Abstract
Abstract The 90-kDa heat shock protein (Hsp90) is the most abundant molecular chaperone of eukaryotic cells. Its chaperone function in folding nascent proteins seems to be restricted to a subset of proteins including major components of signal transduction pathways (eg, nuclear hormone receptors, transcription factors, and protein kinases). Improper function of these proteins can be induced by selective disruption of their complexes with Hsp90 using the benzoquinonoid ansamycin geldanamycin. In this study, we demonstrate that geldanamycin treatment blocks interleukin (IL)-2 secretion, IL-2 receptor expression, and proliferation of stimulated T-lymphocytes. Moreover, geldanamycin decreases the amount and phosphorylation of Lck and Raf-1 kinases and prevents activation of the extracellular signal regulated kinase (ERK)-2 kinase. Geldanamycin also disrupts the T-cell receptor–mediated activation of nuclear factor of activated T-cells (NF-AT). Treatment with geldanamycin, however, does not affect the activation of lysophosphatide acyltransferase, which is a plasma membrane enzyme coupled to the T-cell receptor after T-cell stimulation. Through demonstrating the selective inhibition of kinase-related T-lymphocyte responses by geldanamycin, our results emphasize the substantial role of Hsp90–kinase complexes in T-cell activation.
INTRODUCTION
Activation of T-1 lymphocytes results in the tyrosine phosphorylation of numerous CD3 immunoreceptor tyrosine-based activation motifs and a consequent recruitment and phosphorylation of downstream substrates, adaptor proteins, and protein kinases. During the initial phase of this process, the Src family protein tyrosine kinases, Lck and Fyn, are critical for T-cell receptor (TCR) signaling. Consequent activation of Raf-1, a serine-threonine kinase of the mitogen activated protein (MAP) kinase cascade, leads to the activation of c-Fos and JunB and, finally, to interleukin (IL)-2 receptor expression, IL-2 secretion, and cell proliferation. As an alternative signaling pathway, elevation of the intracellular calcium level leads to the activation of calcineurin and the translocation of nuclear factor of activated T-cells (NF-AT) to the cell nucleus. Binding of NF-AT also triggers IL-2 secretion and promotes cell proliferation (Szamel and Resch 1995; Cantrell 1996; Qian and Weiss 1997).
Molecular chaperones assist in the folding and unfolding of proteins during their transport, assembly, and degradation (Hartl 1996), and they probably played a major role in the development of modern enzymes (Csermely 1997). Chaperones are necessary to refold proteins after most types of cell stress. Hsp60- and Hsp90-based chaperone machineries mediate the folding of some de novo synthesized proteins in the cytoplasm (Hartl 1996; Johnson and Craig 1997; Csermely et al 1998). The 90-kDa heat shock protein (Hsp90) and its cochaperones are necessary to achieve the signal-competent conformation of many key elements of T-cell signaling, such as Lck, Fyn, and Raf-1 kinases (Hartmann et al 1997; Pratt 1997; Csermely et al 1998).
Geldanamycin, which is a benzoquinonoid ansamycin analogue of herbimycin A, was originally developed as a tyrosine kinase inhibitor but was later shown to bind specifically to the noncanonic N-terminal ATP/ADP-binding site of Hsp90 homologues (Whitesell et al 1994; Prodromou et al 1997; Stebbins et al 1997; Roe et al 1999). Geldanamycin does not inhibit purified tyrosine kinases, but it does induce degradation of both tyrosine and serine/threonine kinases by the proteasome in vivo (Uehara et al 1986; Whitesell et al 1994; Pratt 1997; Csermely et al 1998; Ochel et al 1999; Sakagami et al 1999). Thus, its “kinase inhibition” is mediated by the premature disruption of Hsp90–kinase complexes. A direct interaction between geldanamycin and some targets of Hsp90 has been proposed after demonstration of its competition with some folding intermediates (Young et al 1997).
Evidence is increasing that geldanamycin is currently one of the most effective tools to investigate the role of Hsp90 in various in vivo cellular processes. In our work, we have analyzed the effect of geldanamycin on key elements of T-cell signaling, such as cell proliferation, IL-2 receptor expression, IL-2 secretion, tyrosine and serine/threonine kinase activation, IL-2 gene activation, and activation of lysophosphatide acyltransferase. Our results indicate that Hsp90 is selectively involved in most of the kinase-mediated signaling steps of T-cell activation.
MATERIALS AND METHODS
Antibodies and reagents
The OKT3 immunoglobulin (Ig) G2a mouse monoclonal antibody raised against the TCR ε chain was provided by Ortho Pharmaceutical (Raritan, NJ, USA). The BW 828 IgG2a mouse monoclonal antibody was from Behring (Marburg, Germany). For flow cytometric analysis of IL-2 receptor, R-phycoerythrin–conjugated antihuman CD25 mAb (mouse IgG1; PharMingen, San Diego, CA, USA) was used. Anti–Raf-1, anti-Lck rabbit polyclonal antibodies, goat antirabbit, and antimouse IgG–horseradish peroxidase conjugates were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Recombinant antiphosphotyrosine antibody coupled to horseradish peroxidase was purchased from Transduction Laboratories (Lexington, KY, USA). Protein A-Sepharose was from Pharmacia Biotech (Uppsala, Sweden). Recombinant, inactive ERK-2 was from New England Biolabs (Schwalbach/Taunus, Germany). The geldanamycin used was a more than95 %-pure product of GIBCO-BRL (Gaithersburg, MD, USA) as judged by high-pressure liquid chromatography. [3H]-thymidine (20 Ci/mmol), [γ-32P]-ATP (3000 Ci/mmol), and Western blot chemiluminescence reagent were obtained from Amersham (Buckinghamshire, UK). 1-[1-14C]-Palmitoyl-sn-glycero-3-phosphocholine (50 mCi/mmol) was from New England Nuclear (Bad Homburg, Germany). The human IL-2 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Genzyme (West Melling, UK). All other chemicals used were from Sigma Chemicals (St. Louis, MO, USA).
