Abstract
Abstract Induction of the protective heat shock proteins (Hsps), and of Hsp72 in particular, has been reported to be decreased in certain tissues from aged animals. To determine if both fast and slow skeletal muscles from aged animals demonstrate an altered ability to induce and accumulate Hsp72, adult (age, 6 months) and aged (age, 20 months) Fischer 344 rats were subjected to heat stress. At selected times (0, 1, 3, and 24 hours) after a 10-minute, 41°C heat stress, fast (white gastrocnemius [WG]) and slow (soleus) skeletal muscles were examined for either heat shock transcription factor (HSF) activation (trimerization and DNA-binding activity) or Hsp72 content using electrophoretic gel mobility shift assays and Western blotting, respectively. Immediately after heat stress, the level of HSF activation between aged and adult animals was similar for both muscles. HSF activation was undetectable at 1 and 3 hours after heat stress in all cases. Twenty-four hours after heat stress, Hsp72 content in the WG muscles from both aged and adult animals was significantly increased compared with unstressed, age-matched controls (P < 0.05). In contrast, perhaps because of their high constitutive Hsp72 levels, soleus muscles from both aged and adult animals did not demonstrate a significant increase in Hsp72 content after heat shock, but there was a trend toward increased levels. Hsp72 content in both the soleus and WG muscles demonstrated no significant differences between adult and aged animals in either the unstressed state (controls) or after heat shock. These results suggest that skeletal muscles from aged animals are capable of inducing the heat shock response and accumulating Hsp72.
INTRODUCTION
All cells respond to heat and other protein-damaging stresses by the rapid synthesis of “stress” or “heat shock proteins” (Hsps). Overexpression of Hsps, and of Hsp72 in particular, has been shown to protect cells and tissues during episodes of stress (Johnston and Kucey 1988; Riabowol et al 1988; Karmazyn et al 1990; Li et al 1991; Plumier et al 1995). The exact mechanism by which Hsps provide protection remains unknown, but it is thought to relate to their ability to act as molecular chaperones.
Stress-induced transcriptional regulation of Hsps is mediated by activation and binding of the heat shock transcription factor (HSF1) to a specific DNA sequence located upstream from all hsp genes and known as the heat shock element (HSE; Amin et al 1988). In unstressed cells, HSF1 exists as inactive, non–DNA-binding monomers, but after exposure to proteotoxic stresses, HSF1 monomers form DNA-binding trimers capable of binding to the HSE (Sarge et al 1993). This process is referred to as HSF activation, and it can be assessed by electrophoretic mobility shift assays.
Induction of Hsps has been shown to confer protection to cells and tissues from both young and adult animals, but cells and tissues from aged animals have demonstrated a diminished hsp induction and thus a diminished protection (Blake et al 1991; Heydari et al 1993; Nitta et al 1994, Locke and Tanguay 1996b). For example, when both adult and aged animals were heat stressed and allowed to recover, the hearts from the aged animals demonstrated reduced HSF activation, decreased Hsp72 expression, and lack of myocardial protection (Locke and Tanguay 1996b). The decreased ability of aged cells and tissues to mount a stress response and thus synthesize the protective Hsps may render aged organisms more susceptible to certain stresses. Thus, to determine if skeletal muscles from aged animals also demonstrate a diminished ability to induce the protective heat shock response and accumulate the protective Hsps, both fast (white gastrocnemius [WG]) and slow (soleus) skeletal muscles from aged and adult animals were assessed for HSF activation and Hsp72 accumulation after a 10-minute, 41°C heat shock.
MATERIALS AND METHODS
Animals and heat shock
Adult (age, 5–6 months) and aged (age, 21–22 months), barrier-reared, male Fischer 344 rats (National Institute on Aging) were used in these experiments. All experiments and procedures were approved by the Animal Care Committee of the University of Toronto. Animals were maintained on a 12-hour dark/light cycle, housed at 20 ± 1°C and 50% relative humidity, and fed and watered ad libitum. Animals subjected to heat shock were anesthetized with sodium pentobarbital (65 mg/kg Intraperitoneal) and placed on a heating plate until the rectal temperature reached 0.5°C less than the desired temperature. During heat shock, rectal temperature was carefully maintained within 0.5°C of the desired temperature (40, 41, or 42°C) for 10 minutes. Before and throughout the entire heat stress, the rectal temperature was measured using a Thermistor TSD 102C Probe (Santa Barbara, CA, USA) Thermistor connected to a Biopac data acquisition system (Santa Barbara, CA, USA). In the experiments that required recovery after heat shock (10 minutes at 41°C), animals were cooled and revived using oral administration of water. At selected times after heat shock (0, 1, 3, and 24 hours), animals were anesthetized with sodium pentobarbital (65 mg/kg ip) if needed, and the relevant muscles were removed and quickly frozen in liquid nitrogen.
