Abstract
BACKGROUND:
The aim of this study is to assess the effect of pre-pregnancy physical training on metabolic responses and its effects on offspring.
METHODS:
Three groups of rats (n = 7 in each group): sedentary pregnant rats (PS), exercised during pregnancy (PE) and pregnant rats trained before and during pregnancy (PT) were compared. They were separated into three subgroups regarding water temperature: 28°C, 35°C or 39°C. Plasma triglycerides and glucose levels, weight gain during pregnancy and rectal temperature pre and post exercise (swim), as well as the offspring size and weight were analysed.
RESULTS:
Rectal temperature post exercise was lower than pre exercise at 28°C and 35°C, and higher at 39°C. Weight gain was lower at 39°C for the PT group and at 35°C for the PT and PE groups compared to the PS group. Plasma glucose, at 28°C and 39°C for PS and PE groups, was higher than those obtained at 35°C, while triglycerides were lower. For trained rats, plasma glucose and triglycerides were similar at all water temperatures. Trained rats presented lower triglyceride values at 35°C, and higher triglyceride values at 39°C compared to PS group. Glucose presented inverse results. None of the groups presented fetal reabsorption. However, in the PS group, the offspring presented lower weight gain at 28°C than at 35°C and 39°C.
CONCLUSIONS:
These results suggest that pre-pregnancy physical training induces steady values of triglycerides and glucose during exercise at all water temperatures.
Keywords: Physical Training, Pregnant Rats, Glucose Metabolism, Triglycerides Metabolism, Thermal Stress
Pregnancy produces important metabolic responses in the mother, directing an adequate substrate delivery to the fetus in order to enable its development and also prepare the mother for lactation.1–3 On the other hand, it is known that high-intensity exercise during pregnancy can increase core temperature and catecholamine release.3,4 These effects can hamper heat exchange between mother and fetus and constrain the delivery of nutrients due to a reduction of utero-placentary flow.1,3
Exercise in water is suggested to be best physical activity for pregnant women5 because water is a favourable medium for heat dissipation and the greater hydrostatic pressure leads to a fluid flow from extra-vascular to intra-vascular space, which increases blood volume and, consequently, the utero-placentary flow. However, water temperature must be considered because some studies carried out on animals1,6,7 observed deleterious effects on the fetus whose mother was submitted to swimming in water at temperatures different from thermoneutral.
A recent study 8 showed that daily exercise at 80% of maximal work supported into water under high thermal stress (at 22°C and 40°C) for 10-15 minutes did not modify metabolic responses for pregnant rats, but it reduced the litter size when exercise was performed at extreme temperatures, suggesting fetal reabsorbing.
On the other hand, it is also known that physical training can modify the physiological responses to exercise, such as the cardio respiratory and metabolic responses, which become more efficient regarding work performance. These alterations could minimize the effects of exercise performed during pregnancy. However, the interaction between these conditions is complex. For example, a smaller increase of catecholamine is observed during exercises carried out by trained individuals; this effect induces a smaller reduction of blood flow to the splanchnic area. However, other results showed that, in spite of the higher blood flow for trained rats, the flow to the reproductive area was not different between trained and sedentary rats.9 Another example is the alteration in metabolic predominance induced by physical training, increasing lipid utilization. During pregnancy, this effect can represent a safeguard for glucose utilization, since it is the preferential substrate for the fetus.1,10 Nevertheless, the results obtained for pregnant animals suggest that these effects could be attenuated by endocrine alterations produced during pregnancy.11 Considering the complexity of the interaction between physical training and pregnancy, further research is necessary to clarify the factors involved.
The present study aims to analyse the influence of physical training and thermal stress on metabolic responses in pregnant rats as well as the effect on offspring. Pregnant sedentary rats, rats exercised during pregnancy, and rats trained before and during pregnancy, were compared. In order to evaluate metabolic responses, plasma levels of triglycerides and glucose were determined on the 20th day of pregnancy and the offspring were counted and weighed, after a caesarean section. Triglycerides and glucose was chosen to evaluate metabolic responses to the exercise because of their relationship with exercise and fetal development.12,13
Methods
The sample consisted of sixty-three female Wistar rats (190 ± 1 g), kept in individual cages, receiving water and food (ad libitum) (Purina-Brazil), in an artificially illuminated ambience, with a 12 hour day-night cycle and mean temperature of 24°C.
Initially, all animals were submitted to an adaptation period (5 days) of swimming for 5 minutes in water at thermo-neutral temperature in a pool (50 cm × 45 cm). All the animals at the start of experimental procedure weighed between 180g and 220g and were in their reproductive maturity period.