Cell culture, preparation, and stimulation of lymphocytes
The human Jurkat T-cell leukemia subline J32 was provided by M. Kamoun (Department of Pathology and Laboratory Medicine, Philadelphia, PA, USA). Human peripheral blood lymphocytes were isolated by Ficoll gradient centrifugation of heparinized buffy coat and depleted for contaminating, nonadherent mononuclear cells by plastic adherence overnight at 37°C as described elsewhere (Szamel et al 1997). J32 and peripheral blood cells were cultured in RPMI 1640 medium (Bio-Whittaker, Verviers, Belgium) supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM).
To induce lymphocyte activation, cells (2 × 106 cells/mL) were cultured in flat-bottom microtiter plates at 37°C in 10% FCS and stimulated with OKT3 mAb (5 μg/mL) or phorbol myristate acetate (PMA) (1 ng/mL) and ionomycin (0.5 μg/mL) in the presence or absence of geldanamycin (1.78 μM) for the indicated periods of time (see legends of Figures). Cell viability was regularly checked by trypan blue exclusion.
Cell-proliferation assay
Both control and stimulated peripheral lymphocytes were cultured in 96-well, flat-bottom microtiter plates at 4 × 105 cells per well in 200 μL at 37°C. After 44 hours, 0.5 μCi of [3H]-thymidine per well was added for an additional 4 hours. Cells were harvested on glass-fiber filters using an automatic harvester, and [3H]-thymidine incorporation was determined by liquid scintillation counting.
Analysis of IL-2 receptor expression
Both control and stimulated peripheral cells (2 × 106 cells/mL) were incubated in a flat-bottom microtiter plate at 37°C. After 24 hours, lymphocytes were resuspended at 5 × 105 cells per 250 μL in blocking medium (0.1% bovine serum albumin [BSA] in phosphate-buffered saline [PBS]) and stained in round-bottom microtiter plates with a saturating amount of R-phycoerythrin–conjugated anti-CD25 mAb for 15 minutes at room temperature in the dark. Cells were then washed 3 times and resuspended in 250 μL of blocking buffer. Samples were analyzed using a FACScan flow cytometer (Becton-Dickinson, San Jose, CA, USA), and the gating of lymphocytes was set based on the forward- and side-scattering characteristics of the cells.
Measurement of IL-2 secretion
To determine IL-2 production, peripheral cells (106 cells in 500 μL) were cultured in flat-bottom microtiter plates at 37°C for 24 hours. The supernatants were then collected and the IL-2 concentration measured using the commercial human IL-2 ELISA kit according to the manufacturer's recommendations.
Propidium iodide staining
Peripheral blood lymphocytes (2 × 106 cells/mL) were stimulated with OKT3 mAb (5 μg/mL) in the presence or absence of geldanamycin (1.78 μM) in a flat-bottom microtiter plate at 37°C. After 24 hours, lymphocytes were resuspended at 5 × 105 cells per 250 μL in blocking medium (0.1% BSA in PBS) and stained in round-bottom microtiter plates with propidium iodide at a final concentration of 2.5 μg/mL at room temperature in the dark. Samples were analyzed using the FACScan flow cytometer.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting
J32 cells (density, 2 × 106 cells/mL) were incubated in RPMI 1640 with 10% heat-inactivated fetal calf serum at 37°C in the presence or absence of geldanamycin (1.78 μM) for 12 hours. During the last 4 hours of geldanamycin treatment, control and treated cells were serum starved and then stimulated with 5 μg/mL OKT3 mAb at 37°C for the times indicated (see legend of Fig. 5). Cells were then washed with ice-cold PBS and resuspended in lysis buffer (25 mM Tris-HCl [pH, 7.4], 100 mM NaCl, 1% Brij 98, 4 mM ethylenediaminetetraacetic acid, 1 mM 1,4-dithiothreitol, 1 mM sodium vanadate, 10 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mM phenyl methyl sulfonyl-flouride (PMSF), 20 μM leupeptin, 1 μM aprotinin, and 1 μM pepstatin A). Cell lysis was performed at 4°C for 30 minutes and followed by centrifugation at 14 000 × g for 10 minutes at 4°C. Protein concentration of postnuclear supernatants was determined using the method of Bradford (1976). For analysis of Raf-1, Lck, and ERK-2 mobility shift and degradation, 7.5% and 10% sodium dodecyl sulfate (SDS) gels were used, respectively. Proteins were then electrophoretically transfered to polyvinylidene diflouride (PVDF) membranes using a semidry transfer apparatus. All steps of Western blotting were performed at room temperature. The membranes were blocked for 1 hour with washing buffer (20 mM Tris-HCl [pH, 7.6], 137 mM NaCl, 0.1% Tween 20) containing 5% nonfat dry milk and then probed with primary antibodies diluted in washing buffer for 1 hour. After 3 washes with washing buffer containing 1% nonfat dry milk, horseradish peroxidase–conjugated secondary antibodies were added for 30 minutes. After an additional 4 washes, the protein bands were visualized using the enhanced chemiluminescence detection system. Bands were quantified using an LKB-Pharmacia Ultroscan Laser Densitometer (Pharmacia).