Preparation of protein extracts
Protein extracts were prepared according to the method described by Mosser et al (1988). Briefly, portions of skeletal muscles were thawed and homogenized in 15 volumes of extraction buffer (25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid [EDTA; pH, 8.0], 20 mM HEPES [pH, 7.9], 0.5 mM Dithiothreitol, and 0.5 mM phenylmethylsufonylfluoride) at 4°C and 5000 rpm using a Ultra-Turrax T8 (IKA Labortechnik, Staufen, Germany). Tissue lysates were centrifuged at 14 000 rpm at 4°C (16 000 × g) for 20 minutes in an Eppendorf centrifuge (Eppendorf-Brinkman Instruments, Mississaugo, Ontario, Canada). The supernatant was then removed and stored at −70°C.
Mobility shift analyses
Analyses of HSF-HSE binding in extracts was performed according to the procedure described by Locke and Tanguay (1996a). Protein extracts (50 μg) from control and heat shocked rat skeletal muscles were incubated with a 32P-labeled, self-complementary, ideal HSE oligonucleotide (5′-CTA GAA GCT TCT AGA AGC TTC TAG-3′) in binding buffer (10% glycerol, 50 mM NaCl, 1.0 mM EDTA [pH, 8.0], 20 mM Tris [pH, 8.0], 1.0 mM DTT, 0.3 mg/mL bovine serum albumin) with 0.1 ng (50 000 cpm) of 32P-labeled oligonucleotide and 5.0 μg poly (dI dC) (Pharmacia Fine Chemicals, Piscataway, NJ, USA) for 30 minutes at room temperature. Samples were electrophoresed on 4% acrylamide gel at 200 V for 2–3 hours. Gels were then dried and exposed to radiographic film (Amersham-ECL, Mississauga, Ontario, Canada). HSF activation was assessed using band shifts, and correct HSF-HSE interaction was confirmed by incubating extracts with a 200-fold molar excess of unlabeled HSE as described by Locke et al (1995).
Polyacrylamide gel electrophoresis and immunoblotting
Muscle portions were homogenized in 600 mM NaCl and 15 mM Tris (pH, 7.5), and the protein concentration determined using the method of Lowry et al (1951). One dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method described by Laemmli (1970), except that the separating gel (0.15 × 4.5 × 8 cm) consisted of a 5%–15% polyacrylamide gradient. After electrophoretic separation, proteins were transferred to nitrocellulose membranes (thickness, 0.22 μm; Bio-Rad Laboratories) as described by Towbin et al (1979) using the Bio-Rad miniprotean II gel transfer system (Mississauga, Ontario, Canada). After protein transfer, blots were reacted with a polyclonal antibody (SPA-812; Stress-Gen, Victoria, Canada) specific for Hsp72 and diluted to 1:2500 in Tris buffered saline + 0.05% Tween-20 with 2% non fat dried milk as described by Locke et al (1995). To determine nonspecific binding, duplicate gels were run, transferred, and reacted, but the primary antibody was omitted. Immunoblots or autoradiograms were scanned using an Agfa Arcus II scanner (Woburn, MA, USA), and quantification of bands from the immunoblots or exposed film was performed using Kodak 1D Image Analysis Software (Kodak Scientific Imaging Systems, New Haven, CT, USA). Standard curves were constructed to assure linearity.
Statistical analyses
Data obtained from the aged and adult groups were analyzed using an unpaired Student's t-test. For cases in which 4 groups were used, data were analyzed by analysis of variance and then the Tukey's post hoc test. In all cases, the level of significance was set at P < 0.05.
