Abstract
Abstract In this study, we have employed whole-mount, in situ hybridization to study the spatial pattern of hsc70 and hsp70 mRNA accumulation in normal and heat shocked embryos during Xenopus laevis development. Our findings revealed that hsc70 mRNA was constitutively present in a global fashion throughout the embryo and was not heat inducible. Accumulation of hsp70 mRNA, however, was detected only in heat shocked embryos. Furthermore, hsp70 mRNA accumulation was enriched in a tissue-specific manner in X laevis tailbud embryos within 15 minutes of a 33°C heat shock. Abundant levels of heat shock–induced hsp70 mRNA were detected in the head region, including the lens placode, the cement gland, and in the somitic region and proctodeum. Preferential heat-induced accumulation of hsp70 mRNA was first detected at a heat shock temperature of 30°C. Placement of embryos at 22°C after a 1-hour, 33°C heat shock resulted in decreased hsp70 mRNA with time, but the message persisted in selected tissues, including the lens placode and somites. Treatment of tailbud embryos with either sodium arsenite or zinc chloride induced a tissue-specific enrichment of hsp70 mRNA in the lens placode and somitic region. These studies reveal the complex nature of the heat shock response in different embryonic tissues and suggest the presence of regulatory mechanisms that lead to a stressor-induced, tissue-specific enrichment of hsp70 mRNA.
INTRODUCTION
The Hsp70 family, which is the most heavily studied group of heat shock proteins, includes the cytosolic stress-inducible Hsp70, the constitutively expressed Hsc70, mitochondrial p70, and endoplasmic reticulum resident protein, immunoglobulin-binding protein, or glucose-regulated protein 78 (Morimoto et al 1994; Parsell and Lindquist 1994; Feige et al 1996; Morimoto 1998). All these Hsc/Hsp70 isoforms have the ability to act as molecular chaperones that protect nascent or denatured proteins from aggregation and assist their folding or refolding into the correct conformation. Numerous studies have shown that synthesis of Hsp70 and other molecular chaperones is enhanced to protect cells against various stress conditions such as elevated temperature and exposure to sodium arsenite or heavy metals, which usually lead to an accumulation of unfolded protein. In cultured cells, the overexpression of Hsp70 greatly enhances survival of the cells when exposed to elevated temperatures or other stresses. The stress-inducible regulation of hsp gene expression occurs primarily at the transcriptional level, but regulation at the level of mRNA stability and translation has been documented (Morimoto et al 1994; Parsell and Lindquist 1994; Morimoto 1998). Heat shock–induced transcriptional activation of hsp genes is mediated by the interaction of the transcriptional-activating protein, heat shock factor (HSF), with the hsp gene enhancer element, heat shock element, found in the 5′ upstream region of these genes.
Developmental regulation of heat shock–induced hsp70 gene expression has been observed in a number of fungal, plant, and animal systems (Hightower and Nover 1991; Heikkila 1993a, 1993b). For example, hsp70 gene expression is not heat inducible until cycle 12 of Drosophila sp. development (Wang and Lindquist 1998) or the blastula stage of snail and sea urchin embryogenesis (Giudice 1989; Boon-Niermeijer 1991). Our laboratory and others have examined the developmental regulation of hsp70 gene expression during early embryogenesis of the frog, Xenopus laevis, in which 2 inducible hsp70 genes, 2 constitutive hsc70 genes, and one Bip gene have been described (Bienz 1984a, 1984b; Ali et al 1996a, 1996b; Miskovic et al 1997). X laevis hsp70 genes are not heat shock inducible until after the midblastula transition (MBT), which signals the onset of zygotic genome activation, even though HSF is detectable and heat activatable in cleavage-stage embryos (Bienz 1984a; Heikkila et al 1985, 1987, 1997; Krone and Heikkila 1988, 1989; Ovsenek and Heikkila 1990; Ali et al 1996a). In contrast, hsc70 mRNA is detectable throughout development and is not heat inducible (Ali et al 1996a). The acquisition of the ability to express hsp70 genes in response to heat shock as well as the constitutive upregulation of hsc70 mRNA levels in postblastula embryos has been correlated with an increase in thermoresistance (Heikkila et al 1985; Ali et al 1996a). These previous studies have examined the developmental stages at which hsc70/hsp70 genes were expressed or induced, but little information is available regarding their spatial pattern of expression in X laevis embryos. In this study, we have used whole-mount, in situ hybridization to examine the temporal and spatial distribution of X laevis hsc70 and hsp70 mRNA accumulation in normal and stress-treated embryos.
MATERIALS AND METHODS
Maintenance of X laevis embryos
Xenopus laevis eggs were collected, fertilized, and dejellied as described elsewhere (Heikkila et al 1985). Embryos were maintained in Steinberg's solution (60 mM NaCl; 0.7 mM KCl; 0.8 mM MgSO4·7H2O; 0.3 mM CaNO3·4H2O; 1.4 mM Tris base; pH. 7.4) at 22°C, and developmental stages were determined according to external criteria described by Nieuwkoop and Faber (1967). Embryos to be heat treated were sealed with parafilm in petri dishes containing Steinberg's solution. The container was then placed in a water bath at temperatures ranging from 26 to 35°C for 1 hour unless otherwise indicated. Control embryos were maintained at 22°C. Some embryos were treated with either 50 μM sodium arsenite or 100 μM zinc chloride for 2 hours at 22°C in Steinberg's solution. Embryos collected for RNA isolation were frozen and stored at −80°C. Embryos collected for whole-mount, in situ hybridization were fixed for 2 hours in MEMFA (0.1 M 3-morpholino propane sulfonic acid; 2 mM ethylenediaminetetraacetic acid; 1 mM MgSO4; 4% paraformaldehyde; pH, 7.4), rinsed twice in methanol, and then stored at −20°C.