The determination of maximal work load was performed in a swimming pool with water at thermo-neutral temperature. The work load was increased every 3 minutes by weights attached to the animal's tail and corresponding to 1%, 2%, 3%, etc. of the total body weight, until the maximal work load was reached by the animal's exhaustion (unable to surface for 10 s). All rats were kept in cages (3-4 females for each male) during 12 hours (night period). The copulation was confirmed the following morning by the presence of sperm in vaginal smears.
The pregnant rats (n = 63) were then separated into three groups: sedentary rats (PS, n = 21; 188 ± 2 g) submitted to water immersion but did not perform exercise during pregnancy, exercised rats (PE, n = 21; 193 ± 2 g) submitted to swimming sessions at 80% of maximal work load supported for 30 minutes during 19 days of pregnancy, trained rats (PT, n = 21; 188 ± 2 g) submitted to swimming sessions during 45 days before and during pregnancy for 30 minutes, for 19 days of pregnancy, at 80% of maximal work load supported into water, which was calculated for each rat before pregnancy. Each group was divided into three subgroups (n = 7), regarding water temperature: 28°C, 35°C, and 39°C. All swimming sessions were carried out between 8:00 A.M. and 12:00 P.M.
In order to determine weight gain during pregnancy, the rats were weighed on the first and 20th day of the gestational period (Ohaus balance). Rectal temperature was measured daily by thermometer before and after water immersion or swimming.
The rats were decapitated on the 20th day of pregnancy in order to collect a blood sample (5ml) for determining plasma levels of triglycerides and glucose by the enzymatic method.8 Just after decapitation, a caesarean section was carried out and the offspring were counted and weighed.
Statistical analysis was performed by two-way ANOVA and post hoc Tukey test. The null hypothesis was set up at p < 0.05.
Results
Mean values ± standard error for rectal temperatures are shown in table 1. Comparing rectal temperatures, prior and post conditions, showed that, there was a decrease in rectal temperature for all experimental groups at 35°C and 28°C, while an increase was found at 39°C, and temperature (post-prior) was significantly higher at 28°C than at 35°C and 39°C. Weight gain during pregnancy is presented in table 2.
Table 1.
Mean values ± standard error of Rectal temperature (°C) determined during 19 days of pregnancy for sedentary rats (PS), rats exercised during pregnancy (PE) and trained rats exercised before and during pregnancy (PT), prior to and post-immersion or swimming (80% of maximal work load supported) into water at temperatures of 28°C; 35°C or 39°C. (n = 7 for each group)
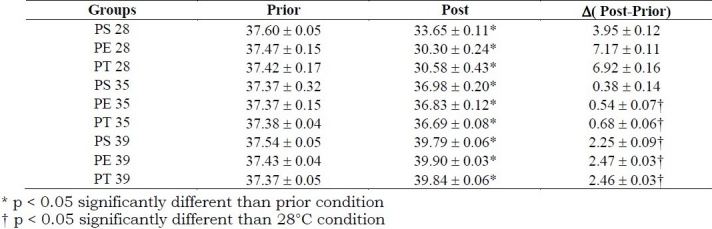
Table 2.
Mean values ± standard error of Weight gain (g) for sedentary pregnant rats submitted to immersion (PS), rats exercised during pregnancy (PE) and trained rats, exercised before and during pregnancy (PT) at 80% of maximal work load supported into water at 28°C, 35°C or 39°C. (n = 7 for each group)
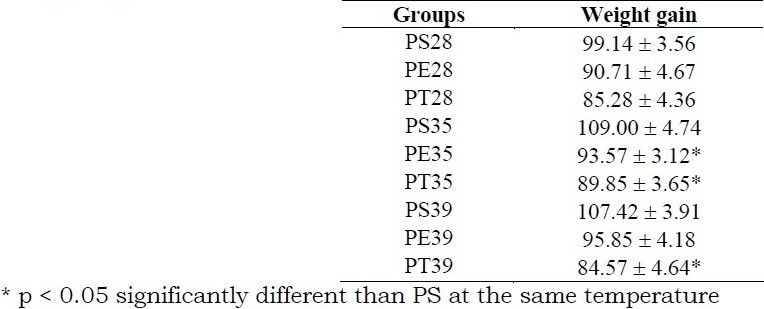
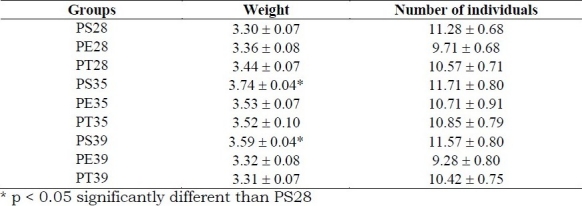
At 28°C, there was no difference between PS, PE and PT groups, but at 35°C, PT and PE presented smaller weight gain than the PS group, and at 39°C, only PT presented a smaller gain. Plasma glucose was higher and plasma triglycerides were lower at 28°C and 39°C than at 35°C for sedentary and exercised rats. Trained rats did not present differences in these variables at any of the water temperatures and had different values than sedentary rats at 35°C and 39°C (Table 3). Offspring from PS28 presented smaller body weight than offspring from PS35 and PS39. The litter size was not different among the groups at any of the water temperatures (Table 4).