RESULTS
Physical characteristics and thermal response during heat shock
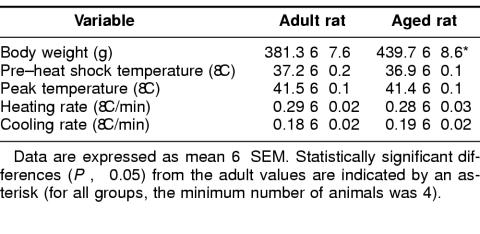
Aged animals demonstrated a greater body weight (P < 0.05) than adult animals (Table 1). No differences, however, were detected in the thermal responses of adult and aged animals. Pre–heat shock rectal temperatures were 37.2 ± 0.2°C for adult animals and 36.9 ± 0.1°C for aged animals, whereas peak temperatures were 41.5 ± 0.1°C for adult animals and 41.4 ± 0.1°C for aged animals. Heating rates for adult and aged animals were similar (0.29 ± 0.02°C/min vs 0.28 ± 0.03°C/min, respectively), as were cooling rates (0.18 ± 0.02°C/min vs 0.19 ± 0.02°C/min, respectively). These results indicate that even though aged animals had a greater body weight, both adult and aged animals experienced a similar thermal stress during heat shock.
Table 1.
Physical characteristics and thermal responses of adult and aged Fischer 344 rats
HSF activation in aged skeletal muscle
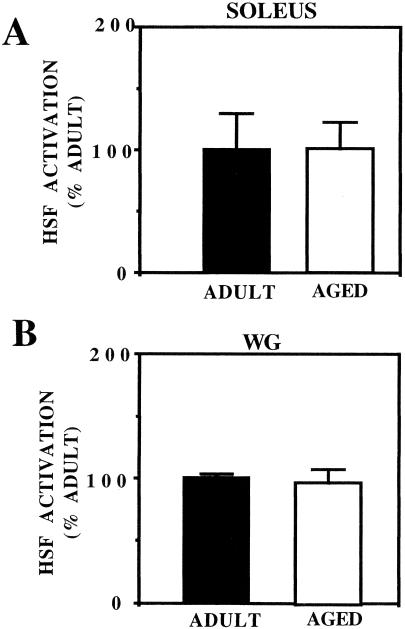
The term HSF activation is used to describe the HSF trimer binding to the HSE. HSF activation in protein extracts from control and heat shocked adult and aged rat skeletal muscles was assessed using mobility shift–polyacrylamide gel electrophoresis (MS-PAGE). No HSF activation was detected in extracts from control adult or aged rat soleus muscles (Fig 1; lanes 2 and 6, respectively). In addition, no HSF activation was detected in extracts from control adult or aged rat WG muscles (Fig 2; lanes 2 and 6, respectively). HSF activation was detected in extracts from adult rat soleus muscles from animals that were heat shocked for 10 minutes to either 40, 41, or 42°C (Fig 1; lanes 3, 4, and 5, respectively) and in extracts from aged rat soleus muscles from animals that were heat shocked for 10 minutes to either 40, 41, or 42°C (Fig 1; lanes 7, 8, and 9, respectively). Similarly, HSF activation was detected in extracts from adult rat WG muscles from animals that were heat shocked for 10 minutes to either 40, 41, or 42°C (Fig 2; lanes 3, 4, and 5, respectively) and in extracts from aged rat WG muscles from animals that were heat shocked for 10 minutes to either 40, 41, or 42°C (Fig 2; lanes 7, 8, and 9, respectively). The level of HSF activation in aged rat soleus and WG muscles at any given temperature was similar to that observed in the corresponding adult rat muscles. When HSF bands from autoradiograms were quantified by densitometry, the level of HSF activation in aged soleus and WG muscles was not significantly different from that in adult muscles (Fig 3). These results suggest that both fast and slow muscles from aged animals are equally capable of activating HSF.
Fig 1.
