Abstract
BACKGROUND:
Dental unit waterlines (DUWLs) are ideal environment for development of microbial biofilms. Microbial contamination of water in DUWLs is thought to be the result of biofilm formation as it could serves as a haven for pathogens. The aim of this study was to assess microbial quality of water in dental unit waterlines of dental units located at the dental school of Isfahan University of Medical Sciences.
METHODS:
Water samples were collected from air/water syringe and high-speed handpiece. Generally, 100-200 ml water samples were collected aseptically in sterile containers with sodium thiosulfate at the beginning of the day after a 2 minute purge. Samples were transferred to the laboratory in insulated box with cooling packs and examined for total viable heterotrophic bacteria and fungi.
RESULTS:
The heterotrophic plate count levels were significantly exceeded the American Dental Association recommendations for DUWL water quality (< 200 CFU/ml), in both air/water syringe (84%, CFU/ml: 500-20000) and high-speed handpiece (96%, CFU/ml: 710-36800) samples. However, there was no significant difference between the level of contamination in the air/water syringe and high-speed handpiece. Fungi were found in 28% and 36% of air/water syringe and high-speed handpiece samples, respectively; and filamentous fungi were the most frequently isolated fungi.
CONCLUSIONS:
DUWLs should be subjected to routine microbial monitoring and to a decontamination protocol in order to minimize the risk of exposure to potential pathogens from dental units.
Keywords: Biofilm, Dental Unit Waterlines, Microbial Quality, Water
Aquatic biofilms, which are well organized communities of microorganisms, are wide spread in the nature. They constitute a major problem in many environmental, industrial and medical settings.1 Special attention was given to the buildup of biofilm in dental unit waterlines (DUWLs), which are small-bore flexible plastic tubing to bring water to the different handpieces, namely the air/water syringe and the high-speed handpiece.2,3 The surfaces of DUWLs provide an ideal environment for developing of microbial biofilms.4,5
The source of microorganisms for biofilm in DUWLs may be 1) municipal water piped into the dental unit and 2) suck back of patient's saliva into the line due to the lack of preventive valves.1,6 It was known that microbial contamination of water in DUWLs is a result of biofilm formation.4,6,7 The bacteria isolated from the water in dental units (DU) included both environmental bacteria and opportunistic and true human pathogens 2 such as pseudomonas,4,6 leptospira,4 legionella pneumophila,6,8 Mycobacterium spp. 6 and Staphylococcus spp.4
Microbiologically contaminated water may be a risk factor for the dental team and patients, since they exposed to water and aerosols generated from dental units.3,9,10 This is particularly important in view of the increasing numbers of medically compromised and immunocompromised patients receiving regular dental treatment.6 For this reason, the water quality of DUWLs has considerable importance and according to the American Dental Association (ADA) recommendations, the microbial loading of dental unit water must be less than 200 colony forming units (CFU) per milliliter.6,11 The aim of this study was to determine the microbial quality of water from DUWLs.
Methods
Water samples were taken from air/water syringe and high-speed handpiece of 25 dental units at the dental school of Isfahan University of Medical Sciences, Isfahan, Iran. As Municipal water supplies all dental units, control samples also obtained from the nearest taps. Generally, 100-200 ml water samples were collected aseptically in sterile containers with sodium thiosulfate at the beginning of the day after a 2 minute purge. Samples were transferred to the laboratory in insulated box with cooling packs and immediately processed in the following way.
Bacteriological Analysis: for total heterotrophic bacteria, the samples were agitated by vortexing for 15 s and ten-fold serial dilutions (10-1 to 10-3) were prepared for each sample. From all, 200 μl volumes of undiluted and diluted samples were spread plated on R2A agar medium (Merck) and incubated at 35°C for 3-5 days. The number of colony forming units was determined in each plate after incubation.
Mycological Analysis: About 100 to 150 ml water samples was concentrated on 0.45 μm pore-size membrane filters. The filter placed in a screw-cap sterile container and washed by shaking in 5 ml of phosphate buffered saline for 10 minutes. Then 200 μl aliquots of suspension plated on sabouraud dextrose agar and the plates were incubated at 25°C for up to 5 days. After incubation, the fungi colonies were counted and identified on the basis of colony and morphological characteristics.
Statistical analysis was performed using SPSS software. Hypothesis of difference in the microbial quality of various samples was tested using t test. A p value of < 0.05 was considered significant.
Results
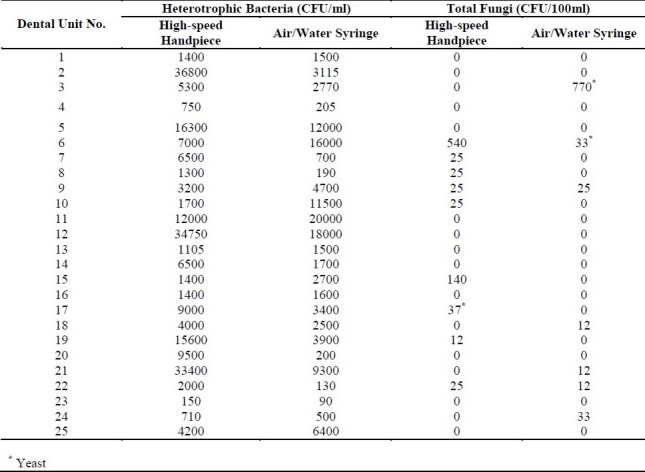
The quantitative bacterial analysis of samples collected from the waterlines of 25 dental units indicated that most of dental units (96%) under study delivered water that couldn't meet the accepted standard of American Dental Association for DU water quality (i.e. less than 200 CFU/ml). Table 1 shows the results of heterotrophic plate count (HPC) in CFU/ml for both air/water syringe and high-speed handpiece of different dental units. The results show %84 (500-20000 CFU/ml) and %96 (710-36800 CFU/ml) of water samples of air/water syringe and high-speed handpiece, respectively, were contaminated to different extents. The mean concentration of HPC in control samples was 190 CFU/ml and there was a significant difference between the HPC levels in control samples and dental unit waterlines. Fungi were found in 28% and 36% of air/water syringe and high-speed handpiece samples, respectively; and filamentous fungi were the most frequently isolated fungi (Table 1).