Riboprobe preparation
In vitro RNA synthesis generating digoxygenin (DIG)-labeled riboprobes for Northern blot, in situ hybridization, or both was performed according to the manufacturer's protocol (Roche Molecular Biochemicals, Laval, Quebec, Canada). The coding region of hsc70.I cDNA (Ali et al 1996a) was cut with XbaI and XhoI and cloned into the corresponding sites of pSP72 (Promega, Madison, WI, USA). To generate hsc70 sense riboprobes, the hsc70.I/pSP72 construct was linearized with XhoI and then transcribed with T7 RNA polymerase. Production of hsc70 antisense riboprobe involved linearization of the vector with XbaI and transcription with SP6 RNA polymerase. To synthesize hsp70 riboprobes, the coding region of hsp70 genomic DNA (pXL16P; Bienz 1984b) was isolated with use of FspI and PstI and then cloned into the SmaI and PstI sites of pSP72 (Promega). The sense riboprobe was generated by linearization of pSP72 with XhoI and transcription with T7 RNA polymerase, whereas the antisense riboprobe was generated by linearization with MluNI (BalI) and transcription with SP6 RNA polymerase.
RNA isolation and Northern blot analysis
Total RNA was isolated from X laevis embryos using the method of Chirgwin et al (1979) as modified by Ohan and Heikkila (1995). The embryos were homogenized in 10 mL of 4 M guanidine isothiocyanate and layered on 3.3 mL of 5.7 M cesium chloride solution. A SW-41 Ti rotor (Beckman, Palo Alto, CA, USA) was used to centrifuge the samples at 30,000 rpm for 23 hours. The RNA pellets were then recovered and precipitated twice in ethanol to remove cesium chloride. Concentration, purity, and integrity of the RNA was established by spectrophotometry and formaldehyde agarose gel electrophoresis. Three micrograms of total RNA was electrophoresed in 1.2% formaldehyde agarose gels (Sambrook et al 1989), transferred to a positively charged nylon membrane (Roche Molecular Biochemicals) and ultraviolet cross-linked with a GS-Gene linker (Bio-Rad, Mississauga, Ontario, Canada). The RNA blots were then subjected to rapid reversible staining with 0.02% methylene blue before hybridization to check for equal sample loading (Herrin and Schmidt 1988). After prehybridization of the membrane in DIG-Easy-Hyb buffer (Roche Molecular Biochemicals) for at least 4 hours at 68°C, the buffer was replaced with the same buffer containing DIG-labeled antisense riboprobe and then incubated overnight at 68°C. Chemiluminescence detection was performed in accordance with the manufacturer's protocol (Roche) after exposure of the blot to Kodak BioMax film (Rochester, NY). The Northern blot hybridization results presented in this study are representative of at least 3 different experiments.
Whole-mount, in situ hybridization
Albino X laevis embryos obtained from the mating of albino females and normal males were used in the whole-mount, in situ hybridization protocol as described elsewhere (Miskovic and Heikkila 1999). The resultant embryos did not show the presence of pigmentation until the late tailbud stage. A nutator (VWR, Mississauga, Ontario, Canada) was used in all parts of the procedure that required shaking. The alkaline phosphatase–conjugated, anti-DIG antibody was used at a 1:12,000 dilution for hsp70 and hsc70. Identical conditions were used regarding the exposure of embryos to chromogenic reagents in all experiments. To document the results, the embryos were rehydrated with decreasing gradations of methanol, counterstained in Bouin's Fixative (VWR), and cleared for viewing in benzyl benozoate and benzyl alcohol at a ratio of 2:1 (Harland 1991; Drysdale et al 1997). The embryos were photographed using a Nikon AFX-11 camera (Mississauga, Ontario) attached to a Nikon dissecting microscope using EPT160T Kodak film. Each of the whole-mount, in situ hybridization results presented in this study are representative of at least 3 different experiments.
Histologic analysis
Whole-mount, in situ hybridized embryos used for histologic serial sectioning were embedded in paraplast, cut in 8- to 10-μm sections using a rotary microtome (American Optical, Leica Microsystems, Willowdale, Ontario), and mounted in Permount (Drysdale et al 1997). Photos of the histologic sections were taken using a Zeiss Axiophot microscope (Carl Zeiss, Don Mills, Ontario) and EPT 200 ASA Kodak film.
RESULTS
In situ hybridization analysis of hsc70 and hsp70 mRNA accumulation during X laevis development
A typical pattern of hsp70 and hsc70 mRNA accumulation in control and heat shocked embryos as determined by Northern blot analysis employing antisense riboprobes is shown in Figure 1. Accumulation of hsp70 mRNA was not detected constitutively in X laevis embryos, but hsp70 mRNA was first heat inducible during the postblastula stages (Fig 1A). The relative levels of heat shock–induced hsp70 mRNA accumulation then increased with development to the midtailbud stage, which was followed by a slight decrease at the late tailbud stage. Throughout development, hsc70 mRNA was detected constitutively, with increased relative levels in the gastrula and later stages that were not enhanced by heat shock treatment (Fig 1B). Given the different sizes of X laevis Hsp70 (2.7 kb) and hsc70 (2.3 kb) mRNA (Ali et al 1996a), we determined that the antisense Hsp70 riboprobe did not cross-react with hsc70 mRNA, or vice versa, under the conditions employed during the sequential RNA blot hybridization experiments (data not shown).
Fig 1.
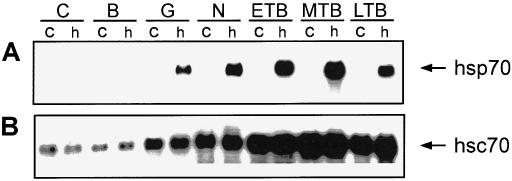
Relative levels of hsp70 and hsc70 mRNA in control and heat shocked Xenopus laevis embryos. Total RNA was isolated from control (c) and heat shocked (h; 1 hour at 33°C) embryos at the cleavage (C; stage 3), early blastula (B; stage 8), gastrula (G; stage 10/11), neurula (N; stage 17/18), early (ETB; stage 22/23), midtailbud (MTB; stage 28), and late tailbud (LTB; stage 35) stage. Three micrograms of RNA was subjected to Northern hybridization analysis employing digoxygenin-labeled X laevis hsp70 (panel A) and hsc70 (panel B) antisense riboprobes as outlined in the text. The hsc70 blot was slightly overexposed to visualize hsc70 mRNA in the earlier embryonic stages. Arrows indicate the positions of the different mRNAs. Transcript sizes: hsp70, 2.7 kb; hsc70, 2.3 kb
To determine the spatial pattern of hsc70 mRNA in X laevis embryos, whole-mount, in situ hybridization using DIG-labeled hsc70 antisense riboprobes was used (Fig 2). These studies demonstrated that hsc70 mRNA was distributed globally in gastrula and late tailbud embryos, and that the relative levels were not enhanced by heat shock (Fig 2B,C,E,F). Similar results were obtained at other embryonic stages of X laevis development, from early cleavage to late tadpole (data not shown). Histologic analysis revealed that hsc70 mRNA accumulation was not as intense in the internal yolk regions of the embryos. In control experiments, incubation of embryos with hsc70 sense riboprobe did not reveal the presence of any hybridizable RNA (Fig 2A,D).
Fig 2.
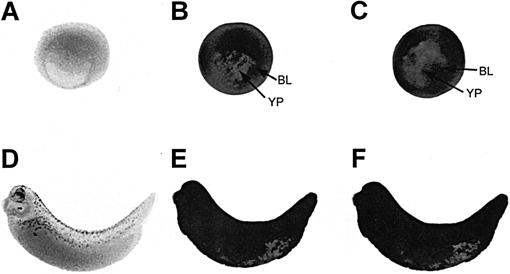
Effect of heat shock on the spatial pattern of hsc70 mRNA accumulation in Xenopus laevis embryos. Whole-mount, in situ hybridization with digoxygenin (DIG)-labeled hsc70 antisense riboprobe was performed with either control (22°C; panels B and E) or heat shocked (33°C for 1 hour; panels C and F) X laevis gastrula (stage 10/11; panels A, B, and C) and late tailbud (stage 34; panels D, E, and F) embryos. Panels A and D represent heat shocked X laevis embryos hybridized with DIG-labeled hsc70 sense riboprobe. At the late tailbud stage, embryos (D) display a natural increase in eye pigmentation and melanocyte production. BL, blastopore lip; YP, yolk plug
In contrast to the pattern of hsc70 mRNA accumulation, hsp70 mRNA was not detectable in control embryos at any of the developmental stages examined from cleavage to late tailbud (Fig 3A,C,E,G,I). In late tailbud–stage embryos, there was a natural increase in pigmentation of the lens placode as well as an increase in melanocyte production (Fig 3I). Heat shock treatment (33°C for 1 hour) of gastrula embryos induced hsp70 mRNA accumulation throughout the embryo, with an enrichment from the dorsal region to the marginal zone (Fig 3D). Heat shocked neurula-stage embryos showed a relatively uniform distribution of hsp70 mRNA on the surface (epidermal cells) of the embryo (Fig 3F), which was verified at histologic analysis (data not shown). In heat shocked midtailbud embryos (Fig 3H), hsp70 mRNA accumulation was distributed across the embryo surface, with enrichment in the anterior region, including the heart, the cement gland, the olfactory pit, the lens placode, the pronephros, and in the somites, the spinal cord, and the proctodeum. A similar phenomenon was observed in late tailbud–stage embryos, as was an increase in accumulation of the mRNA along the surface.
Fig 3.
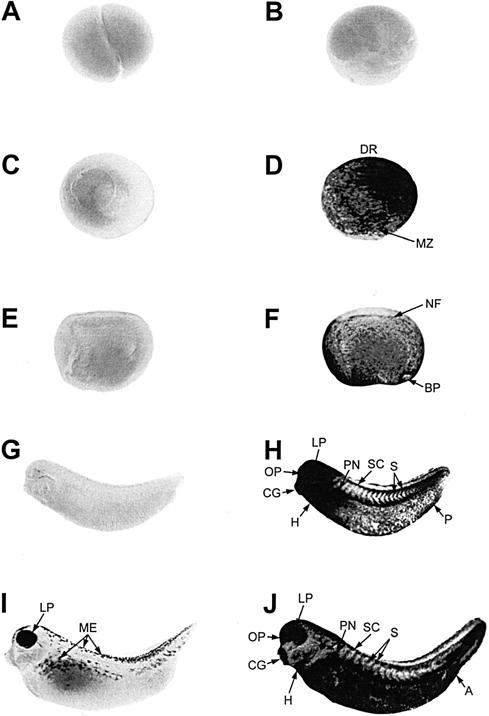
Spatial pattern of hsp70 mRNA accumulation during early Xenopus laevis development. Whole-mount, in situ hybridization with digoxygenin-labeled hsp70 antisense riboprobe was performed with control (22°C for 1 hour; panels A, C, E, G, and I) and heat shocked (33°C for 1 hour; panels B, D, F, H, and J) X laevis embryos at cleavage (stage 3; panels A and B), gastrula (stage 10/11; panels C and D [dorsomedial view]), neurula (stage 17/18; panels E and F), midtailbud (stage 28, panels G and H), and late tailbud (stage 35; panels I and J) stages. At the late tailbud stage, embryos (I) display a natural increase in eye pigmentation and melanocyte production (ME). A, anus; BP, blastopore; CG, cement gland; DR, dorsal region; H, heart; LP, lens placode; MZ, marginal zone; NF, neural fold; OP, olfactory pit; P, proctodeum; PN, pronephros; S, somites; SC, spinal cord
Histologic analysis of the DIG-labeled, in situ hybridized embryos was performed to verify the heat shock–induced preferential enrichment of hsp70 mRNA accumulation in specific tissues. An anterior cross-section revealed hsp70 mRNA in the heart region and in the epidermis (Fig 4A). An enlarged medial region of a heat shocked, midtailbud, whole-mount, in situ hybridized embryo (Fig 4B) indicated the presence of hsp70 mRNA in the somites and ectoderm. Heat shock–induced accumulation of hsp70 mRNA in this region was confirmed histologically (compare Fig 4C and 4D). The hsp70 mRNA was also enriched in the pronephric duct after heat shock (compare Fig 4E and 4F) and in the lens placode and optic cup of midtailbud embryos (compare Fig 4G and 4H). In control experiments, hybridizable RNA was not detected in histologic sections of heat shocked, midtailbud embryos incubated with DIG-labeled, hsp70 sense riboprobe (data not shown).
Fig 4.
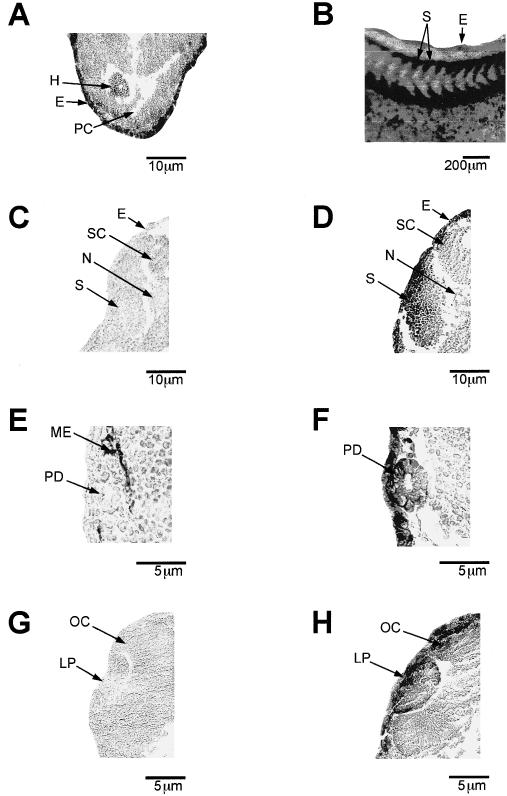
Pattern of hsp70 mRNA accumulation in histologic sections from Xenopus laevis tailbud embryos. Whole-mount, in situ hybridization with digoxygenin-labeled hsp70 antisense riboprobe was performed, and histologic sections were taken from control (1 hour at 22°C; panels C, E, and G) and heat shocked (1 hour at 33°C; panels A, B, D, F, and H) X laevis tailbud embryos. (A) Anterior histologic section that shows hsp70 mRNA accumulation in the heart and ectoderm of a midtailbud embryo (stage 28). (B) An enlarged medial region of a midtailbud embryo. (C) and (D) Histologic cross-sections taken from control and heat shocked midtailbud embryos that show hsp70 mRNA in the somitic region. (E) and (F) Cross-sections of a late tailbud embryo (stage 35) that shows hsp70 mRNA in the pronephric duct. (G) and (H) Accumulation of hsp70 mRNA in the eye and optic cup of a midtailbud embryo. Magnification bars are indicated in each panel. E, ectoderm; H, heart; LP, lens placode; ME, melanocytes; S, somite; SC, spinal cord; N, notochord; OC, optic cup; PC, pericardial coelom; PD, pronephric duct
Spatial pattern of Hsp70 mRNA accumulation in X laevis tailbud embryos during continuous heat shock and recovery from heat shock
The spatial pattern of heat shock–induced hsp70 mRNA accumulation in X laevis embryos was also characterized by continuous heat shock and recovery experiments. Midtailbud embryos were used to avoid complications resulting from a natural increase of pigmentation in the lens placode and melanocytes in late tailbud embryos. As shown in Figure 5B, heat shock–induced hsp70 mRNA accumulation was strongly induced in the lens placode, with relatively lower amounts in the otic vesicle and somites after 15 minutes. After 30 minutes of heat shock, hsp70 mRNA was strongly enriched in the head region, somites, and tail region of the embryo (Fig 5C). A 1-hour heat shock produced a similar phenomenon, with slightly less hsp70 mRNA accumulation in the tail region (Fig 5D). After 2 hours of heat shock, there was a general reduction in hsp70 mRNA levels, with the exception of the somitic region (Fig 5E), and a further decrease in hsp70 mRNA levels was observed after 4 hours of heat shock (Fig 5F). These experiments indicate a transient accumulation of hsp70 mRNA in the different tissues of the midtailbud embryo during continuous exposure to a 33°C heat shock. We have observed this phenomenon previously in Northern blot hybridization studies (Krone and Heikkila 1988). The present study also demonstrate that selected regions, such as the somites, lens placode, and cement gland, maintained abundant relative levels of hsp70 mRNA longer than other regions of the embryo.
Fig 5.
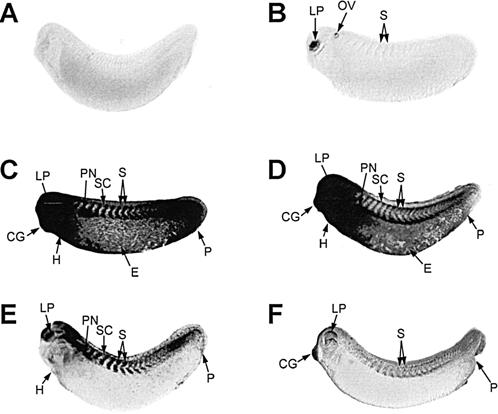
Time course of heat shock–induced hsp70 mRNA accumulation in Xenopus laevis tailbud embryos. Whole-mount, in situ hybridization was performed with digoxygenin-labeled hsp70 antisense riboprobe. Midtailbud embryos (stage 28) were exposed to either 22°C (panel A) or 33°C for 15 minutes (panel B), 30 minutes (panel C), 1 hour (panel D), 2 hours (panel E) or 4 hours (panel F). The low amount of somitic staining in panel B was more easily discernable in the original color slide. CG, cement gland; E, epidermis; H, heart; LP, lens placode; OV, otic vesicle; P, proctodeum; PN, pronephros; S, somites; SC, spinal cord
The pattern of hsp70 mRNA accumulation during recovery at 22°C after a heat shock of 33°C for 1 hour is shown in Figure 6. After 1–3 hours of recovery at 22°C, the relative levels of hsp70 mRNA decreased in all regions, with the exception of the somitic region (Fig 6C,D). Elevated relative levels of hsp70 mRNA were still detected in the somitic region, spinal cord, and lens placode after 7 hours (Fig 6E), and a trace amount was still visible in the lens placode and spinal cord region after 11 hours (Fig 6F).
Fig 6.
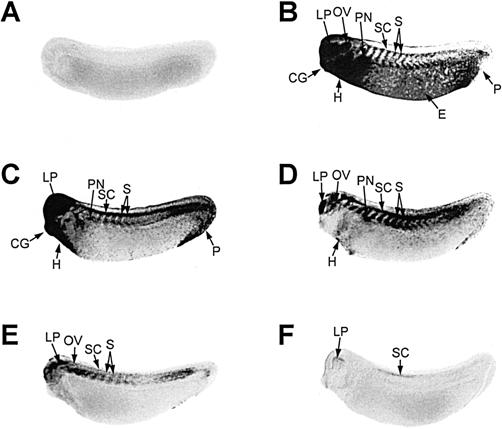
Pattern of hsp70 mRNA accumulation in tailbud embryos during recovery from heat shock. Whole-mount, in situ hybridization was performed with digoxygenin-labeled hsp70 antisense riboprobe. Midtailbud Xenopus laevis embryos (stage 28) were exposed to either 22°C (panel A) or 33°C (panel B) for 1 hour followed by recovery at 22°C for either 1 hour (panel C), 3 hours (panel D), 7 hours (panel E), or 11 hours (panel F). CG, cement gland; E, epidermis; H, heart; LP, lens placode; OV, otic vesicle; P, proctodeum; PN, pronephros; S, somites; SC, spinal cord
Effect of different temperatures on the spatial pattern of hsp70 mRNA accumulation
We examined the effect of a range of heat shock temperatures (26°C–35°C) on hsp70 mRNA accumulation (Fig 7). Elevation of the incubation temperature from 22°C to 26°C for 1 hour did not induce the accumulation of hsp70 mRNA (compare Fig 7A and 7B). Placement of midtailbud embryos at 30°C, however, resulted in enhanced hsp70 mRNA accumulation in the heart, lens placode, otic vesicle, pronephros, somites, and proctodeum (Fig 7C). A similar but more enhanced pattern was observed in midtailbud embryos incubated at 33°C, as was an enrichment of hsp70 mRNA in the head region, with additional localization to the spinal cord and ectoderm (Fig 7D). At 35°C, the accumulation pattern of hsp70 mRNA was similar to but more intense than that found at 33°C (Fig 7E).
Fig 7.
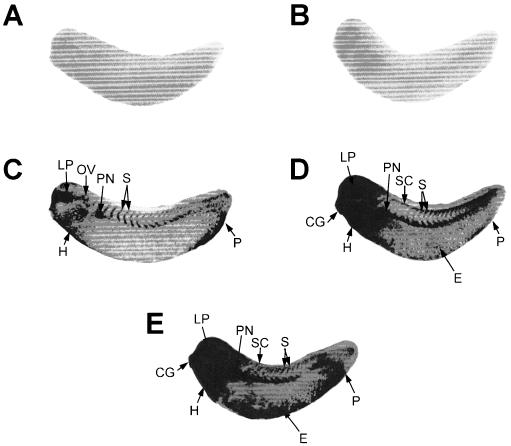
Effect of different temperatures on the spatial patterns of hsp70 mRNA accumulation in Xenopus laevis tailbud embryos. Whole-mount, in situ hybridization was performed with digoxygenin-labeled hsp70 antisense riboprobe. The midtailbud X laevis embryos (stage 28) were exposed to 1-hour heat treatments at either 22°C (panel A), 26°C (panel B), 30°C (panel C), 33°C (panel D), or 35°C (panel E). The slight increase in density at the anterior region in panel B is nonspecific. CG, cement gland; E, epidermis; H, heart; LP, lens placode; OV, otic vesicle; P, proctodeum; PN, pronephros; S, somites; SC, spinal cord
Effect of sodium arsenite and zinc chloride treatment of the accumulation of hsp70 mRNA in tailbud embryos
In previous Northern blot hybridization studies, we have found that treatment of X laevis embryos with stresses other than heat shock, such as sodium arsenite and zinc chloride, can also induce hsp70 mRNA accumulation (Heikkila et al 1987; Ovsenek and Heikkila, unpublished results). In this study, we examined the effect of a 2-hour treatment with either 50 μM sodium arsenite or 100 μM zinc chloride at 22°C on the spatial pattern of hsp70 mRNA accumulation in X laevis tailbud embryos (Fig 8). In our earlier studies, these concentrations of chemical stressors induced maximal accumulation of hsp70 mRNA in X laevis embryos, but the relative levels were much lower than that found with a 33°C heat shock. As shown in Figure 8, sodium arsenite and zinc chloride both induced hsp70 mRNA accumulation in the head region, including the lens placode, as well as in the somitic region of the tailbud embryos. Enrichment of hsp70 mRNA was also observed in the proctodeum. These results indicate that chemical stressors induce a preferential accumulation of hsp70 mRNA in selected tissues of the tailbud embryo.
Fig 8.
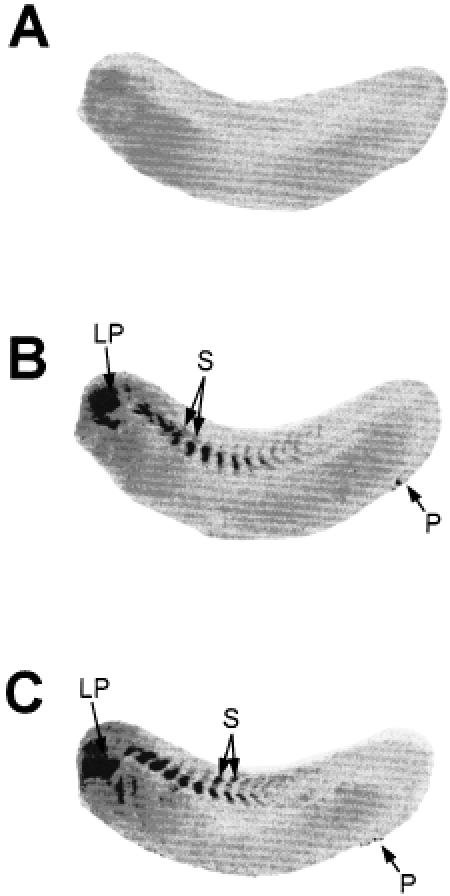
Effect of sodium arsenite and zinc chloride on the spatial pattern of hsp70 mRNA accumulation in tailbud embryos. Midtailbud embryos (stage 28) were either maintained at 22°C (panel A) or exposed to 50 μM sodium arsenite (panel B) or 100 μM zinc chloride (panel C) for 2 hours at 22°C. Whole-mount, in situ hybridization was performed with digoxygenin-labeled hsp70 antisense riboprobe as outlined in the text. LP, lens placode; P, proctodeum; S, somites
DISCUSSION
In this study, we have used whole-mount, in situ hybridization to examine the spatial pattern of hsc70 and hsp70 mRNA accumulation in control and stress-treated X laevis embryos. The hsc70 mRNA was detected in cleavage-stage embryos, indicating a maternal origin for these transcripts that is followed by an increase during the postblastula stages after activation of the zygotic genome at the MBT. The detection of constitutive hsc70 gene expression in X laevis embryos is in agreement with results from previous studies of this gene using Northern blot analysis (Ali et al 1996a) and immunohistochemistry (Herberts et al 1993). The relatively high levels of hsc70 gene expression during the gastrula and later stages may be in response to an increased demand for molecular chaperone activity resulting from the synthesis of new protein after zygotic genome activation (Ali et al 1996a). The spatial pattern of hsc70 mRNA accumulation in X laevis embryos was essentially global, but with less mRNA in the internal yolk cell regions. This type of hybridization pattern was observed with X laevis actin mRNA (Lang et al, 1999) and X laevis ribosomal L8 protein (Miskovic and Heikkila 1999). Additionally, the in situ hybridization studies showed that X laevis hsc70 gene expression in postblastula embryos was not induced by heat shock. A lack of heat inducibility for hsc70 gene expression has also been reported in embryos of the amphibian Pleurodeles waltl (Delelis-Fanien et al 1997) and in Chinook salmon embryo cells (Zafarullah et al 1992). In zebrafish embryos, rat PC12, and human HeLa cells, however, hsc70 transcript levels were increased after heat shock (Santacruz et al 1997; O'Malley et al 1985).
In contrast to hsc70 mRNA, hsp70 mRNA was not detected constitutively at any of the developmental stages we examined. Furthermore, heat shock–induced hsp70 mRNA was detected only after the midblastula stage. It is likely that the mechanism responsible for activation of the embryonic genome at this stage also applies to the hsp70 genes, as has been suggested by Heikkila et al (1997). One model for the activation of genes at MBT suggests that the rapid cell division in pre-MBT embryos prevents RNA transcription, and that the lengthening of the cell cycle at MBT permits the onset of transcription (Kimelman et al 1987). Another, more recent theory suggests that chromatin domain structure, DNA accessibility, and transcription complex–chromatin dynamic competition interact to regulate transcription in the X laevis embryo both before and after MBT (Hair et al 1998). In this latter study, Hair et al suggested that the large pool of histone in early cleavage-stage embryos binds to DNA and blocks binding of transcription factor. At the midblastula stage, this pool of histone is depleted, a situation that in turn increases the accessibility of transcription factor. Wang and Lindquist (1998) have proposed that the lack of heat-inducible expression for hsp70 genes during early Drosophila sp. development may result from an inability of transcription factors, including HSF, to enter the nucleus. Whether a similar mechanism is involved in the developmental stage–dependent regulation of heat shock–induced expression of hsp70 genes in X laevis, however, remains to be determined.
This study also determined that heat shock–induced hsp70 mRNA accumulation was enriched in a tissue-specific manner in X laevis embryos. For example, in heat shocked (33°C) early tailbud embryos, hsp70 mRNA was found to accumulate primarily in the anterior head region, including the lens placode, the cement gland, and the heart as well as in the somites, spinal cord, and proctodeum. Tissue-specific enrichment of hsp70 mRNA was detected within 15 minutes of heat shock in the somites, lens placode, and otic vesicle. The lowest temperature that induced tissue-specific enrichment of hsp70 mRNA was 30°C for 1h. Development of Hsp70 isoform–specific antibodies will be required to determine the spatial pattern of hsp70 gene expression at the protein level. Recently, we have observed a similar, but not identical, pattern of tissue-specific enrichment of hsp30 mRNA and Hsp30 protein in heat shocked X laevis embryos (Lang et al, 1999). Tissue-specific enhancement of hsp70 mRNA has also been documented in 2-day-old zebrafish embryos (Lele et al 1997); for example, hsp70 mRNA accumulation was enriched in epidermal epithelial cells and in the outermost cells of the eye and retina. Additionally, a cell type–specific pattern of hsp70 mRNA accumulation has been observed during Drosophila sp. embryogenesis (Wang and Lindquist 1998).
The mechanism involved in the heat shock–induced, tissue-specific enrichment of hsp70 mRNA in X laevis tailbud embryos is not known. It is possible that these tissues have a lower temperature set point than other tissues for the activation of HSF, as has been shown previously in adult X laevis heart (Ali et al 1997) and mouse pachytene spermatocytes (Sarge 1995). In the latter study, Sarge suggested that the temperature of HSF activation may not necessarily have a fixed value and can vary in a tissue-dependent manner. Therefore, it is possible that selected tissues, such as somite, lens placode, cement gland, heart, and proctodeum, may have a lower HSF activation temperature compared with that of other tissues. One cannot exclude, however, the possible involvement of other X laevis transcription factors in this phenomenon. In Drosophila melanogaster, it has been suggested that the cell-specific heat shock induction of Hsp23 in the eye and testes may require other transcription factors in addition to HSF (Marin et al 1996; Michaud et al 1997). Further work is required to determine the mechanism for heat shock–induced, tissue-specific enrichment of hsp70 mRNA in X laevis embryos.
This study has also shown that stressors other than heat shock can induce a tissue-specific enrichment of hsp70 mRNA in X laevis tailbud embryos. For example, treatment of embryos with either sodium arsenite or zinc chloride induced hsp70 mRNA primarily in the lens placode and the somitic region. The magnitude of the responses with the sodium arsenite and zinc chloride was not as intense as that observed with a 33°C, or even with a 30°C, heat shock. It has been suggested that environmental and chemical stressors may have different stimulatory pathways that ultimately converge and result in the unfolding of protein, which in turns triggers HSF activation (Morimoto 1998). Heat shock may be a more efficient inducer of HSF given its direct effect on the cell compared with chemical stressors, which need to gain entry into the cell and accumulate.
This study has illustrated the complexity of the heat shock response in X laevis embryos. A natural question that arises, however, is why certain tissues in the developing organism have an enhanced heat shock response compared with other tissues. The tissues in X laevis tailbud embryos that are relatively sensitive to stress treatment are the somitic region and the lens placode. For example, both these regions accumulated hsp70 mRNA at 30°C as well as within 15 minutes of a 33°C heat shock. Additionally, these regions contained relatively abundant levels of the mRNA during continuous heat shock and recovery experiments, even though the mRNA had significantly decayed in other regions. These tissues were also targeted by sodium arsenite and zinc chloride treatment. The visual system of the early embryo is of obvious importance once feeding behavior has initiated at the tadpole stage, whereas the somitic region gives rise to key structures such as the axial skeleton, trunk, and limb muscle. Severe heat shock temperatures have been shown to disrupt the somitic region of X laevis embryos and of mammals, causing skeletal malformations (Danker et al 1992; Fisher et al 1996). It is possible that preferential Hsp70 accumulation in these tissues during heat shock or other stresses may function in a protective manner, presumably in the role of molecular chaperone.
Acknowledgments
This research was supported by a Natural Sciences and Engineering Research Council grant to J.J.H. The authors would like to thank Dr Tom Drysdale for assistance with in situ hybridization and Dale Weber for technical aid in preparation of the histologic sections.
REFERENCES
- Ali A, Fernando P, Smith WL, Ovsenek N, Lepock JR, Heikkila JJ. Preferential activation of HSF-binding activity and hsp70 gene expression in Xenopus heart after mild hyperthermia. Cell Stress Chap. 1997;2:229–237. doi: 10.1379/1466-1268(1997)002<0229:paohba>2.3.co;2.1355-8145(1997)002<0229:PAOHBA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Salter-Cid L, Flajnik MF, Heikkila JJ. Isolation and characterization of a cDNA encoding a Xenopus 70-kDa heat shock cognate protein, hsc70. I. Comp Biochem Physiol. 1996a;113B:681–687. doi: 10.1016/0305-0491(95)02081-0.0010-406X(1996)113<0681:IACOAC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ali A, Salter-Cid L, Flajnik MF, Heikkila JJ. Molecular cloning of a cDNA encoding a Xenopus laevis 70-kDa heat shock cognate protein, hsc70. II. Biochim Biophys Acta. 1996b;1309:174–178. doi: 10.1016/s0167-4781(96)00156-x.0006-3002(1996)1309<0174:MCOACE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984a;81:3138–3142. doi: 10.1073/pnas.81.10.3138.0027-8424(1984)081<3138:DCOTHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp70 genes are constitutively expressed in injected oocytes. EMBO J. 1984b;3:2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x.0261-4189(1984)003<2477:XHGACE>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon-Niermeijer EK 1991 Heat shock effects in snail development. In: Heat Shock and Development, ed. Hightower L, Nover L. Springer-Verlag, New York, NY, 7–28. [DOI] [PubMed] [Google Scholar]
- Chirgwin J, Przbyla A, MacDonald R, Rutter W. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005.0006-2960(1979)018<5294:IOBARA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Danker K, Hacke H, Wedlich D. Effects of heat shock on the pattern of fibronectin and laminin during somitogenesis in Xenopus laevis. Dev Dynamics. 1992;193:136–144. doi: 10.1002/aja.1001930205.1058-8388(1992)193<0136:EOHSOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Delelis-Fanien C, Penrad-Mobayed M, Angelier N. Molecular cloning of a cDNA encoding the amphibian Pleurodeles waltl 70-kDa heat shock cognate protein. Biochem Biophys Res Commun. 1997;238:159–164. doi: 10.1006/bbrc.1997.7255.0006-291X(1997)238<0159:MCOACE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Drysdale TA, Patterson KD, Saha M, Krieg PA. Retinoic acid can block differentiation of the myocardium after heart specification. Dev Biol. 1997;188:205–215. doi: 10.1006/dbio.1997.8623.0012-1606(1997)188<0205:RACBDO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Feige U, Morimoto RI, Yahara I, and Polla BS 1996 Stress-Inducible Cellular Responses. Birkhauser-Verlag, Basel, Switzerland. [PubMed] [Google Scholar]
- Fisher BR, Heredia DJ, Brown KM. Heat-induced alterations in embryonic cytoskeletal and stress proteins precede somite malformation in rat embryos. Teratogenesis Carcinog Mutagen. 1996;16:49–64. doi: 10.1002/(SICI)1520-6866(1996)16:1<49::AID-TCM6>3.0.CO;2-G.0270-3211(1996)016<0049:HIAIEC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Giudice G. Heat shock proteins in sea urchin embryos. Dev Growth Diff. 1989;31:103–106. doi: 10.1111/j.1440-169X.1989.00103.x.0012-1592(1989)031<0103:HSPISU>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hair A, Prioleau M-N, Vassetzky Y, Mechali M. Control of gene expression in Xenopus early development. Dev Genet. 1998;22:122–131. doi: 10.1002/(SICI)1520-6408(1998)22:2<122::AID-DVG2>3.0.CO;2-8.0192-253X(1998)022<0122:COGEIX>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Harland RM 1991 In situ hybridization: an improved whole-mount method for Xenopus embryos. In: Methods in Cell Biology, Xenopus laevis: Practical Uses in Cell and Molecular Biology, vol 36, ed. Kay BK, Peng HB. Academic Press, Toronto, Canada, 685–694. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ. Heat shock gene expression and development. I. An overview of fungal, plant, and poikilothermic animal developmental systems. Dev Genet. 1993a;14:1–5. doi: 10.1002/dvg.1020140102.0192-253X(1993)014<0001:HSGEAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heikkila JJ. Heat shock gene expression and development. II. An overview of mammalian and avian developmental systems. Dev Genet. 1993b;14:87–91. doi: 10.1002/dvg.1020140202.0192-253X(1993)014<0087:HSGEAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heikkila JJ, Kloc M, Bury J, Schultz GA, Browder L. Aquisition of the heat shock response and thermotolerance in Xenopus laevis. Dev Biol. 1985;107:483–489. doi: 10.1016/0012-1606(85)90329-x.0012-1606(1985)107<0483:AOTHSR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heikkila JJ, Ohan N, Tam Y, Ali A. Heat shock protein gene expression during Xenopus development. Cell Mol Life Sci. 1997;53:114–121. doi: 10.1007/PL00000573.1420-682X(1997)053<0114:HSPGED>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila JJ, Ovsenek N, Krone P. Examination of heat shock protein mRNA accumulation in early Xenopus laevis embryos. Biochem Cell Biol. 1987;65:87–94. doi: 10.1139/o87-013.0829-8211(1987)065<0087:EOHSPM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Herberts C, Moreau N, Angelier N. Immunolocalization of Hsp70-related proteins constitutively expressed during Xenopus laevis oogenesis and development. Int J Dev Biol. 1993;37:397–406.0214-6282(1993)037<0397:IOHRPC>2.0.CO;2 [PubMed] [Google Scholar]
- Herrin DL, Schmidt GW. Rapid, reversible staining of Northern blots prior to hybridization. Biotechnology. 1988;6:196–199.0168-1656(1988)006<0196:RRSONB>2.0.CO;2 [PubMed] [Google Scholar]
- Hightower L, Nover L 1991 Heat Shock and Development. Springer-Verlag, Heidelberg, Germany. [PubMed] [Google Scholar]
- Kimelman D, Kirschner M, Scherson T. The events of the midblastula transition in Xenopus are regulated by changes in the cell cycle. Cell. 1987;48:399–407. doi: 10.1016/0092-8674(87)90191-7.0092-8674(1987)048<0399:TEOTMT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krone PH, Heikkila JJ. Analysis of hsp 30, hsp 70, and ubiquitin gene expression in Xenopus laevis tadpoles. Development. 1988;103:59–67. doi: 10.1242/dev.103.1.59.0950-1991(1988)103<0059:AOHHAU>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krone PH, Heikkila JJ. Expression of microinjected hsp 70/CAT and hsp 30/CAT chimeric genes in developing Xenopus laevis embryos. Development. 1989;106:271–281. doi: 10.1242/dev.106.2.271.0950-1991(1989)106<0271:EOMHCA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lang L, Miskovic D, Fernando P, and Heikkila JJ. 1999 Spatial pattern of constitutive and heat shock-induced expression on the small heat shock protein gene family, hsp30, in Xenopus laevis tailbud embryos. Dev Genet. in press. [DOI] [PubMed] [Google Scholar]
- Lele Z, Engel S, Krone PH. Hsp47 and hsp70 gene expression is differentially regulated in a stress- and tissue-specific manner in zebrafish embryos. Dev Genet. 1997;21:123–133. doi: 10.1002/(SICI)1520-6408(1997)21:2<123::AID-DVG2>3.0.CO;2-9.0192-253X(1997)021<0123:HAHGEI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marin R, Demers M, Tanguay RM. Cell-specific heat shock induction of Hsp23 in the eye of Drosophila melanogaster. Cell Stress Chap. 1996;1:40–46. doi: 10.1379/1466-1268(1996)001<0040:cshsio>2.3.co;2.1355-8145(1996)001<0040:CSHSIO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Marin R, Westwood TJ, Tanguay RM. Cell-specific expression and heat shock induction of Hsps during spermatogenesis in Drosophila melanogaster. J Cell Sci. 1997;110:1989–1997. doi: 10.1242/jcs.110.17.1989.0021-9533(1997)110<1989:CSEAHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Miskovic D, Heikkila JJ. Constitutive and stress-inducible expression of the endoplasmic reticulum heat shock protein 70 gene family member, immunoglobulin-binding protein (BiP), during Xenopus laevis early development. Dev Genet. 1999;25:31–39. doi: 10.1002/(SICI)1520-6408(1999)25:1<31::AID-DVG4>3.0.CO;2-M.0192-253X(1999)025<0031:CASIEO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Miskovic D, Salter-Cid L, Ohan N, Flajnik MF, Heikkila JJ. Isolation and characterization of a cDNA encoding a Xenopus immunoglobulin binding protein, BiP (GRP78) Comp Biochem Physiol. 1997;116B:227–234. doi: 10.1016/s0305-0491(96)00219-2.0010-406X(1997)116<0227:IACOAC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788.0890-9369(1998)012<3788:ROTHST>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissieres A, and Georgopoulos C 1994 The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Nieuwkoop PD, Faber J 1967 Normal Table of Xenopus laevis (Daudin). North Holland Publishing, Amsterdam, the Netherlands. [Google Scholar]
- Ohan NW, Heikkila JJ. Involvement of differential gene expression and mRNA stability in the developmental regulation of the hsp30 gene family in heat shocked Xenopus laevis embryos. Dev Genet. 1995;17:176–184. doi: 10.1002/dvg.1020170209.0192-253X(1995)017<0176:IODGEA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- O'Malley K, Mauron A, Barchas JD, Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat shock-like protein. Mol Cell Biol. 1985;5:3476–3483. doi: 10.1128/mcb.5.12.3476.0270-7306(1985)005<3476:CERMEA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsenek N, Heikkila JJ. DNA sequence-specific binding activity of the heat shock transcription factor is heat-inducible before the midblastula transition of early Xenopus development. Development. 1990;110:427–433. doi: 10.1242/dev.110.2.427.0950-1991(1990)110<0427:DSSBAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parsell DA, Linquist S. The function of heat shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1994;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253.0066-4197(1994)027<0437:TFOHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritisch EF, and Maniatis T 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sanatacruz H, Vriz S, Angelier N. Molecular characterization of a heat shock cognate cDNA of zebrafish, hsc70, and developmental expression of the corresponding transcripts. Dev Genet. 1997;21:223–233. doi: 10.1002/(SICI)1520-6408(1997)21:3<223::AID-DVG5>3.0.CO;2-9.0192-253X(1997)021<0223:MCOAHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sarge KD. Male germ cell-specific alteration in temperature set point of the cellular stress response. J Biol Chem. 1995;270:18745–18748. doi: 10.1074/jbc.270.32.18745.0021-9258(1995)270<18745:MGCSAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang Z, Lindquist S. Developmentally regulated nuclear transport of transcription factors in Drosophila embryos enable the heat shock response. Development. 1998;125:4841–4850. doi: 10.1242/dev.125.23.4841.0950-1991(1998)125<4841:DRNTOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zafarullah M, Wisniewski J, Shworak NW, Schieman S, Misra S, Gedamu L. Molecular cloning and characterization of a constitutively expressed heat shock cognate hsc71 gene from rainbow trout. Eur J Biochem. 1992;204:893–900. doi: 10.1111/j.1432-1033.1992.tb16709.x.0014-2956(1992)204<0893:MCACOA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]