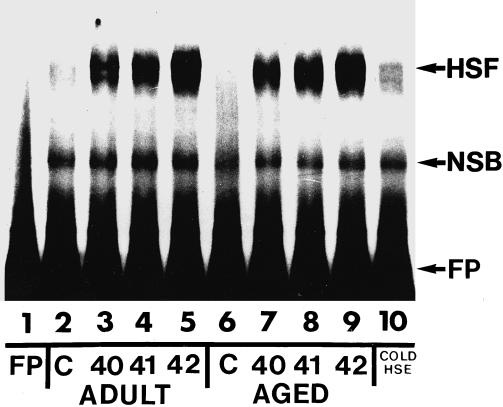
Heat shock factor activation in adult and aged rat soleus muscles after heat shock. Protein extracts from adult and aged rat soleus muscles were incubated with a 32P-labeled heat shock element (HSE) and analyzed by mobility shift–polyacrylamide gel electrophoresis as described in the text. Lane 1: free probe (FP); lane 2: unstressed (control) adult rat soleus muscle; lane 3: adult rat soleus muscle after a 10-minute, 40°C heat shock; lane 4: adult rat soleus muscle after a 10-min, 41°C heat shock; lane 5: adult rat soleus muscle after a 10-minute, 42°C heat shock; lane 6: unstressed (control) aged rat soleus muscle; lane 7: aged rat soleus muscle after a 10-minute, 40°C heat shock; lane 8: aged rat soleus muscle after a 10-minute, 41°C heat shock; lane 9: aged rat soleus muscle after a 10-minute, 42°C heat shock; lane 10: protein extract from aged rat soleus muscle after a 10-minute, 42°C heat shock incubated with a 200-fold molar excess of unlabeled HSE. HSF, heat shock transcription factor complex; NSB, non–specific-binding
Fig 2.
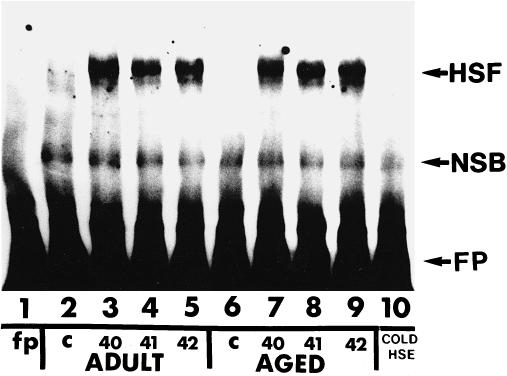
Heat shock factor activation in adult and aged rat white gastrocnemius muscles after heat shock. Protein extracts from white gastrocnemius muscles were incubated with a 32P-labeled heat shock element (HSE) and analyzed by mobility shift–polyacrylamide gel electrophoresis as described in the text. Lane 1: free probe (FP); lane 2: unstressed (control) adult rat white gastocnemius muscle; lane 3: adult rat white gastocnemius muscle after a 10-minute, 40°C heat shock; lane 4: adult rat white gastocnemius muscle after a 10-minute, 41°C heat shock; lane 5: adult rat white gastocnemius muscle after a 10-minute, 42°C heat shock; lane 6: unstressed (control) aged rat white gastocnemius muscle; lane 7: aged rat white gastocnemius muscle after a 10-minute, 40°C heat shock; lane 8: aged rat white gastocnemius muscle after a 10-minute, 41°C heat shock; lane 9: aged rat white gastocnemius muscle after a 10-minute, 42°C heat shock; lane 10: protein extract from aged rat white gastocnemius muscle after a 10-minute, 42°C heat shock incubated with a 200-fold molar excess of unlabeled HSE. HSF, heat shock transcription factor complex; NSB, non–specific-binding
Fig 3.
Graphic representation of the densitometric scans from autoradiograms similar to those presented in Figures 2 and 3. Data are expressed as mean ± SEM (n = 4 for all groups). (A) Soleus muscle. (B) White gastrocnemius muscle
To determine if the time course of HSF activation and inactivation differed in the aged muscles, HSF activation was also assessed at 1 and 3 hours after heat shock. MS-PAGE analyses of skeletal muscle extracts from adult and aged rats that were heated to 41°C for 10 minutes and then allowed to recover demonstrated that HSF activation was only detectable directly after heat shock and not at 1 or 3 hours after heat shock in either adult or aged rat soleus and WG muscles (Fig 4; lanes 3 and 7, respectively). These results demonstrate that the kinetics of HSF activation and inactivation are similar in both the soleus and WG muscles from adult and aged rats.
Fig 4.
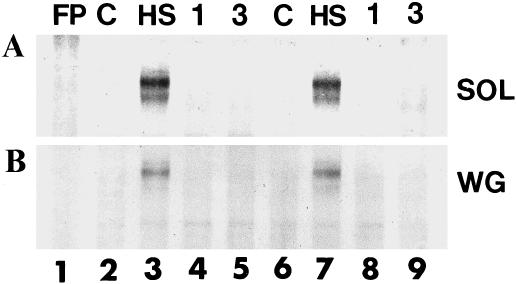
Heat shock factor inactivation follows similar kinetics in aged and adult skeletal muscles. Protein extracts from aged and adult white gastrocnemius and soleus muscles were incubated with a 32P-labeled heat shock element and analyzed by mobility shift–polyacrylamide gel electrophoresis as described in the text. Lane 1: free probe; lane 2: unstressed (control) adult rat skeletal muscle; lane 3: adult rat skeletal muscle after a 10-minute, 41°C heat shock; lane 4: adult rat skeletal muscle 1 hour after a 10-minute, 41°C heat shock; lane 5: adult rat skeletal muscle 3 hours after a 10-minute, 41°C heat shock; lane 6: unstressed (control) aged rat skeletal muscle; lane 7: aged rat skeletal muscle after a 10-minute, 41°C heat shock; lane 8: aged rat skeletal muscle 1 hour after a 10-minute, 41°C heat shock; lane 9: aged rat skeletal muscle 3 hours after a 10-minute, 41°C heat shock. (A) Soleus muscle. (B) White gastrocnemius muscle
Hsp72 protein accumulation after heat shock
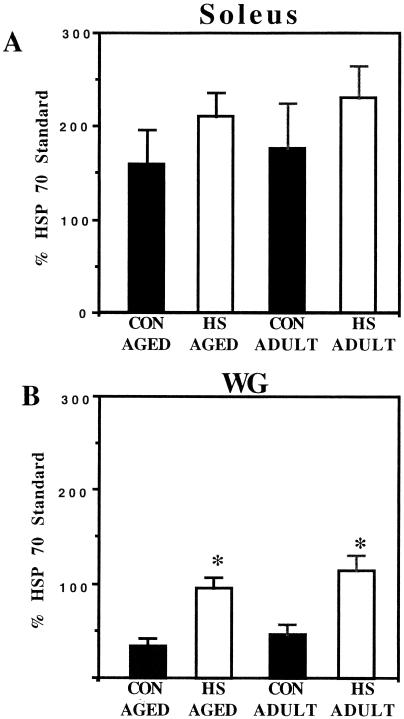
To determine the Hsp72 content in skeletal muscles from unstressed and heat shocked (24 hours after 10 minutes at 41°C) adult and aged animals (n = 4 for each group), portions of the soleus and WG muscles were analyzed for Hsp72 (the inducible member of the Hsp70 family) by Western blotting. Representative Western blots containing the SDS-PAGE–separated proteins from aged skeletal muscles (soleus and WG) reacted with antibody for Hsp72 are shown in Figure 5. Hsp72 content in the soleus muscles from unstressed, control aged and adult animals was readily detectable (Fig 5A; lanes 1 and 3, respectively). Twenty-four hours after heat shock, the Hsp72 content in soleus muscles from adult and aged animals was only slightly increased. When similar blots were quantified by densitometric scanning, the Hsp72 content in the soleus from heat shocked aged and adult animals was not elevated compared with that in unstressed, age-matched controls (Fig 6A). A trend toward increased Hsp72 content, however, was apparent.
Fig 5.
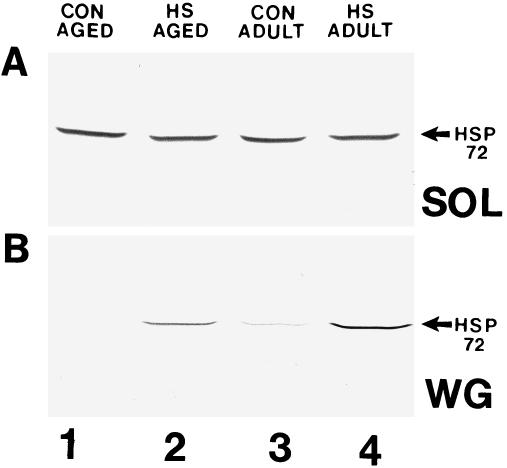
The amount of Hsp72 increases to a similar extent in adult and aged rat skeletal muscles after heat shock. Proteins from muscles were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and reacted with a Hsp72-specific antibody as described in the text. Lane 1: unstressed (control) aged rat skeletal muscle; lane 2: heat shocked aged rat skeletal muscle; lane 3: unstressed (control) adult rat skeletal muscle; lane 4: heat shocked adult rat skeletal muscle. (A) Soleus muscle. (B) White gastrocnemius muscle
Fig 6.