Table 1.
Mean concentration of heterotrophic bacteria and fungi in water from DUWLs
Discussion
The bacterial number reported here were comparable to those found in a number of other studies 2,6 and lower than one other report.9 There was no significant statistical difference between the level of contamination in the air/water syringe and high-speed handpiece. However, in some studies bacterial counts of water samples from high-speed handpiece issued higher mean HPC than the air/water syringe.2,5
In DUWLs biofilm there are always present primarily bacteria of saprophytic gram-negative species well adapted to growth in aquatic systems.1 In this study the predominant bacterial species recovered from the dental unit water samples also were gram-negative rods.
The number of colony forming fungi in the water samples from high-speed hand-piece and air/water syringe varied from 0 to 540 and 0 to 770 CFU/100ml, respectively. These results differ from those presented by Szymanska (2005), who has reported variation between 0 and 375 CFU/ml in water flowing from high-speed handpieces.12
Conclusions
The nature of DUWLs is such that they will develop a biofilm, and water flowing down the biofilm-coated waterlines will contribute to the microbial load in the water as it exits the tubing.1,5 The high levels of bacterial contamination found in this study indicate on the development of biofilm in the DUWLs. Thus, DUWLs should be subjected to routine microbial monitoring and to a decontamination protocol. Microbial control of water in DUWLs minimizes the risk of exposure to potential pathogens and creates a safe working environment for treatment of patients.
Authors’ Contributions
MN Supervised the research and writing of the manuscript. MH was the technician of the laboratory and supervised microbial analysis. ZS and OZ collected the samples and did the bacterial analysis. All authors have read and approved the content of the manuscript.
Footnotes
Conflict of Interests
Authors have no conflicts of interests.
References
- 1.Szymanska J. Biofilm and dental unit waterlines. Ann Agric Environ Med. 2003;10(2):151–7. [PubMed] [Google Scholar]
- 2.Barbeau J, Tanguay R, Faucher E, Avezard C, Trudel L, Cote L, et al. Multiparametric analysis of waterline contamination in dental units. Appl Environ Microbiol. 1996;62(11):3954–9. doi: 10.1128/aem.62.11.3954-3959.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szymanska J. Control methods of the microbial water quality in dental unit waterlines. Ann Agric Environ Med. 2003;10(1):1–4. [PubMed] [Google Scholar]
- 4.Singh R, Stine OC, Smith DL, Spitznagel JK, Jr, Labib ME, Williams HN. Microbial diversity of biofilms in dental unit water systems. Appl Environ Microbiol. 2003;69(6):3412–20. doi: 10.1128/AEM.69.6.3412-3420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noce L, Di Giovanni D, Putnins EE. An evaluation of sampling and laboratory procedures for determination of heterotrophic plate counts in dental unit waterlines. J Can Dent Assoc. 2000;66(5):262. [PubMed] [Google Scholar]
- 6.Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martin MV, Marsh PD. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66(8):3363–7. doi: 10.1128/aem.66.8.3363-3367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpay RI, Plamondon TJ, Mills SE. Comparison of methods to enumerate bacteria in dental unit water lines. Curr Microbiol. 1999;38(2):132–4. doi: 10.1007/s002849900416. [DOI] [PubMed] [Google Scholar]
- 8.Atlas RM, Williams JF, Huntington MK. Legionella contamination of dental-unit waters. Appl Environ Microbiol. 1995;61(4):1208–13. doi: 10.1128/aem.61.4.1208-1213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza-Gugelmin MC, Lima CD, Lima SN, Mian H, Ito IY. Microbial contamination in dental unit waterlines. Braz Dent J. 2003;14(1):55–7. doi: 10.1590/s0103-64402003000100010. [DOI] [PubMed] [Google Scholar]
- 10.Barbeau J, Gauthier C. Payment P. Biofilms, infectious agents, and dental unit waterlines: a review. Can J Microbiol. 1998;44(11):1019–28. doi: 10.1139/cjm-44-11-1019. [DOI] [PubMed] [Google Scholar]
- 11.Karpay RI, Plamondon TJ, Mills SE, Dove SB. Combining periodic and continuous sodium hypochlorite treatment to control biofilms in dental unit water systems. J Am Dent Assoc. 1999;130(7):957–65. doi: 10.14219/jada.archive.1999.0336. [DOI] [PubMed] [Google Scholar]
- 12.Szymanska J. Evaluation of mycological contamination of dental unit waterlines. Ann Agric Environ Med. 2005;12(1):153–5. [PubMed] [Google Scholar]


