Abstract
BACKGROUND:
Despite recent technologic improvements in identifying mycobacterium tuberculosis, we are still facing problems in rapid diagnosis of tuberculosis. The objective of this study is to determine the diagnostic value of a new rapid screening test (Patho-TB™) for diagnosis of pulmonary tuberculosis.
METHODS:
Between September 2006 to August 2007, 178 patients were enrolled in the study who were finally classified into two groups; a group of documented pulmonary tuberculosis (n = 67) and a group of non-tuberculous pulmonary infection (n = 111). Patho-TB™ test, Ziehl-Neelsen staining and culture were done on all specimens.
RESULTS:
Of all, 43 patients with pulmonary tuberculosis were sputum smear positive for acid fast bacilli and the rest were smear negative. Mean age of the patients was 59.8 ± 16.1 years and 44% of them were men. The results of Patho-TB™ test were positive in 40 of smear positive and 20 of smear negative tuberculous patients and 33 cases of non-tuberculous control group. The sensitivity, specificity, positive and negative predictive values and accuracy of Patho-TB™ test were estimated 89.5%, 70.2%, 64.5%, 91.7% and 77.5%, respectively.
CONCLUSIONS:
According to the present study it would be suggested that Patho-TB™ test could be a rapid and inexpensive method for diagnosis of pulmonary tuberculosis, given by its high sensitivity and negative predictive value. Concerning the high number of false positive results, using a confirmatory diagnostic procedure is mandatory.
Keywords: Pulmonary Tuberculosis, Rapid Diagnosis, Mycobacterium Tuberculosis Antigens, Iran
Despite intense efforts to find an accurate and rapid diagnostic method for pulmonary tuberculosis, the classic laboratory tests such as sputum smear and mycobacterial culture still remain the most widely used diagnostic procedures. An estimated 5,000 to 10,000 organisms/ml of sputum are required for smear positivity.1 The sensitivity of acid-fast smears varies from 47% to 65% in the diagnosis of pulmonary tuberculosis (PTB).2 Direct observation under the microscope is not specific for tuberculous bacilli because mycobacteria are acid fast and they cannot easily be differentiated by physical characteristics. Culture is the gold standard for the detection of mycobacteria with a sensitivity between 70% and 90% in adult active pulmonary tuberculosis. Solid media culture require 3 to 8 weeks of incubation for detection of Mycobacterium tuberculosis (MTB) and liquid broth culture takes 1 to 3 weeks for growing the organism.3 Polymerase chain reaction (PCR) offers another technique for the direct detection of MTB in clinical specimens. For smear positive specimens, the sensitivity of PCR exceeds 95% but for smear negative cases, it varies from 40% to 77%. In both positive and negative sputum smears, the specificity of PCR remains over 95%.4,5 Other diagnostic methods such as the efficacy of different mycobacterial specific antigens by serodiagnosis vary in their sensitivity.6–9
Patho-TB™ test is a direct observational method which can detect bacilli retained on a filter with antibodies directed against them. These antibodies are raised and purified against TB bacilli, especially 35, 65 and 85 kDa mycobacterial antigens. These antibodies are then made to react with a gold conjugate. The presence of bacilli is evidenced by the appearance of a central red-pink color on the filter. The objective of this study is to determine test performance characteristics of this rapid flow-through diagnostic method for the diagnosis of pulmonary tuberculosis.
Methods
After getting the university ethics committee's approval, a prospective case-control study was conducted. All patients who admitted to the Boo-Ali hospital, Zahedan, Southeastern Iran, with a clinical suspicion of pulmonary tuberculosis or non tuberculous pulmonary infections from September 2006 to August 2007 were included for comparison.
One hundred and seventy eight patients were included in the study in which 67 were documented cases of PTB and 111 were patients with pulmonary infections other than tuberculosis as control group (mostly acute pneumonia and acute exacerbation of chronic bronchitis) (Figure 1). Multiple sputum samples are usually collected from a single patient in the process of TB diagnosis. Patho-TB™ test, Ziehl-Neelsen staining and culture for mycobacterium tuberculosis were done on all specimens. Polymerase chain reaction based on detecting a 123-bp DNA segment belonging to insertion sequence IS6110 specific for MTB was just done in 42 sputum smear negative samples. Analysis of data was performed in confirmed PTB patients (63 culture-positive and 4 PCR-positive cases). Patients with pulmonary infections with negative acid fast bacilli (AFB) smear and culture results were included in the control group. None of the patients were HIV positive. Sputum samples from all the subjects were given code numbers. Decoding was done at the time of data analysis. Forty three patients in the TB group were sputum smear positive and the rest were sputum smear negative. We used the Patho-TB™ kits (Anda-RT mycobacteria Patho-TB, ANDA biologicals, Strasbourg, France) which were developed in collaboration with the Pasteur Institute of Iran. The whole test takes about 40 minutes at room temperature.
Figure 1.
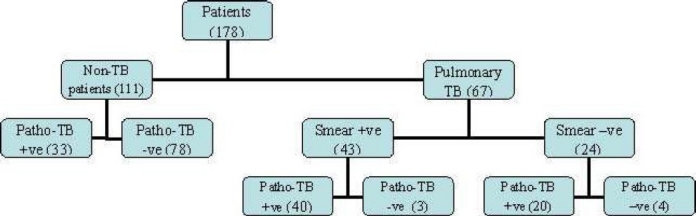
Trial profile
Laboratory Technique
The Patho-TB™ test was performed as the manufacturer brochure's following stages:
1- Sample Decontamination and Neutralization:
The sputum sample was treated for decontamination by the KUBICA method in which 2 ml of sample was put in a 50 ml conical centrifuge tube. An equal volume of Solublization/Decontamination Solution was added and mixed well by vortexing and incubating for 20 minutes on an orbital or turning agitator.
Neutralization was done with 18 ml dNS. Centrifugation was done for 20 minutes at 4000 rpm at room temperature. The supernatant was discarded and 1 ml of pellet was kept. At least 0.2 ml aliquot of the re-suspended pellet was kept to perform the rapid Patho-TB™ test and the rest was used to seed culture and perform other required diagnostic test.
2- Re-suspended Pellet Treatment:
The re-suspended 0.2 ml aliquot was diluted in 1 to 5 volumes of dNS. Then 0.1 ml of diluted aliquot was transferred to a boiling-resistant microtube. Subsequently, an equal volume of negative control and rehydrated positive control were transferred to two other boiling resistant microtubes. From dissolving solution, 0.1 ml was added into each microtube and vortexed. A small hole was made in the cap of the microtube with a needle. The microtube was placed on a floating support and boiled for 20 minutes. The rapid Patho-TB™ test was performed with those treated samples following the procedure presented in the following section.
3- Rapid Test on The Cartridge:
Only one pre-filter was placed in the center of each cartridge (Filtering Unit). The pre-filter square should be imprinted side up. This pre-filter was essential to retain large particulate matter that would clog the filter. A funnel was made on the pre-filter by snapping it securely in place on the cartridge without damaging the pre-filter. The treated aliquot was transferred in the funnel immediately after the boiling step. Then the pre-filter was washed with 2 drops of washing solution. Thereafter, the funnel and the pre-filter were removed and a second wash was done with 2 drops of washing solution. Then 3 drops of antibody solution was added. After absorption and washing, 3 drops of the gold conjugate solution was added. After the last liquid washing and absorption, the results were read immediately. A red-pink color at the filter center, as mentioned earlier, indicated the presence of tuberculous bacilli in the processed sample.
4- Result Interpretation:
The color intensity is proportional to the number of antigens in the sample. It is classified into three categories: (+) a visible central pinkish color, lighter than the control; (++) a pronounced central pinkish red color, similar to the control; and (+++) a central purple-red color, darker than the control. When the result of a test was compatible with the first category, the test should be repeated. The second and the third categories were recorded as positive. The negative samples yielded a white central region. The appearance of a red or pink ring with or without a red-pink central area is an artifact that does not influence the results and should be considered negative.