Fig 5.
Effect of geldanamycin (GA) on protein kinases involved in T-lymphocyte signaling. J32 cells (5 × 106 cells/mL) were incubated in the presence or absence of 1.78 μM GA at 37°C for 12 hours. Cells were serum starved during the last 4 hours of incubation and then stimulated with the T-cell receptor (TCR) ε-specific mAb OKT3 (OKT) at a final concentration of 5 μg/mL for 0 to 20 minutes as indicated. Cells were then washed and lysed in the presence of 1% Brij 98, and postnuclear supernatants of detergent extracts were prepared. In Western blot analysis (panels A, B, and D), 30 μg of postnuclear supernatant of detergent extracts were separated by 7.5% (panels A and B) or 10% (panel D) sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with the respective antibodies as described in the text. In the MAPR kinase (MEK) kinase assay, 0.5 μg inactive ERK-2 was added to 5 μg of postnuclear supernatants of cell lysates and incubated in the presence of 50 μM [γ-32P]ATP for 20 minutes at 30°C as described in the text. (A) Effect of GA on Lck hyperphosphorylation. (B) Effect of GA on Raf-1 hyperphosphorylation. (C) Effect of GA on MEK activity as measured by the phosphorylation of inactive ERK-2. (D) Effect of GA on the endogenous ERK-2 hyperphosphorylation. Western blots and the autoradiogram are representatives of 5 independent experiments
Assay of MAPR kinase (MEK) activity
After geldanamycin treatment of J32 cells as described earlier, equal amounts (5 μg) of postnuclear supernatants were incubated with 0.5 μg of inactive ERK-2 in kinase buffer (50 mM Tris-HCl [pH, 7.4], 10 mM MgCl2, 50 μM ATP, and 2 μCi [γ-32P]ATP) at 30°C for 20 minutes. Reactions were terminated by the addition of SDS sample buffer and boiling. Proteins were resolved by electrophoresis on 10% SDS-polyacrylamide gels. Autoradiograms were quantified by laser densitometry.
Reporter gene construction and assay
The constructs containing 3 times the distal NF-AT site (−284 to −258) of the IL-2 enhancer [p(NFAT)3LUC] and the 5′ −326 to +47 nontranslated region of the human IL-2 gene (pIL2LUC) coupled to the reporter gene firefly luciferase were kindly provided by D.A. Cantrell (Imperial Cancer Research Fund, London UK; Williams et al 1995). Transfection of Jurkat T-cells was made by B. Schraven (Institute of Immunology, Ruprecht Karls University, Heidelberg, Germany). Cellular extracts were made using the Promega lysis mix. Luciferase activity of extracts containing 20 μg of protein was determined using the Promega luciferase substrate mix as described by Williams et al (1995).
Determination of lysophosphatide acyltransferase activity
Cells were disrupted by the nitrogen cavitation method, and plasma membrane vesicles were isolated and measurement of lysophosphatide acyltransferase (EC 2.3.1.23) activity performed as described elsewhere (Szamel et al 1986, 1989, 1993). Enzyme activity was calculated from the amount of radioactive phosphatidylcholine formed from 1-[1-14C]palmitoyl-sn-glycero-3-phosphocholine (50 μM) and arachidonoyl coenzyme A (30 μM).
RESULTS
Geldanamycin inhibits proliferation of activated T-cells
Stimulation of peripheral T-lymphocytes via the TCR with OKT3 mAb resulted in a marked, 16-fold increase in cell proliferation (Fig 1). Simultaneous treatment of cells with geldanamycin decreased the incorporation of [3H]-thymidine to the basal level. Addition of the phorbol ester, PMA, and ionomycin induced an even more prominent, 22-fold increase in proliferation, which was also completely abolished by geldanamycin treatment (Fig 1).
Fig 1.
Effect of geldanamycin (GA) on stimulation-induced T-cell proliferation. Peripheral lymphocytes (2 × 106 cells/mL) were stimulated with the T-cell receptor (TCR) ε-specific mAb OKT3 (OKT), and phorbol myristate acetate (PMA) with ionomycin (PMA/iono) at final concentrations of 5 μg/mL, 1 ng/mL, and 0.5 μg/mL, respectively, in the presence or absence of 1.78 μM GA. After 44 hours of incubation, [3H]-thymidine (0.5 μCi per well) was added for 4 hours. Cell harvesting and assessment of [3H]-thymidine incorporation were performed as described in the text. Results are means ± SD of 3 independent experiments performed in triplicate cultures
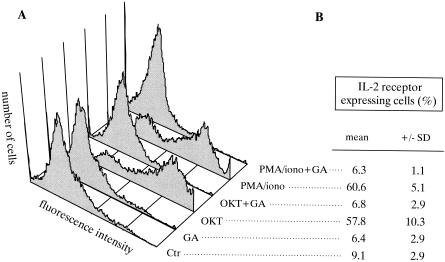
Geldanamycin prevents expression of IL-2 receptors on stimulated T-lymphocytes
T-cell receptor engagement by the OKT3 antibody led to a 7-fold increase in surface expression of IL-2 receptors. PMA and ionomycin treatment resulted in essentially similar changes. Both ways of T-cell activation proved to be geldanamycin sensitive, as revealed by the lack of receptor expression in the presence of the drug (Fig 2).
