psychiatry. 2008;50(1):16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki E, Nakaki T, Nakamura M, Miyaoka H. Plasma nitrate levels in deficit versus non-deficit forms of schizophrenia. J Psychiatry Neurosci. 2003;28(4):288–92. [PMC free article] [PubMed] [Google Scholar]
- 27.Davies IJT. Springfield, Illinois: C.C.Thomas; 1972. The clinical significance of the essential biological metals; p. 74. [Google Scholar]
- 28.Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl) 2005;182(4):494–8. doi: 10.1007/s00213-005-0117-1. [DOI] [PubMed] [Google Scholar]