Statistical Analysis
Data were analyzed using the SPSS software for Windows, version 11.5 (SPSS Inc, Chicago, IL, USA). We carried out Chi squared or Fisher exact tests for comparing categorical data. The diagnostic performances of the test characteristics including sensitivity, specificity and positive and negative predictive values were measured. A two-sided significance level of 0.05 was used.
Results
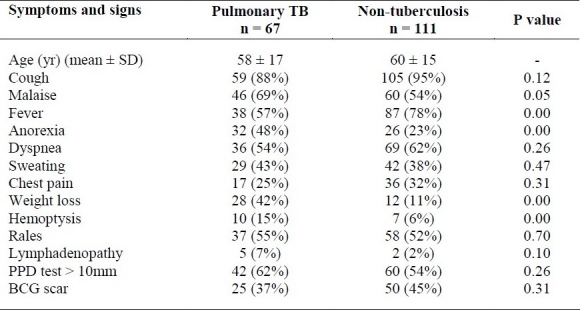
One hundred and seventy eight patients were enrolled in the study who were finally classified into two groups; a group of documented pulmonary tuberculosis (n = 67) and a group of non-tuberculous pulmonary infection (n = 111). The mean age of the TB patients was 58 ± 17 years and 42% of them (28 patients) were men. The mean age in non TB patients was 60 ± 15 and 46% of them (51 patients) were men. Twenty (11%) of the patients were from Afghanistan and the rest were Iranian. The major presenting clinical features were cough and fever. Anorexia, weight loss, hemoptysis and malaise were significantly higher in the TB group but fever was more presented in the non-TB group (Table 1). Other clinical manifestations between two groups were not statistically relevant. Positive reactions to PPD skin test (more than 10 mm) and presence of BCG scar were seen in 62% and 37% of PTB group comparing to 54% and 45% in non-TB group, respectively (p > 0.05) (Table 1). The sensitivity, specificity, PPV and NPV of PPD test versus culture were 63%, 46%, 41% and 67%, respectively. As it was shown in table 1, 43 out of 67 PTB patients had positive direct smear examination. The sensitivity of sputum smear examination was 64% versus 90% in patho-TB test but the specificity was 100% versus 70%.
Table 1.
The most common clinical findings of pulmonary tuberculosis and non-tuberculous patients
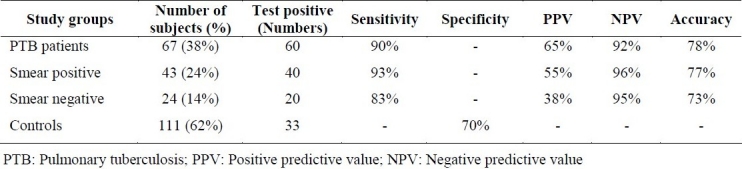
The results of Patho-TB™ test were positive in 40 of smear positive and 20 of smear negative tuberculous patients and 33 cases of non-tuberculous control group. Four specimens in control group were positive which were retested and only one specimen was reported positive at the end. The sensitivity of Patho-TB™ test in sputum smear positive and negative PTB was 93% and 83%, respectively. Specificity of the test was 70% and positive predictive value ranged from 38% in sputum smear negative PTB to 65% in the total number of PTB patients. The negative predictive value was more than 90% in each group (Table 2).
Table 2.
Test performance characteristics of PATHO-TB test in PTB patients and control subjects
Discussion
Among communicable diseases, tuberculosis (TB) is the second leading cause of death worldwide, killing nearly 2 million people each year. About one-third of the world population is infected with TB in which 10% progress toward active disease. Most of these patients are living in underdeveloped countries of the world.10 The incidence of total PTB and sputum smear positive PTB were estimated about 53.4 and 22.4 per 100,000 respectively in the study region during 2006.11 The definite diagnosis of pulmonary tuberculosis is difficult because of low sensitivity of sputum direct examination. Despite the low cost and easy performance of direct sputum smear microscopy, it has a sensitivity of 61.3-63.4% and specificity of 97.3-97.4% and is being used as the primary diagnostic test for the diagnosis of PTB.12 Culture requires several weeks to provide results and other complex tests such as PCR are expensive and rarely available in low income countries. World Health Organization targets 70% of case detections and 85% of successful treatments and they are not likely to be achieved with the existing methods of diagnosis.13 Attempts to develop a rapid and inexpensive diagnostic method with high sensitivity and specificity are still highly desirable for the diagnosis of PTB.
Serological tests might be based on antigen or antibody. Different methods by using new reagents or purified antibodies try to provide high sensitivity and specificity for the tests comparable to direct smear microscopy or AFB culture.14 There are many different free mycobacterial antigens which can be detected in different types of body fluid. The most commonly used antigens are lipoarabinomannan (LAM, MycoDot™ serologic test), antigen 5 (38 kDa, Pathozyme-TB™ ELISA test and ICT diagnostics™), LAM and 38 kDa (Pathozyme-Myco™ ELISA test), A-60 antigen (Antigen A-60™ ELISA test), 45/47 kDa antigen, KP90 antigen, 30 kDa antigen, P32 antigen and some other antigens.The standard methods used for those different antigen assays include sandwich ELISA, inhibition ELISA, latex agglutination and reverse passive hemagglutination tests.14–16 Dipstick method had a sensitivity and specificity of 93% and 95%, respectively.17 Recently it was reported that Patho-TB™ test has a reasonable sensitivity and specificity. Among 310 TB patients, the sensitivity of Patho-TB™ was 91.1% comparing to direct examination that was 91.8% and the specificity was 85.5% versus 100%.18 Direct examination in that study was done by auramine staining which was confirmed by Ziehl-Neelsen in positive specimens but in the present study only Ziehl-Neelsen staining for direct examination was performed. Sensitivities in both of these studies revealed approximately similar results but the specificity was lower in the present study.
In the present study, the sensitivity of Patho-TB™ test was 90% in contrast with 64.1% for the direct smear method. The higher sensitivity, coupled with prompt response, low cost and easy performance, will enable the identification of a sizeable number of pulmonary tuberculosis. Another advantage of the test is using just a small amount of sputum (0.2 ml). In order to have good sensitivity, the antibodies used in this test are polyclonal. These antibodies are raised and purified against TB bacilli, especially 35, 65 and 85 kDa mycobacterial antigens, but can also react with other mycobacterial species. Thus it is necessary to analyze results together with the clinical data of each patient to obtain a complete diagnosis. Concerning the high number of false positive, using a confirmatory diagnostic procedure like culture in association with clinical features is mandatory.
None of the patients in this study were HIV positive so a study in immune compromised patients with several opportunistic infections is recommended. As mentioned earlier, performance of anti-mycobacterial antibody test revealed contradictory results, regardless of type of tuberculosis. The value of detection of specific IGG or IGM antibodies against antigenic particles of Mycobacterium tuberculosis revealed different results.19–23
Rapid immunochromatographic test (ICT) for detection of antibodies directed against MTB antigens and MTB-specific enzyme-linked immunospot assay, with early antigenic target-6 and culture filtrate protein-10 peptides are two other different new diagnostic methods for TB.24,25 Despite all these different types of rapid diagnostic tests, newer and more accurate methods of diagnosis are still needed.
Although clinical manifestations in table 1 such as malaise, anorexia, weight loss and hemoptysis showed a significant difference between PTB and control groups, they are not characteristic for TB. Positive tuberculin skin test in our study also did not show any significant difference and the results of diagnostic performance characteristics were much lower than Patho-TB™ test results. Although Sputum smear examination was highly specific (100%), it was just 64% sensitive.
Conclusions
Higher sensitivity of this new rapid flow-through method comparing to direct smear microscopy of AFB, might enable using Patho-TB™ test for rapid diagnosis of PTB as a screening test along with traditional diagnostic tests. Positive predictive values of the test are low therefore patients with positive Patho-TB™ test need a confirmatory test such as sputum culture to confirm the diagnosis of PTB. Concerning the high percentage of NPVs, it would be possible to rely on them for patients with a negative Patho-TB™ test and clinical manifestations which are not suggestive of PTB. Further studies on larger number of sputum smear negative PTB patients are required to clear the characteristic performance of Patho-TB™ test.
Authors’ Contributions
RAN was the main researcher, wrote the manuscript and coordinated in most of the experiments. MM edited the article. EA was the laboratory association. HM collected data. All authors have read and approved the content of the manuscript.
Acknowledgments
Authors want to thank Dr A.R. Bahremand, director of respiratory diseases unit in Pasteur Institute of Iran. We are also grateful to Dr A. Moghtaderi for critically reviewing the manuscript. And we want to thank Deputy of Research at Zahedan University of Medical Sciences for financial support; and A.R. Dashi-poor and A. Zahabi for statistical and technical assistance.
Footnotes
Conflict of Interests
Anda biologicals® provided Patho-TB™ kits for the study. It had no role in study design, data collection, data analysis, data interpretation or writing of the report.
References
- 1.Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999.This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 2.Gordin F, Slutkin G. The validity of acid-fast smears in the diagnosis of pulmonary tuberculosis. Arch Pathol Lab Med. 1990;114(10):1025–7. [PubMed] [Google Scholar]
- 3.Hale YM, Pfyffer GE, Salfinger M. Laboratory diagnosis of mycobacterial infections: new tools and lessons learned. Clin Infect Dis. 2001;33(6):834–46. doi: 10.1086/322607. [DOI] [PubMed] [Google Scholar]
- 4.Rapid diagnostic tests for tuberculosis: what is the appropriate use? American Thoracic Society Workshop. Am J Respir Crit Care Med. 1997;155(5):1804–1814. doi: 10.1164/ajrccm.155.5.9154896. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PF. Rapid diagnostic tests for tuberculosis: progress but no gold standard. Am J Respir Crit Care Med. 1997;155(5):1497–8. doi: 10.1164/ajrccm.155.5.9154847. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Kumari S, Banwalikar JN, Gupta SK. Diagnostic utility of the estimation of mycobacterial Antigen A60 specific immunoglobulins IgM, IgA and IgG in the sera of cases of adult human tuberculosis. Tuber Lung Dis. 1995;76(5):418–24. doi: 10.1016/0962-8479(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 7.Chiang IH, Suo J, Bai KJ, Lin TP, Luh KT, Yu CJ, et al. Serodiagnosis of tuberculosis.A study comparing three specific mycobacterial antigens. Am J Respir Crit Care Med. 1997;156(3 Pt 1):906–11. doi: 10.1164/ajrccm.156.3.9607122. [DOI] [PubMed] [Google Scholar]
- 8.Delacourt C, Gobin J, Gaillard JL, de Blic J, Veron M, Scheinmann P. Value of ELISA using antigen 60 for the diagnosis of tuberculosis in children. Chest. 1993;104(2):393–8. doi: 10.1378/chest.104.2.393. [DOI] [PubMed] [Google Scholar]
- 9.Luh KT, Yu CJ, Yang PC, Lee LN. Tuberculosis antigen A60 serodiagnosis in tuberculous infection: application in extrapulmonary and smear-negative pulmonary tuberculosis. Respirology. 1996;1(2):145–51. doi: 10.1111/j.1440-1843.1996.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 10.Bello AK, Njoku CH. Tuberculosis: current trends in diagnosis and treatment. Niger J Clin Pract. 2005;8(2):118–24. [PubMed] [Google Scholar]
- 11.Internal Bulletin. Zahedan (Iran): Zahedan University of Medical Sciences; 2006. Ministry of Health and Treatment. Centers for Disease Control. (Persian) [Google Scholar]
- 12.Tiwari V, Jain A, Verma RK. Application of enzyme amplified mycobacterial DNA detection in the diagnosis of pulmonary and extra-pulmonary tuberculosis. Indian J Med Res. 2003;118:224–8. [PubMed] [Google Scholar]
- 13.WHO. Global tuberculosis control- surveillance, planning, financing. 2008. Available at: http://www. who.int/gtb/publications/globrep02/index.html .