Table 3.
Mean values ± standard error of Plasma glucose (mg/dl) and triglycerides (mg/dl) on the twentieth day of pregnancy, determined in blood samples collected 24 hours after the last immersion or swimming session (80% of maximal work load supported) of pregnant sedentary rats (PS), rats exercised during pregnancy (PE) and trained rats exercised before and during pregnancy (PT) in water at temperatures of 28°C, 35°C or 39°C. (n = 7 for each group)
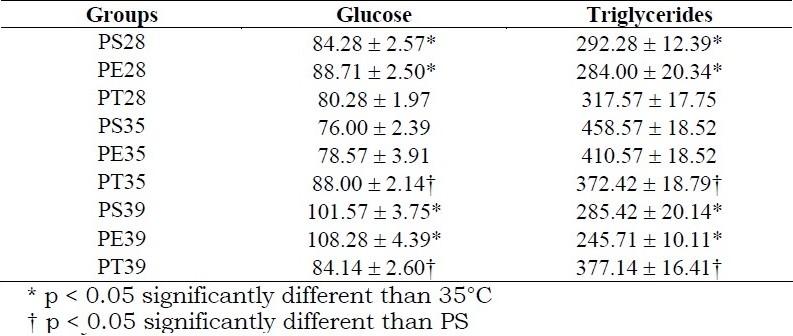
Table 4.
Mean values ± standard error of Offspring weight (g) and size of litter in pregnant sedentary rats (PS) submitted to daily immersion, rats exercised during pregnancy (PE) and trained rats exercised before and during pregnancy (PT) at 80% of maximal work load supported into water at 28°C, 35°C or 39°C. (n = 7 for each group)
Discussion
The literature demonstrates that pregnant rats have lower glucose levels than non-pregnant ones submitted to the same exercise conditions, which means they are not influenced by water temperature.8 Our findings indicate that, under thermal stress (28°C or 39°C), sedentary and exercised pregnant rats present higher plasma glucose values than at 35°C. These results suggest that they have either a smaller uptake or an increase of glyconeogenesis from lactate, alanine and piruvate.14,15
An increase in homeostasis alterations (exercises in high or low temperatures) induces higher increases in catecholamine and cortisol release.16–19 A raise in adrenalin induces pancreas beta cells to reduce the insulin release, which induce alpha cells to increase glucagon.20 Catecholamines, cortisol and glucagon are gluconeogenese and hepatic glycogenolysis inducers, contributing to glicemic levels rise.16,19,21,22
Yet, for trained animals, plasma glucose was kept steady, suggesting that daily thermal stress did not modify the regulatory mechanism of plasma glucose in these animals. The results obtained from pregnant sedentary rats and exercised rats showed alterations of plasma glucose according to the temperatures to which they were submitted daily. It suggests that the duration of immersion or swimming was more important than the temperature concerning the determination of plasma glucose levels, since in the previous study, which used more extreme temperatures with a shorter duration of exposition, pregnant rats showed steady levels of glucose.8
By determining the glucose level during exercise performed by pregnant women, greater hypoglycemia was recorded compared to that observed in resting condition, but this hypoglycemia is smaller during water exercises than land exercises.2,23,24
Glucose returned to resting level within 24 hours of the last session of exercise, indicating the efficiency of the glucose level adjustments.25 This quick regulation is made mainly by the hyperglycemiant hormones action (catecholamine, cortisol and glucagon) that have their serum concentrations increased by exercise.16,22
In the present study, trained rats had lower values of plasma triglycerides at 35°C than sedentary rats, indicating an increase of lipid utilization in this condition. Plasma triglyceride levels were also lower at extreme temperatures rather than at 35°C for exercised and sedentary rats, but trained rats did not present differences in plasma triglycerides at any of the water temperatures. When the current results were compared to our previous study,8 in which pregnant rats were submitted to more extreme temperatures but for a smaller duration, it was observed that both glucose and triglycerides were more influenced by the duration of exposure (30 minutes) rather than by the temperature, which was warmer than in the previous research.
Increased serum concentrations of triglycerides during the exercise are also induced by the hormones cited above. Catecholamines, cortisol and glucagon act on adiposity cells rising the lipolysis and lipids release to blood. Concomitantly the decrease in insulin concentration increases the hormone-sensitive lipase, augmenting the fat acids availability too.26
Increased triglyceride levels were observed in pregnant rats, when compared with non-pregnant animals, especially at the end of pregnancy.2,25 This increase was produced by the endogenous triglycerides from the blood stream.27 Therefore, a lipid represents an alternative substrate for the mother, safeguarding glucose for the fetus. On the other hand, aerobic training modifies the substrate utilization during exercise, increasing lipid mobilization and safeguarding glucose.1
In a previous study carried out in our laboratory,8 we did not observe any alteration in weight gain of rats submitted to daily swimming sessions of 10-15 minutes, at different water temperatures (22°C, 35°C and 40°C). In the present study, using water at more agreeable temperatures for a longer exercise period, it was observed that exercised rats (PE) at 35°C and trained rats (at 35°C and 39°C) had smaller weight gain than sedentary pregnant rats, indicating that substrate mobilization for exercise interferes with total weight gain.
The effect of exercise on maternal weight gain is contradictory. A smaller weight gain was found by some authors.25,28–33 Nonetheless, other authors did not observe any alterations.34–36
In the present study, as in our previous paper,8 after immersion or swimming, pregnant rats modified their rectal temperature with relation to water temperature: decreasing at 28°C and 35°C and increasing at 39°C. In spite of these alterations, a reduction in the litter size was not observed at extreme temperatures, although the duration of immersion or exercise was greater than that employed in our previous study. Nevertheless, if the length of exposure is much longer, where the rats exercise daily for one hour at temperatures of 34.6°C and 37.6°C, fetal abnormalities and reabsorption can be found, probably due to an increase of maternal core temperature.37
Regarding exposition to the cold and heat during pregnancy, some studies showed that hypothermia as well as hyperthermia could produce deleterious effects in fetus.6,7,38–42
There is a temperature gradient between mother and fetus (the fetus presents a temperature 0.4 to 0.6°C greater than the mother), allowing heat transference from the fetus to the mother.43 During high-intensity exercise, this gradient could be reversed, modifying the heat exchange between mother and fetus.6,7 This response could lead to fetal hyperthermia and consequent deleterious effects on offspring.44
When the weight of offspring was investigated, it was verified that sedentary pregnant rats submitted daily to water immersion at 28°C presented litters with smaller weight in relation to offspring from mothers submitted to immersion at 35°C and 39°C. This result suggests that maternal hypothermia, leading to reduction in utero-placentary flow, could induce an inadequate delivery of substrate to the fetus and, in consequence, decrease its weight. Besides, such an effect was not found in exercised and trained rats, suggesting that compensatory mechanisms to safeguard an adequate delivery of nutrients to the fetus were developed by training.
Artal et al45 published a guideline for exercise during pregnancy of the American College of Obstetricians and Gynecologists and advocated the benefits of an exercise program to the pregnant and to the fetus. In a recent study Barakat et al46 concluded that the regular practice of exercise during the pregnancy did not influence the gestational age, but this study included only sedentary participants. Our results demonstrated that an exercise program before and during the pregnancy produced better results than an exercise program only during pregnancy. Further studies are necessary to find the effect of pre pregnancy training on other variables (hormonal responses, cardiovascular and respiratory responses, gestational age, etc.) in animal and human models, considering the great similar physiology between rat and human pregnancy.
Conclusions
We concluded that physical training before pregnancy induces a greater stability of plasma triglycerides and glucose, regardless of daily swimming sessions at different water temperatures. These exercise conditions before pregnancy interfere with total weight gain during pregnancy, without deleterious effects on offspring.
Authors’ Contributions
DALO and IDCP carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. JSC, AKR and WR carried out all the experiments and participated in manuscript preparation. MM and RP provided assistance for all experiments and participated in manuscript preparation specially the interpretation and description of the results and discussion.
All authors have read and approved the content of the manuscript.
Acknowledgments
The authors wish to thank Dr. Neil Ferreira Novo for his statistical assistance.
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.McMurray RG, Mottola MF, Wolfe LA, Artal R, Millar L, Pivarnik JM. Recent advances in understanding maternal and fetal responses to exercise. Med Sci Sports Exerc. 1993;25(12):1305–21. [PubMed] [Google Scholar]
- 2.McMurray RG, Katz VL, