Abstract
BACKGROUND:
To determine the prevalence of metabolic syndrome in survivors of childhood leukemia in Isfahan, Iran.
METHODS:
During a 4-year period (2003 to 2007), 55 children (33 male and 22 female) diagnosed with ALL at Unit of Hematology/ Oncology, Department of Pediatrics, Isfahan University of Medical Science, were enrolled in this cross-sectional study. Metabolic syndrome was defined using the modified version of Adult Treatment Panel (ATP III) crite-ria. Insulin resistance was defined based on the homeostasis model assessment index (HOMA-IR).
RESULTS:
The mean age of participates was 10.4 years (range 6-19 years) and the mean interval since completion of chemotherapy was 35 months. Twenty percent (11/55) of survivors (10 male, 1 female) met criteria for diagnosis of metabolic syndrome. Obesity was observed in one forth of patients and nearly 3/4 of obese patients had metabolic syndrome. High serum insulin levels were found in 16% of participants and in 63% of obese survivors. The mean insulin levels in survivors with metabolic syndrome was three-times more than those without (28.3 mu/l vs. 9.57 mu/l, p = 0.004). Insulin resistance was detected in 72.7% of survivors with metabolic syndrome and it was positively correlated with serum triglycerides (0.543, p ≤ 0.001), systolic and diastolic BP (0.348, p = 0.01 and 0.368, p = 006 respectively), insulin levels (0.914, p < 0.001) and blood sugar (0.398, p = 003).
CONCLUSIONS:
The prevalence of metabolic syndrome in survivors of childhood leukemia in Iran is higher than developed countries. Nearly all of the obese patients had metabolic syndrome. Weight control and regular physical exercise are recommended to the survivors.
Keywords: Acute lymphoblastic leukemia, metabolic syndrome, obesity, children
Acute lymphoblastic leukemia is the most common malignancy in children.1 It accounts for one fourth of all childhood cancer cases.1,2 Using modern treatment regimens, the 5-year survival rate of ALL has improved from virtually zero (in the 1950's) to approximately 80%.3 As the population of ALL survivors increases, the adverse effects of their treatment become apparent. Numerous endo-crine/metabolic adverse effects such as deficient secretion of growth hormone, gonadotropins and thyroid hormones and metabolic syndrome have been demonstrated in survivors.4–6
Metabolic syndrome, a group of disorders related to insulin resistance, is characterized clinically by central (abdominal) obesity, elevated plasma glucose, dyslipidemia, hypertension, and a prothrombotic and proinflammatory state.7 Metabolic syndrome is an important risk factor for cardiovascular disease and diabetes mellitus and the link between obesity, metabolic syndrome and type2 diabetes has been described in children.8 In the developed countries, the overall prevalence of metabolic syndrome in healthy children and adolescents based on modified ATP III criteria is 2.0-11.5%.9–11 Cruz et al demonstrated that metabolic syndrome was present in 30% of the overweight and obese patients.12 Growth hormone (GH) deficiency has been expected as a likely contributor to obesity and its related metabolic disorders, such as insulin resistance and dyslipidemia. Talvensaari et al demonstrated that decreased GH secretion is related to metabolic abnormalities among long term survivors of childhood cancer.13
The aim of this study was to determine the prevalence of metabolic syndrome in survivors of childhood leukemia in Iran and to assess the relation between obesity and metabolic syn-drome. Early identification of metabolic syndrome among them can reduce the risk of cardiovascular morbidity and mortality.
Methods
During a 4-year period (2003 to 2007), 75 children who have been diagnosed with ALL at Unit of Hematology/ Oncology, Department of Pediatrics; Isfahan University of Medical Science were called by phone to participate in this study. Seven patients died. Patients who had relapse of leukemia (6 patients) were excluded from the study. From the remaining 62 patients, seven refused to participate in the study because of distance, time or lack of interest. The remaining 55 patients were enrolled (33 males, 60%) in the study. The mean age of patients at the time of study entry was 10.4 ± 4.1 years and the mean interval since completion of chemotherapy was 35 months. The mean age at diagnosis was 5.8 ± 2.2 years. All patients had been treated according to the ALL-BFM chemotherapy protocol. Before commencing the study approval was granted by the University Ethics Committee. Written informed consent was obtained from each subject. All patients had physical examination and measurement of weight, height, and blood pressure (BP). Height and weight were measured with a calibrated scale to the nearest 0.5 kg and 0.5 cm, respectively. Body mass index (BMI, kg/m2) was calculated from these measurements and was categorized by age and sex. Blood pressure was measured with standard mercury sphygomanometers and appropriate sized cuffs. After 5 minutes of rest, 2 measurements were performed in the right arm in the sitting position. The first and fifth Korotkoff sounds were recorded for the systolic and diastolic BP, respectively. A mean of the 2 measurements was used in the analysis. All measurements were given as percentiles for age, sex, and height according to data from a population study in children and adolescents of Iranian origin.14
Venous blood samples were drawn for determinations of serum glucose, serum total cholesterol, triglycerides, high-density lipoprotein cho-lesterol (HDL-C) and insulin after a 12-hour overnight fasting. The samples centrifuged and the plasma was stored until assayed. Serum concentrations of triglycerides were measured enzymatically. (Parsazmun GPO-PAP and GOD kit, Karaj, IRAN) and HDL cholesterol levels were assessed after precipitation of very low-density lipoproteins with dextran sulfate and magnesium chloride. Plasma insulin concentration were determined by enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle (DRG Insulin ELISA kit EIA-2935, Germany).
Patients with BMI above 95th percentile were defined as obese, whereas patients with 75th < BMI < 95th percentile were defined as overweight. Metabolic syndrome was defined using the modified version of ATP III criteria.9 Children with 3 or more of the following criteria were considered positive for metabolic syndrome: 1) Obesity or waist circumference ≥ 75th percentile for age and gender; 2) triglyceride levels ≥ 100 mg/dL; 3) Fasting blood glucose ≥ 100 mg/dL; 4) Hypertesion: systolic and diastolic blood pressure more than 90th percentile for age, gender and height; 5) HDL-C < 50 mg/dL. Hyperinsulinema was defined as a single fasting insulin level > 24 mu/L and IR based on the HOMA-IR that estimates IR with the expression FI (MU/ml. FG (mmol/L)/22.5.15
Statistical analysis: The data were complied and analyzed using the Statistical Package for Social Sciences (SPSS 11.5) program. The metabolic syndrome parameters in ALL survivors with and without metabolic syndrome and patients who received chemotherapy with cranial radiation and those received chemotherapy alone was compared using t-test if all values were assumed to be normally distributed. If not, the non-parametric analysis such as Mann-Whitney U was used. The p < 0.05 was considered statistically significant.
Results
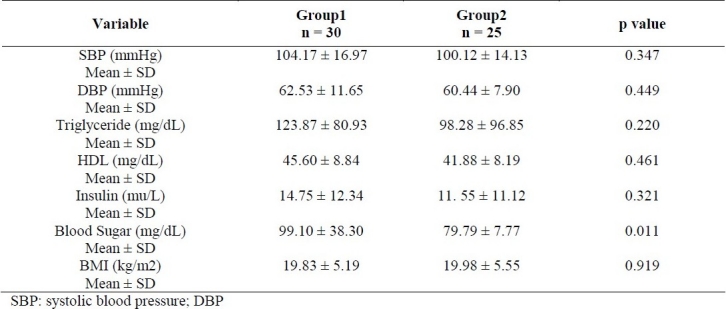
The mean age of participants was 10.4 years (range 6-19). Mean Values of Triglycerides, HDL-C, Glucose, Insulin, BMI and Blood Pressure in survivors of childhood ALL with and without metabolic syndrome are presented in table 1. Prevalence of metabolic syndrome criteria in young survivors of childhood ALL were as follows: Obesity (90.9%), low HDL-C levels (81.8%), hypertriglyceridemia (54.5%), high blood pressure (72.7%) and hyperglycemia (18.2%). Overall, 28 /55 subjects (50.9 %) had one risk factor for metabolic syndrome and 15/55 (27.3%) had two risk factors. Twenty percent (11/55) of survivors (10 male, 1 female) met criteria for diagnosis of metabolic syndrome. The prevalence of metabolic syndrome was higher in male than female (30% Vs 4.5%, p = 0.02). Obesity was observed in 14/55 (25%) patients (confidence interval 95% CI: 6.74-11.87) whereas 11/55 (20%) were overweight. Seventy-one percent (10/14) of obese patients had metabolic syndrome with at least three criteria of modified ATP III. The most common component of metabolic synrome in obese survivors was high blood pressure (80 %), low HDL-C levels (80%) and high triglyceride (50%). The prevalence of metabolic syndrome was higher in patients who received chemotherapy with cranial radiotherapy versus chemotherapy alone 26% vs. 12% respectively (p = 0.15). Comparison of metabolic syndrome parameters between ALL survivors who received chemotherapy with cranial radiation (group1) and those who received chemotherapy alone (group 2) is shown in table 2. High serum insulin levels were found in 16% of participants and 63% of obese survivors. Insulin resistance was detected in 72.7% of survivors with metabolic syndrome.
Table 1.
Mean values of BMI, blood pressure, triglycerides, HDL, LDL, glucose and insulin in survivors of childhood ALL with and without metabolic syndrome
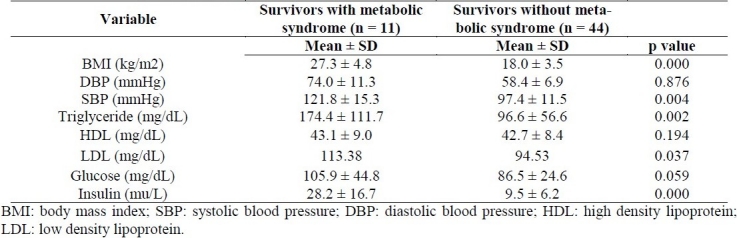
Table 2.
Comparison of metabolic syndrome parameters between ALL survivors who received chemotherapy with cranial radiation (group1) and those who received chemotherapy alone (group 2)
BMI was positively correlated with systolic and diastolic BP (0.545, 0.651, p < 0.001), fasting insulin (0.728, p < 0.001), serum triglycerides (0.384, p = 0.004) and blood sugar (0.037, p = 0.791) whereas it was inversely correlated with HDL-C (-0.020, p = 0.885). HOMA index was positively correlated with serum triglycerides (0.543, p ≤ 0.001), systolic and diastolic BP (0.348, p = 0.01 and 0.368, p = 0.06 respectively), insulin levels (0.914, p < 0.001) and blood sugar (0.398, p = 003).
Discussion
ALL is the most common childhood malignancy.1 With current chemotherapy regimens, prolonged survival is anticipated in approximately 80% of patients.3 However, treatment of ALL has a number of long-term sequels including obesity, the metabolic syndrome, secondary malignancies, cardiotoxicity, growth and puberty disorders, and educational and psychological dysfunction. Our results indicate an increased frequency of metabolic syndrome in long-term survivors of childhood ALL. Obesity was observed in one forth of children and adolescents previously treated for ALL. Nearly 3/4 of obese patients had metabolic syndrome. The metabolic syndrome was present very early after completion of ALL therapy.
The prevalence of both the metabolic syndrome and its components may influence by differences in genetic background, dietary habits, levels of physical activity, population age and sex structure and levels of over and under-nutrition.16 In the developing countries, the overall prevalence of the metabolic syndrome in healthy children and adolescents based on modified ATP III criteria ranges between 2.2-14.1%.17–20 The prevalence of metabolic syndrome was higher in overweight and obese children in this study as was reported by Goodman and crutz.12,21 In Kourti et al study the metabolic syndrome prevalence in young ALL survivors was 5.76%.22 This rate is much lower than the overall metabolic syndrome prevalence of Trimis et al16 patients (11%). However, Trimis et al found that this rate was higher in patients who received combination of chemotherapy and cranial irradiation (22%) versus chemotherapy alone (8%).6 In contrast, in our study the overall prevalence of metabolic syndrome was found quite high (20%). Whether differences in genetic predisposition or mode of treatment can explain this needs further investigation. Likewise, in our study the prevalence of metabolic syndrome was higher in patients who received combination of chemotherapy and cranial irradiation versus chemotherapy alone.
Obesity is a well known adverse consequence of ALL and an unusually high proportion of survivors are overweight and obese.6,22 Children treated for ALL gain weight from the time of diagnosis and it continues well beyond the end of treatment.23 A number of risk factors predisposed the patients to excess weight gain, including a low BMI standard deviation score at diagnosis, younger age at diagnosis, gender, cranial radiotherapy and reduced total energy expenditure secondary to reduced habitual physical activity.22–24 Our results are in accordance with other studies that documented an increased prevalence of obesity in the initial years of follow-up among survivors of child-hood ALL.
It was shown that GH deficiency was strongly associated with cranial radiation treatment in young ALL survivors who presented with an abnormal pattern of serum lipids and obesity.6,25 Abnormally low GH was also associated with higher fasting insulin, and higher HOMA index.26 In our study, nearly one forth of children who received cranial radiotherapy developed metabolic syndrome. One of the limitations of this study is the lack of control group. In addition, we did not measure the GH level.
In Talvensaari study,13 a higher fasting plasma insulin level was seen in survivors of child-hood cancer than compared to a control group. In the present study, fasting plasma insulin level was significantly higher in children with metabolic syndrome than those without. Hyperinsulinemia in the survivors of ALL may be partly due to obesity and partially secondary to hepatotoxic effect of chemotherapy. Synthesis of insulin-like growth factor binding protein-1(IGFBP-1) and sex hormone binding globulin (SHBG) in the liver has been shown to be regulated by insulin and hyperinsulinemia is associated with reduced levels of these proteins after chemotherapy.27,28 Obesity is a strong independent risk factor for insulin resistance.13 In our study, insulin resistance was detected in nearly 3/4 of survivors with metabolic syndrome and all of them were obese.
In conclusion, metabolic syndrome in survivors of childhood leukemia in Iran is not uncommon. Nearly all of the obese patients had metabolic syndrome. Early identification of metabolic syndrome among survivors of childhood leukemia can reduce the risk of cardiovascular morbidity and mortality. Weight control and regular physical exercise should be emphasized in their follow-up.
Authors‘ Contributions
NR carried out the design and coordinated the study, participated in most of the experiments and participated in manuscript preparation.
AAz provided assistance in the design, coordinated the study and prepared the manuscript.
AAm provided assistance in the design and experiments and data collection.
AM provided assistance in the design and experiments and data collection.
MH provided assistance in the design and experiments.
PR provided assistance in the statistical issue of the research.
All authors have read and approved the content of the manuscript.
Footnotes
Conflict of Interests
The authors have no conflicts of interest.
References
- 1.Kadan-Lottick NS. Cancer and Benign tumor. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Saunders: Nelson textbook of pediatrics; 2007. pp. 2097–100. [Google Scholar]
- 2.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. National Cancer Institute; 2005. SEER cancer statistics review, 1975-2002. [Google Scholar]
- 3.Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–8. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 4.Davies HA. Late problems faced by childhood cancer survivors. Br J Hosp Med. 1993;50(2-3):137–40. [PubMed] [Google Scholar]
- 5.Gleeson HK, Shalet SM. Endocrine complications of neoplastic diseases in children and adolescents. Curr Opin Pediatr. 2001;13(4):346–51. doi: 10.1097/00008480-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Trimis G, Moschovi M, Papassotiriou I, Chrousos G, Tzortzatou-Stathopoulou F. Early indicators of dysmetabolic syndrome in young survivors of acute lymphoblastic leukemia in childhood as a target for preventing disease. J Pediatr Hematol Oncol. 2007;29(5):309–14. doi: 10.1097/MPH.0b013e318059c249. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8(5):299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 9.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110(16):2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 10.Lambert M, Paradis G, O'Loughlin J, Delvin EE, Hanley JA, Levy E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int J Obes Relat Metab Disord. 2004;28(7):833–41. doi: 10.1038/sj.ijo.0802694. [DOI] [PubMed] [Google Scholar]
- 11.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr. 2008;152(2):165–70. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4(1):53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- 13.Talvensaari KK, Lanning M, Tapanainen P, Knip M. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J Clin Endocrinol Metab. 1996;81(8):3051–5. doi: 10.1210/jcem.81.8.8768873. [DOI] [PubMed] [Google Scholar]
- 14.Ataei N, Aghamohammadi A, Yousefi E, Hosseini M, Nourijelyani K, Tayebi M, et al. Blood pressure nomograms for school children in Iran. Pediatr Nephrol. 2004;19(2):164–8. doi: 10.1007/s00467-003-1275-1. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Pitsavos C, Panagiotakos D, Weinem M, Stefanadis C. Diet, exercise and the metabolic syndrome. Rev Diabet Stud. 2006;3(3):118–26. doi: 10.1900/RDS.2006.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HM, Park J, Kim HS, Kim DH. Prevalence of the metabolic syndrome in Korean adolescents aged 12-19 years from the Korean National Health and Nutrition Examination Survey 1998 and 2001. Diabetes Res Clin Pract. 2007;75(1):111–4. doi: 10.1016/j.diabres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Esmaillzadeh A, Mirmiran P, Azadbakht L, Etemadi A, Azizi F. High prevalence of the metabolic syndrome in Iranian adolescents. Obesity (Silver Spring) 2006;14(3):377–82. doi: 10.1038/oby.2006.50. [DOI] [PubMed] [Google Scholar]
- 19.Agirbasli M, Cakir S, Ozme S, Ciliv G. Metabolic syndrome in Turkish children and adolescents. Metabolism. 2006;55(8):1002–6. doi: 10.1016/j.metabol.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Kelishadi R, Ardalan G, Gheiratmand R, Adeli K, Delavari A, Majdzadeh R, et al. Paediatric metabolic syndrome and associated anthropometric indices: the CASPIAN Study. Acta Paediatr. 2006;95(12):1625–34. doi: 10.1080/08035250600750072. [DOI] [PubMed] [Google Scholar]
- 21.Goodman E, Dolan LM, Morrison JA, Daniels SR. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation. 2005;111(15):1970–7. doi: 10.1161/01.CIR.0000161957.34198.2B. [DOI] [PubMed] [Google Scholar]
- 22.Odame I, Reilly JJ, Gibson BE, Donaldson MD. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1994;71(2):147–9. doi: 10.1136/adc.71.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warner JT, Gregory JW, Webb DK. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1995;72(1):97. doi: 10.1136/adc.72.1.97-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ventham JC, Reilly JJ. Childhood leukaemia: a model of pre-obesity. Proc Nutr Soc. 1999;58(2):277–81. doi: 10.1017/s0029665199000385. [DOI] [PubMed] [Google Scholar]
- 25.Jarfelt M, Bjarnason R, Lannering B. Young adult survivors of childhood acute lymphoblastic leukemia: spontaneous GH secretion in relation to CNS radiation. Pediatr Blood Cancer. 2004;42(7):582–8. doi: 10.1002/pbc.20020. [DOI] [PubMed] [Google Scholar]
- 26.Gurney JG, Ness KK, Sibley SD, O’Leary M, Dengel DR, Lee JM, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107(6):1303–12. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 27.Suikkari AM, Koivisto VA, Rutanen EM, Yki-Jarvinen H, Karonen SL, Seppala M. Insulin regulates the serum levels of low molecular weight insulin-like growth factor-binding protein. J Clin Endocrinol Metab. 1988;66(2):266–72. doi: 10.1210/jcem-66-2-266. [DOI] [PubMed] [Google Scholar]
- 28.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67(3):460–4. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]