- 14.Tiwari RP, Hattikudur NS, Bharmal RN, Kartikeyan S, Deshmukh NM, Bisen PS. Modern approaches to a rapid diagnosis of tuberculosis: promises and challenges ahead. Tuberculosis (Edinb) 2007;87(3):193–201. doi: 10.1016/j.tube.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Daleine G, Lagrange PH. Preliminary evaluation of a Mycobacterium tuberculosis lipooligosaccharide (LOS) antigen in the serological diagnosis of tuberculosis in HIV seropositive and seronegative patients. Tuber Lung Dis. 1995;76(3):234–9. doi: 10.1016/s0962-8479(05)80011-5. [DOI] [PubMed] [Google Scholar]
- 16.Chandramuki A, Bothamley GH, Brennan PJ, Ivanyi J. Levels of antibody to defined antigens of Mycobacterium tuberculosis in tuberculous meningitis. J Clin Microbiol. 1989;27(5):821–5. doi: 10.1128/jcm.27.5.821-825.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Prete R, Picca V, Mosca A, D’Alagni M, Miragliotta G. Detection of anti-lipoarabinomannan antibodies for the diagnosis of active tuberculosis. Int J Tuberc Lung Dis. 1998;2(2):160–3. [PubMed] [Google Scholar]
- 18.Fabre M, Gerome P, Maslin J, Herve V, Vong R, Carpentier G, et al. Assessment of the Patho-TB kit for diagnosis of tuberculosis. Pathol Biol (Paris) 2007;55(10):482–5. doi: 10.1016/j.patbio.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hajjaj MS, Gad-el-Rab MO, al-Orainey IO, al-Kassimi FA. Improved sensitivity for detection of tuberculosis cases by a modified Anda-TB ELISA test. Tuber Lung Dis. 1999;79(3):181–5. doi: 10.1054/tuld.1998.0205. [DOI] [PubMed] [Google Scholar]
- 20.Turneer M, Van Nerom E, Nyabenda J, Waelbroeck A, Duvivier A, Toppet M. Determination of humoral immunoglobulins M and G directed against mycobacterial antigen 60 failed to diagnose primary tuberculosis and mycobacterial adenitis in children. Am J Respir Crit Care Med. 1994;150(6):1508–12. doi: 10.1164/ajrccm.150.6.7952608. [DOI] [PubMed] [Google Scholar]
- 21.Kalantri Y, Hemvani N, Bhatia GC, Chitnis DS. Elisa kit evaluation for IGG and IGM antibodies to A-60 tubercular protein antigen. Indian J Med Sci. 2005;59(8):337–46. [PubMed] [Google Scholar]
- 22.Breen RA, Hardy GA, Perrin FM, Lear S, Kinloch S, Smith CJ, et al. Rapid diagnosis of smear-negative tuberculosis using immunology and microbiology with induced sputum in HIV-infected and uninfected individuals. PLoS ONE. 2007;2(12):e1335. doi: 10.1371/journal.pone.0001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gounder C, De Queiroz Mello FC, Conde MB, Bishai WR, Kritski AL, Chaisson RE, et al. Field evaluation of a rapid immunochromatographic test for tuberculosis. J Clin Microbiol. 2002;40(6):1989–93. doi: 10.1128/JCM.40.6.1989-1993.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartoloni A, Strohmeyer M, Bartalesi F, Messeri D, Tortoli E, Farese A, et al. Evaluation of a rapid immunochromatographic test for the serologic diagnosis of tuberculosis in Italy. Clin Microbiol Infect. 2003;9:632–639. doi: 10.1046/j.1469-0691.2003.00574.x. [DOI] [PubMed] [Google Scholar]
- 25.Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, et al. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med. 2006;174(9):1048–54. doi: 10.1164/rccm.200604-465OC. [DOI] [PubMed] [Google Scholar]