Graphic representation of the densitometric scans from blots similar to those presented in Figure 5. Data are expressed as mean ± SEM. Statistical significance is indicated by an asterisk (n = 4 for all groups). (A) Soleus muscle. (B) White gastrocnemius muscle
The Hsp72 content in WG muscles from aged and adult, unstressed control animals was barely detectable (Fig 5B; lanes 1 and 3, respectively). At 24 hours after heat shock, however, the Hsp72 content in WG muscles from aged and adult animals was greatly increased (Fig 5B; lanes 2 and 4, respectively). When similar blots were quantified by densitometric scanning, the Hsp72 content in WG muscles from heat shocked aged and adult animals was elevated at least 2-fold compared with the content in unstressed, age-matched controls (P < 0.05; Fig 6B). There was no statistically significant difference between unstressed adult and aged animals in the amount of Hsp72 in the WG muscles, nor was there any statistically significant difference between aged and adult animals in the amount of Hsp72 accumulated in the WG muscles 24 hours after heat shock (Fig 6B). These results suggest that the WG muscle in aged animals is capable of accumulating Hsp72 to a similar extent as that observed in adult animals, and that both fast and slow muscles from aged and adult animals respond in a similar manner to heat stress. In addition, it also suggests that slow muscles, such as the soleus, may not accumulate Hsp72 to the same magnitude as that observed in fast muscles, such as the WG.
DISCUSSION
Cells and tissues from aged animals have been reported to exhibit decreased Hsp induction and expression after heat shock (Liu et al 1989; Choi et al 1990; Fargnoli et al 1990; Blake et al 1991; Heydari et al 1993; Nitta et al 1994; Kregal et al 1995; Locke and Tanguay 1996b). The diminished ability to induce Hsps in aged cells and tissues appears to result from decreased HSF activation (Heydari et al 1993; Locke et al 1996). The novel feature of this study is that after heat shock, both fast and slow skeletal muscles from aged animals demonstrated similar HSF activation and Hsp72 accumulation as the same muscles from adult animals. These results may appear to contrast with those of previous studies (Liu et al 1989; Choi et al 1990; Fargnoli et al 1990; Blake et al 1991; Heydari et al 1993; Nitta et al 1994; Kregal et al 1995), but a number of factors may explain these apparent differences. First, most studies that have examined the heat shock response and aging have used cultured cells or cells removed from aged animal tissues (Liu et al 1989; Choi et al 1990; Fargnoli et al 1990; Heydari et al 1993), but the in vivo heat shock response has been reported to lack the coordinated control that is characteristic of cultured cell populations (Blake et al 1990; Locke and Tanguay 1996a). Thus, it remains possible, and even probable, that mitigating physiologic factors such as redundant controls are at play in the intact animal. Second, the heat shock response is known to be tissue (Blake et al 1991; Tanguay et al 1993) and even muscle specific (Locke and Tanguay, 1996b). To our knowledge, no studies have examined the heat shock response in aged skeletal muscle after whole-animal heat shock. Thus, terminally differentiated skeletal muscle cells and fibers may be unique in their ability to maintain a heat shock response with increasing age. In this study and others (Locke and Tanguay, 1996a), Hsp72 content before heat shock and Hsp72 accumulation after heat shock demonstrated a muscle-specific pattern of expression. Thus, Hsp72 may be activated and accumulate differently in skeletal muscles than in other tissues and cells. Lastly, because heat shock response can be influenced by a number of different factors, including specific proteins involved in negative regulation (Satyal et al 1998), it remains possible that age- and/or tissue-specific alterations in the content of such proteins may influence the ability of certain cells and tissues to mount a heat shock response and thus accumulate Hsps.
In this study, a high constitutive level of Hsp72 was detected in the slow soleus muscles from both aged and adult animals, whereas a low level of Hsp72 expression was observed in the fast WG muscles from both aged and adult animals. The exact reason (or reasons) for these muscle-specific differences in Hsp72 expression remains unknown, but muscles such as the soleus, which are constantly being recruited and thus continually “stressed,” may require a greater level of protection or chaperone function. In contrast, muscles such as the WG, which are only occasionally recruited or “stressed,” may require a lower level of the protective Hsps.
In rat tissues, heat shock and the concomitant induction of the “protective” Hsps, and of Hsp72 in particular, have been associated with cellular protection (Currie et al 1988, 1993; Karmazyn et al 1990; Locke et al 1996b). In the heart, a direct correlation between Hsp72 content and protection has been observed (Hutter et al 1994). Thus, it would seem to follow that an increased Hsp72 content in skeletal muscle may also provide a similar level of protection. Indeed, a previous heat shock treatment has been shown to confer a significant biochemical protection to rat muscle against ischemic injury (Garramone et al 1994). After heat shock in this study, however, there was only a slight increase in the Hsp72 content of the soleus muscles, whereas there was a robust increase in the Hsp72 content of the WG muscles. The exact reason (or reasons) for this differential induction of Hsp72 remains to be determined, but muscles with a high constitutive Hsp72 content before heat stress may not require the same amount of Hsp72 protein to be synthesized as muscles with a low constitutive Hsp72 content. In agreement with this are studies showing that cells overexpressing Hsp70 demonstrate a reduced HSF activation and subsequent Hsp70 accumulation when heat shocked (Baler et al 1992; Mosser et al 1993). These studies suggest a regulatory mechanism in which cells and tissues accumulate Hsp72 to a certain level, which then feeds back to shut down further Hsp72 production. It remains possible that such a mechanism is operating in skeletal muscle. According to the proposed Hsp72 feedback mechanism, tissues with a high constitutive Hsp72 content, such as the soleus muscle, would require a greater absolute stress to experience the same relative level of stress as tissues with a low constitutive Hsp72 content, such as the WG muscle. This may explain why even though there was a trend toward an elevated Hsp72 content in adult and aged soleus muscles after heat shock, the Hsp72 content was not significantly different from that in “unstressed” controls.
In conclusion, this study suggests that after heat shock, the ability to activate the protective hsp72 gene and accumulate the major protective Hsp protein is not reduced in aged skeletal muscle. Similar levels of HSF activation and Hsp72 accumulation were observed in aged and adult rat fast and slow skeletal muscles, suggesting that the ability to mount a stress response and accumulate Hsps is not compromised in aged skeletal muscle. This implies that aged skeletal muscles may have little or no impairment in translating stress signals into the biochemical steps necessary for induction of the stress response. In addition, aged skeletal muscles may be capable of coping with disturbances to homeostasis from physiologically relevant stresses, such as hyperthermia or strenuous exercise.
Acknowledgments
The work was supported by Natural Science and Engineering Research Council of Canada grant OGP0183742.
REFERENCES
- Amin J, Anathan J, Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988;8:3761–3769. doi: 10.1128/mcb.8.9.3761.0270-7306(1988)008<3761:KFOHSR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler R, Welch WJ, Voellmy R. Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp 70 as a potential autoregulatory factor. J Cell Biol. 1992;117:1141–1159. doi: 10.1083/jcb.117.6.1151.0021-9525(1992)117<1141:HSGRBN>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Fargnoli J, Gershon D, and Holbrook NJ 1991 Concomitant decline in heat-induced hyperthermia and HSP70 messenger RNA expression in aged rats. Am J Physiol. 260 (Reg Integ Comp Physiol 29). R663–R667. [DOI] [PubMed] [Google Scholar]
- Blake MJ, Gershon D, Fargnoli J, Holbrook NJ. Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem. 1990;265:15275–15279.0021-9258(1990)265<15275:DEOHSP>2.0.CO;2 [PubMed] [Google Scholar]
- Choi H-S, Lin Z, Li B, Liu AY-C. Age-dependent decrease in the heat-inducible DNA sequence-specific binding activity in human diploid fibroblasts. J Biol Chem. 1990;265:18005–18011.0021-9258(1990)265<18005:ADDITH>2.0.CO;2 [PubMed] [Google Scholar]
- Currie RW, Karmazyn M, Kloc M, Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543.0009-7330(1988)063<0543:HSRIAW>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG Jr.. Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit skeletal muscles. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963.0009-7322(1993)087<0963:HSRALO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fargnoli J, Kunisada T, Fornace AJ Jr, Schneider EL, Holbrook NJ. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A. 1990;87:846–850. doi: 10.1073/pnas.87.2.846.0027-8424(1990)087<0846:DEOHSP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garramone RR, Winters RM, Das D, Deckers PJ. Reduction of skeletal muscle injury through stress conditioning using the heat shock response. Plast Reconstr Surg. 1994;93:1242–1247. doi: 10.1097/00006534-199405000-00021.0032-1052(1994)093<1242:ROSMIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13:2909–2918. doi: 10.1128/mcb.13.5.2909.0270-7306(1993)013<2909:EOHSPI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter MM, Sievers RE, Barbosa V, Wolfe CL. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein and the degree of myocardial protection. Circulation. 1994;89:355–360. doi: 10.1161/01.cir.89.1.355.0009-7322(1994)089<0355:HSPIIR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnston RN, Kucey BL. Competitive inhibition of HSP70 gene expression causes thermosensitivity. Science. 1988;242:1551–1554. doi: 10.1126/science.3201244.0036-8075(1988)242<1551:CIOHGE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Mailer K, and Currie RW 1990 Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 259 (Skeletal Muscle Circ Physiol 28) . H424–H431. [DOI] [PubMed] [Google Scholar]
- Kregal KC, Moseley PL, Skidmore R, Gutierrez JA, Guerriero V Jr.. HSP70 accumulation in tissues of heat-stressed rats is blunted with advancing age. J Appl Physiol. 1995;97:1673–1678. doi: 10.1152/jappl.1995.79.5.1673.8750-7587(1995)097<1673:HAITOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0.0028-0836(1970)227<0680:COSPDT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Li GC, Li LG, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts stabily transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991;88:1681–1685. doi: 10.1073/pnas.88.5.1681.0027-8424(1991)088<1681:TRORFS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Lin Y-CZ, Choi H-S, Sorhage F, Li B. Attenuated induction of heat shock gene expression in aging diploid fibroblasts. J Biol Chem. 1989;264:12037–12045.0021-9258(1989)264<12037:AIOHSG>2.0.CO;2 [PubMed] [Google Scholar]
- Locke M, Noble EG, Tanguay RM, Feild MR, Ianuzzo SE, and Ianuzzo CD 1995 Activation of the heat shock transcription factor in the rat skeletal muscle following heat shock and exercise. Am J Physiol. 268 (Cell Physiol 37). C1387–C1394. [DOI] [PubMed] [Google Scholar]
- Locke M, Tanguay RM. Increased HSF activation in muscles with a high constitutive HSP 70 expression. Cell Stress Chaperones. 1996a;1:189–196. doi: 10.1379/1466-1268(1996)001<0189:ihaimw>2.3.co;2.1355-8145(1996)001<0189:IHAIMW>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M, Tanguay RM. Diminished heat shock response in the aged myocardium. Cell Stress Chaperones. 1996b;1:251–260. doi: 10.1379/1466-1268(1996)001<0251:dhsrit>2.3.co;2.1355-8145(1996)001<0251:DHSRIT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275.0021-9258(1951)193<0265:PMWTFP>2.0.CO;2 [PubMed] [Google Scholar]
- Mosser DD, Duchaine J, Massie B. The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by Hsp70. Mol Cell Biol. 1993;13:5427–5438. doi: 10.1128/mcb.13.9.5427.0270-7306(1993)013<5427:TDBAOT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Theodorakis G, Morimoto RI. Coordinate changes in heat shock element-binding activity and HSP70 transcription rates in human cells. Mol Cell Biol. 1988;8:4736–4744. doi: 10.1128/mcb.8.11.4736.0270-7306(1988)008<4736:CCIHSE>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta Y, Abe K, Aoki M, Ohno I, and Isoyama S 1994 Diminished heat shock protein 70 mRNA induction in aged rat skeletal muscles after ischemia. Am J Physiol. 267 (Skeletal Muscle Circ Physiol 36). H1795–H1803. [DOI] [PubMed] [Google Scholar]
- Plumier J-C, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865.0021-9738(1995)095<1854:TMETHH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol KT, Mizzen LA, Welch WJ. Heat shock is lethal to fibroblasts microinjected with antibodies against HSP 70. Science. 1988;242:433–436. doi: 10.1126/science.3175665.0036-8075(1988)242<0433:HSILTF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor-1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392.0270-7306(1993)013<1392:AOHSGT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyal SH, Chen D, Fox SG, Kramer JM, Morimoto RI. Negative regulation of the heat shock transcriptional response by HSBP1. Genes Dev. 1998;12:1962–1974. doi: 10.1101/gad.12.13.1962.0890-9369(1998)012<1962:NROTHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–188. doi: 10.1002/dvg.1020140205.0192-253X(1993)014<0112:TSEOHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350.0027-8424(1979)076<4350:ETFPGT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]