Fig 2.
Effect of geldanamycin (GA) on stimulation-induced interleukin (IL)-2 receptor expression. Peripheral lymphocytes (2 × 106 cells/mL) were stimulated with the T-cell receptor (TCR) ε-specific mAb OKT3 (OKT), and phorbol myristate acetate (PMA) with ionomycin (PMA/iono) at final concentrations of 5 μg/mL, 1 ng/mL and 0.5 μg/mL, respectively, in the presence or absence of 1.78 μM GA. After 24 hours of incubation, expression of the α-chain of the IL-2 receptor was measured by flow cytometric analysis as described in the text. (A) Flow cytometric diagrams of a representative experiment. (B) Percentage of IL-2 receptor–expressing cells. Results are means ± SD of 3 independent experiments
Geldanamycin blocks the IL-2 response of activated T cells
OKT3 stimulation of peripheral lymphocytes caused a 13-fold increase in IL-2 secretion. As shown in Figure 3, geldanamycin reduced this IL-2 response by 84%. Treatment with phorbol ester plus ionomycin led to a supraphysiologic amount of IL-2 secretion. Even in the presence of geldanamycin, very high residual IL-2 levels could be detected; however, the extent of the inhibition by geldanamycin was essentially the same (81%) as that with OKT3 stimulation. Propidium iodide staining followed by flow cytometric analysis indicated a 6.5% decrease in the amount of propidium iodide–negative (ie, living) cells after geldanamycin treatment (Fig 4), which showed that the geldanamycin-induced inhibition of T-cell responses does not result from the toxic effect of the drug. Similar data were obtained using trypan blue exclusion (data not shown).
Fig 3.
Effect of geldanamycin (GA) on stimulation-induced interleukin (IL)-2 secretion. Peripheral lymphocytes (2 × 106 cells/mL) were stimulated with the T-cell receptor (TCR) ε-specific mAb OKT3 (OKT), and phorbol myristate acetate (PMA) with ionomycin (PMA/iono) at final concentrations of 5 μg/mL, 1 ng/mL, and 0.5 μg/mL, respectively, in the presence or absence of 1.78 μM GA. After 24 hours of incubation, supernatants were harvested and assayed for IL-2 levels as described in the text. Results are means ± SD of 3 independent experiments performed in duplicate cultures
Fig 4.
Propidium iodide staining of stimulated T-cells. Peripheral lymphocytes (2 × 106 cells/mL) were stimulated with the T-cell receptor (TCR) ε-specific mAb OKT3 at a final concentration of 5 μg/mL in the absence (OKT) or the presence (OKT+GA) of 1.78 μM geldanamycin (GA). After 24 hours of incubation, cells were stained with propidium iodide and analyzed by flow cytometry as described in the text.
Effect of geldanamycin on protein kinases involved in T-cell early activation steps
To elucidate the mechanism of the inhibitory effect exerted by geldanamycin, we analyzed the early signaling events of T-lymphocyte activation. TCR engagement results in tyrosine phosphorylation of CD3 ε, δ, and ξ chains and a consequent recruitment and phosphorylation of downstream substrates, adaptor proteins, and protein kinases (Szamel and Resch 1995; Cantrell 1996; Qian and Weiss 1997). The Src family protein tyrosine kinases, Lck and Fyn, are critical for initiating TCR signaling. Lck is known to require Hsp90 for its proper folding and function, and the kinase is a target protein of geldanamycin treatment (Pratt 1997; Csermely et al 1998). Consistent with the previous results of Hartson et al (1996), a 12-hour pretreatment of J32 cells with geldanamycin significantly decreased the amount of Lck, and geldanamycin pretreatment largely abolished T-cell activation–induced Lck phosphorylation (Fig 5A). Similar results were obtained with peripheral blood lymphocytes (data not shown), indicating the resemblance of the mechanism of geldanamycin inhibition in the 2 different systems. Even so, 4- and 12-hour geldanamycin treatment did not induce degradation of the ZAP-70 tyrosine kinase (data not shown).
Analyzing the TCR-associated phosphotyrosine pattern, we found that OKT3 stimulation of J32 Jurkat cells caused increased tyrosine phosphorylation of protein substrates and/or increased association of phosphotyrosine-containing proteins coprecipitated with the TCR. The stimulation-induced change in the phosphotyrosine pattern was partially inhibited by pretreatment of T-cells with geldanamycin (data not shown). Geldanamycin pretreatment of J32 cells did not result in any gross changes, however, in the phosphotyrosine pattern of the whole-cell lysates of resting cells (data not shown).
The Raf-MEK-ERK pathway plays an important role in mediation of the TCR signal (Szamel and Resch 1995; Cantrell 1996; Qian and Weiss 1997). The Raf kinase itself is an important target of geldanamycin treatment, because the drug disrupts Hsp90–Raf complexes and promotes degradation of the kinase, which in turn impairs the EGF-induced MAP–kinase signaling pathway (Stancato et al 1997). The OKT3-induced activation of Raf in J32 cells was detected by the reduction of electrophoretic mobility of the kinase because of its hyperphosphorylation. As a result of geldanamycin pretreatment, both the amount and the hyperphosphorylation of Raf-1 were markedly decreased (Fig 5B). The change in Raf hyperphosphorylation supports the existence of an inhibition upstream of Raf-1. In light of these findings, it is not surprising that the signaling downstream of Raf-1 was also influenced, as indicated by a marked decrease in the total MEK activity of the lysates (Fig 5C) as well as by a decrease in ERK-2 hyperphosphorylation and mobility shift, which indicates inhibition of the MAP-kinase activity (Fig 5D). We observed similar degradation of Raf and inhibition of MEK activity after geldanamycin treatment in peripheral lymphocytes (data not shown). Despite the geldanamycin-induced Lck and Raf degradation, however, the ansamycin drug did not induce degradation of the ERK-2 kinase (Fig 5D).
Geldanamycin inhibits IL-2 gene activation
To follow the signaling pathway from the TCR to the activation of the IL-2 gene, we used reporter gene constructs to monitor IL-2 gene promoter activity in Jurkat cells (Table 1). As expected, pretreatment with geldanamycin significantly attenuated activation of the IL-2 gene in the construct containing the major enhancer/promoter region of the human IL-2 gene (pIL2LUC) as well as in the construct containing only the distal NF-AT site of the IL-2 enhancer (p[NFAT]3LUC).
Table 1.
Effect of geldanamycin on IL-2 transcription
Geldanamycin does not affect activation of lysophosphatide acyltransferase
To find a proximal and kinase-independent element of T-cell signaling, we analyzed the activation of lysophosphatide acyltransferase, a plasma membrane enzyme coupled to the TCR (Szamel et al 1986, 1989, 1993) (Table 2). Geldanamycin did not affect activation of lysophosphatide acyltransferase after TCR stimulation. Cyclosporin A, however, caused a large inhibition of enzyme activation, which is in accordance with previous results (Szamel et al 1986, 1993). In our present experiments, we have omitted the stimulation with PMA and ionomycin, because lysophosphatide acyltransferase can not be stimulated by these agents (Szamel et al 1986, 1989, 1993), which shows that its coupling to the TCR is independent of protein kinase C.
Table 2.
Effect of geldanamycin on the activation of lysophosphatide acyltransferase
DISCUSSION
The Hsp90-specific drug geldanamycin (Whitesell et al 1994; Stebbins et al 1997) inhibits nuclear hormone receptor– and EGF-induced signaling (Schulte et al 1996; Chen et al 1997; Czar et al 1997; Segnitz and Gehring 1997; Stancato et al 1997). Similar to these effects, geldanamycin in our experiments blocked TCR-mediated cell proliferation, IL-2 receptor expression, and the signaling pathway leading to IL-2 gene activation and IL-2 secretion. Our results (including the inhibition of NF-AT activation after anti-CD28 antibody treatment) are in agreement with those of other studies showing inhibition of cell proliferation, IL-2 receptor expression, and IL-2 secretion after CD28 stimulation (Schnaider et al 1998). The geldanamycin-induced inhibition of various signaling pathways was not a consequence of geldanamycin toxicity, because both trypan blue exclusion studies and propidium-iodide flow cytometric analysis indicated an overall cell death of less than 7% after geldanamycin treatment. Moreover, geldanamycin did not prevent the operation of all activation pathways, because it blocked neither the tyrosine phosphorylation of several molecules nor the elevation of lysophosphatide acyltransferase activity.
The primary targets of geldanamycin are the various tyrosine and serine/threonine kinases associated with Hsp90. Treatment with the drug induces the polyubiquitination and degradation of Raf-1 kinase or steroid receptors by the proteasome (Schulte et al 1997; Segnitz and Gehring 1997). Geldanamycin-induced degradation seems to be a fairly general phenomenon, because it occurs after EGF (Stancato et al 1997), glutamate (Xiao et al 1999), steroid treatment (Czar et al 1997; Segnitz and Gehring 1997), and T-cell activation. Among the kinases involved in T-cell activation, the degradation of Lck and Raf-1 was significantly enhanced after geldanamycin treatment in our studies. This kinase-targeted, facilitated degradation, however, did not affect all kinases involved in TCR signaling, because the total amount of the ZAP-70 and ERK-2 kinases did not change appreciably in the presence of the drug. This increased geldanamycin sensitivity of Lck and Raf-1 kinases is in good agreement with the results of previous in vitro (Hartson et al 1996) and in vivo (June et al 1990; Ohtsuka et al 1996; Czar et al 1997; Segnitz and Gehring 1997; Stancato et al 1997) studies on Lck or Raf-1 in other signaling systems using geldanamycin or the analogous ansamycin herbimycin A. During the preparation of this manuscript, Losiewicz et al (1999) published a report in which they used numerous tyrosine kinase inhibitors, including geldanamycin, to block the activation of E6-1 Jurkat cells. In contrast to our results, which were obtained after 12 hours of geldanamycin treatment, Losiewicz et al could not demonstrate a geldanamycin-induced inactivation of ERK-2 after 4 hours of incubation. The main differences in the experimental conditions (ie, an almost 20-fold lower concentration of geldanamycin, lack of serum starvation before stimulation, and analysis of ERK-2 activity instead of MEK assay after a more prolonged stimulation in Losiewicz et al) probably explain the difference in results. In our studies, after 4 hours of geldanamycin treatment, the depletion of Lck and Raf-1 kinases is significant, and the inhibition of MEK activity is approximately 30%–40% (data not shown). The degradation of Lck and Raf-1 kinases and the inhibition of the MAPK pathway, however, become much greater only after 12 hours of incubation (Fig 5), and this progressive and rather prolonged action of geldanamycin gives further proof that it acts predominantly not as a direct tyrosine kinase inhibitor but as an agent interfering with kinase targeting by the disruption of kinase–chaperone complexes. In another recent, parallel study, the immunosuppressive effects of geldanamycin have been also demonstrated on enriched rat spleen T cells (Sugita et al 1999), which is in agreement with our own results obtained using a human T-cell line and human peripheral blood lymphocytes.
Interestingly, our negative result showing the inability of geldanamycin to block activation of lysophosphatide acyltransferase after TCR stimulation provides indirect evidence that coupling of this plasma membrane enzyme to the TCR does not involve any Hsp90-related (and thus geldanamycin-sensitive) protein kinases, such as Raf or Lck.
Though geldanamycin has been developed as a member of the tyrosine kinase inhibitor ansamycins, such as the widely used herbimycin A, it does not directly inhibit kinases, such as the p60src kinase (Uehara et al 1986). Geldanamycin binds exclusively to members of the 90-kDa chaperone family, Hsp90, and its homologue in the endoplasmic reticulum, Grp94. Geldanamycin binding occurs with high affinity, and the drug–Hsp90 complex is fairly stable (Whitesell et al 1994; Stebbins et al 1997; Csermely et al 1998; Roe et al 1999). The differential targeting of geldanamycin action compared with that of other, bona fide tyrosine kinase inhibitors might explain that geldanamycin-inhibited phorbol ester induced T-cell activation in contrast to previous results obtained with herbimycin A (June et al 1990; Tsubuki et al 1994).
Our data suggest the involvement of Hsp90 in the antigen receptor signaling apparatus in T-cells, but what is the role of this abundant cytoplasmic chaperone in the signaling process? Our data are in agreement with the general view that assumes a role for Hsp90 as a “docking protein” for many (but not all) important kinases in TCR signaling, such as Lck and Raf. Hsp90 most probably stabilizes a metastable conformation of these proteins and keeps them in a “signaling competent” form until the stimulus arrives. Administration of geldanamycin induces premature disruption of this complex and leads to degradation of the respective kinases, most probably by the proteasome, which is a multifunctional protease complex of the cytoplasm known to be associated with Hsp90 (Tsubuki et al 1994; Wagner and Margolis 1995; Conconi et al 1996; Schnaider et al, unpublished observations). Hsp90, however, may well be involved more in signaling than a mere “folding relay” of certain signaling molecules. Hsp90 contains a so-called immunoreceptor tyrosine-based inhibition motif sequence (the highly conserved IRYESL motif in the N-terminal domain of Hsp90 between amino acids 58–63 of human Hsp90α or 53–58 of human Hsp90β; Gupta 1995; Vivier and Daëron 1997), which suggests its direct involvement in the regulation of T-cell signaling complexes. On the other hand, the Hsp90–chaperone complex, which is associated with the microfilamental and microtubular network, might actually exist as a highly dynamic, flexible extension of these structures in the cytosol (Pratt 1997; Csermely et al 1998) similar to the microtrabecular lattice proposal of Wolosewick and Porter (1979). Filament-bound chaperone structures might be involved in the (pre)organization and targeting of various signaling components, such as Lck and Raf-1 kinases in T-lymphocytes. The exploration of the dynamic meshwork involved in the spatiotemporal organization of signaling pathways is a challenging task for future research, in which chaperones may provide an excellent means to find the solution.
Acknowledgments
Financial support for this research was provided by the Deutsche Forschungsgemeinschaft (SFB 265/A9) and by research grants from the Volkswagen Foundation (I/73612), Hungarian Science Foundation (OTKA-T25206), Hungarian Ministry of Social Welfare (ETT-493/96), Hungarian Ministry of Culture and Education (FKFP 761/97), and the ICGEB. P.C. is a recipient of an International Research Scholar's Award (HHMI 75195-541701) of the Howard Hughes Medical Institute. T.S. acknowledges the support of the Soros Foundation and Semmelweis University. The authors thank Marina Golombek and Annette Garbe (Division of Molecular Pharmacology, Medical School Hanover, Hanover, Germany) for excellent technical assistance. We are also thankful to Drs. D.A. Cantrell (Imperial Cancer Research Fund, London, UK) and B. Schraven (Institute of Immunology, Ruprecht Karls University, Heidelberg, Germany) for providing the Jurkat cells transfected with the NF-AT/luciferase reporter gene. T.S. is thankful to Dr. Georg Varga (Division of Molecular Pharmacology, Medical School Hanover) and Dr. Csaba Söti (Department of Medical Chemistry, Semmelweis University) for helpful discussions.
REFERENCES
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3.0003-2697(1976)072<0248:ARASMF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cantrell D. T cell receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259.0732-0582(1996)014<0259:TCRSTP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen H-S, Singh SS, Perdew GH. The Ah receptor is a sensitive target of geldanamycin-induced protein turnover. Arch Biochem Biophys. 1997;348:190–198. doi: 10.1006/abbi.1997.0398.0003-9861(1997)348<0190:TARIAS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Conconi M, Szweda LI, Levine RL, Stadtman ER, Friguet B. Age-related decline of rat liver multicatalytic proteinase activity and protection from oxidative inactivation by heat-shock protein 90. Arch Biochem Biophys. 1996;331:232–240. doi: 10.1006/abbi.1996.0303.0003-9861(1996)331<0232:ARDORL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Csermely P. Proteins, RNA-s, chaperones and enzyme evolution: a folding perspective. Trends Biochem Sci. 1997;22:147–149. doi: 10.1016/s0968-0004(97)01026-8.0968-0004(1997)022<0147:PRSCAE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Söti CS, Prohászka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8.0163-7258(1998)079<0129:TKMCFS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Czar MJ, Galigniana MD, Silverstein AM, Pratt WB. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x.0006-2960(1997)036<7776:GAHSPB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gupta RS. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol Biol Evol. 1995;12:1063–1073. doi: 10.1093/oxfordjournals.molbev.a040281.0737-4038(1995)012<1063:PAOTKH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl F-U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0.0028-0836(1996)381<0571:MCICPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartmann F, Horak EM, Cho C, et al. Effects of the tyrosine-kinase inhibitor geldanamycin on ligand-induced Her-2/neu activation, receptor expression and proliferation of Her-2-positive malignant cell lines. Int J Cancer. 1997;70:221–229. doi: 10.1002/(sici)1097-0215(19970117)70:2<221::aid-ijc14>3.0.co;2-l.0020-7136(1997)070<0221:EOTTKI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartson SD, Barrett DJ, Burn P, Matts RL. Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry. 1996;35:13451–13459. doi: 10.1021/bi961332c.0006-2960(1996)035<13451:HMFOTL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnson JL, Craig EA. Protein folding in vivo: unraveling complex pathways. Cell. 1997;90:201–204. doi: 10.1016/s0092-8674(00)80327-x.0092-8674(1997)090<0201:PFIVUC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- June CH, Fletcher MC, Ledbetter JA, Schieven GL, Siegel JN, Phillips AF, Samelson LE. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990;87:7722–7726. doi: 10.1073/pnas.87.19.7722.0027-8424(1990)087<7722:IOTPPT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losiewicz MD, Kaur G, Sausville EA. Different early effects of tyrphostin AG957 and geldanamycins on mitogen-activated protein kinase and p120cbl phosphorylation in anti CD-3-stimulated T-lymphoblasts. Biochem Pharmacol. 1999;57:281–289. doi: 10.1016/s0006-2952(98)00293-7.0006-2952(1999)057<0281:DEEOTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ochel HJ, Schulte TW, Nguyen P, Trepel J, Neckers L. The benzoquinone ansamycin geldanamycin stimulates proteolytic degradation of focal adhesion kinase. Mol Genet Metab. 1999;66:24–30. doi: 10.1006/mgme.1998.2774.1096-7192(1999)066<0024:TBAGSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Kaziro Y, Satoh T. Analysis of the T-cell activation signaling pathway mediated by tyrosine kinases, protein kinase C, and Ras protein, which is modulated by intracellular cyclic AMP. Biochim Biophys Acta. 1996;1310:223–232. doi: 10.1016/0167-4889(95)00172-7.0006-3002(1996)1310<0223:AOTTCA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pratt WB. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326. doi: 10.1146/annurev.pharmtox.37.1.297.0362-1642(1997)037<0297:TROTHB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1.0092-8674(1997)090<0065:IASCOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Qian D, Weiss A. T cell receptor signal transduction. Curr Opin Cell Biol. 1997;9:205–212. doi: 10.1016/s0955-0674(97)80064-6.0955-0674(1997)009<0205:TCRST>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y.0022-2623(1999)042<0260:SBFIOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sakagami M, Morrison P, Welch WJ. Benzoquinoid ansamycins (herbimycin A and geldanamycin) interfere with the maturation of growth factor receptor tyrosine kinases. Cell Stress Chaperones. 1999;4:19–28. doi: 10.1006/csac.1998.0115.1355-8145(1999)004<0019:BAHAAG>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaider T, Somogyi J, Csermely P, Szamel M. The Hsp90-specific inhibitor, geldanamycin, blocks CD28-mediated activation of human T lymphocytes. Life Sci. 1998;63:949–954. doi: 10.1016/s0024-3205(98)00352-x.0024-3205(1998)063<0949:THSIGB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schulte TW, An WG, Neckers LM. Geldanamycin-induced destabilization of Raf-1 involves the proteasome. Biochem Biophys Res Commun. 1997;239:655–659. doi: 10.1006/bbrc.1997.7527.0006-291X(1997)239<0655:GIDORI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Romanova L, et al. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signaling pathway. Mol Cell Biol. 1996;16:5839–5845. doi: 10.1128/mcb.16.10.5839.0270-7306(1996)016<5839:DORBGL>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segnitz B, Gehring U. The function of steroid hormone receptors is inhibited by the hsp90-specific compound geldanamycin. J Biol Chem. 1997;272:18694–18701. doi: 10.1074/jbc.272.30.18694.0021-9258(1997)272<18694:TFOSHR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stancato LF, Silverstein AM, Owens-Grillo JK, Chow YH, Jove R, Pratt WB. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–4020. doi: 10.1074/jbc.272.7.4013.0021-9258(1997)272<4013:THBAGD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl F-U, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2.0092-8674(1997)089<0239:CSOAHG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sugita T, Tanaka S, Murakami T, Miyoshi H, Ohnuki T. Immunosuppressive effects of the heat shock protein 90-binding antibiotic geldanamycin. Biochem Mol Biol Int. 1999;47:587–595. doi: 10.1080/15216549900201633.1039-9712(1999)047<0587:IEOTHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Szamel M, Bartels F, Resch K. Cyclosporin A inhibits T cell receptor-induced interleukin-2 synthesis of human T lymphocytes by selectively preventing a transmembrane signal transduction pathway leading to sustained activation of a protein kinase C isoenzyme, protein kinase C-β. Eur J Immunol. 1993;23:3072–3081. doi: 10.1002/eji.1830231205.0014-2980(1993)023<3072:CAITCR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Szamel M, Berger P, Resch K. Inhibition of T lymphocyte activation by cyclosporin A: interference with the early activation of plasma membrane phospholipid metabolism. J Immunol. 1986;136:264–269.0022-1767(1986)136<0264:IOTLAB>2.0.CO;2 [PubMed] [Google Scholar]
- Szamel M, Ebel U, Uciechowski P, Kaever V, Resch K. T cell antigen receptor dependent signaling in human lymphocytes: cholera toxin inhibits interleukin-2 receptor expression but not interleukin-2 synthesis by preventing activation of a protein kinase C isotype, PKC-α. Biochim Biophys Acta. 1997;1356:237–248. doi: 10.1016/s0167-4889(96)00174-7.0006-3002(1997)1356<0237:TCARDS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Szamel M, Resch KT. T-cell antigen receptor induced signal transduction pathways: activation and function of protein kinases C in T lymphocytes. Eur J Biochem. 1995;228:1–15. doi: 10.1111/j.1432-1033.1995.tb20221.x.0014-2956(1995)228<0001:TCARIS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Szamel M, Rehermann B, Krebs B, Kurrle R, Resch K. Activation signals in human lymphocytes. Incorporation of polyunsaturated fatty acids into plasma membrane phospholipids regulates IL-2 synthesis via sustained activation of protein kinase C. J Immunol. 1989;143:2806–2813.0022-1767(1989)143<2806:ASIHLI>2.0.CO;2 [PubMed] [Google Scholar]
- Tsubuki S, Saito Y, Kawashima S. Purification and characterization of an endogenous inhibitor specific to the Z-Leu-Leu-Leu-MCA degrading activity in proteasome and its identification as heat-shock protein 90. FEBS Lett. 1994;344:229–233. doi: 10.1016/0014-5793(94)00388-2.0014-5793(1994)344<0229:PACOAE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Uehara Y, Hori M, Takeuchi T, Umezawa H. Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol Cell Biol. 1986;6:2198–2206. doi: 10.1128/mcb.6.6.2198.0270-7306(1986)006<2198:PCFTTN>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Daëron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4.0167-5699(1997)018<0286:ITBIM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wagner BJ, Margolis JW. Age-dependent association of isolated bovine lens multicatalytic proteinase complex (proteasome) with heat-shock protein 90, an endogenous inhibitor. Arch Biochem Biophys. 1995;323:455–462. doi: 10.1006/abbi.1995.0067.0003-9861(1995)323<0455:ADAOIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, de Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324.0027-8424(1994)091<8324:IOHSPH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DH, Woodrow M, Cantrell DA, Murray EJ. Protein kinase C is not a downstream effector of p21ras in activated T cells. Eur J Immunol. 1995;25:42–47. doi: 10.1002/eji.1830250109.0014-2980(1995)025<0042:PKCINA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wolosewick JJ, Porter KR. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality? J Cell Biol. 1979;82:114–139. doi: 10.1083/jcb.82.1.114.0021-9525(1979)082<0114:MLOTCG>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Callaway CW, Lipinski CA, Hicks SD, DeFranco DB. Geldanamycin provides posttreatment protection against glutamate-induced oxidative toxicity in a mouse hippocampal cell line. J Neurochem. 1999;72:95–101. doi: 10.1046/j.1471-4159.1999.0720095.x.0022-3042(1999)072<0095:GPPPAG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Young JC, Schneider C, Hartl F-U. In vitro evidence that hsp90 contains two independent chaperone sites. FEBS Lett. 1997;418:139–143. doi: 10.1016/s0014-5793(97)01363-x.0014-5793(1997)418<0139:IVETHC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]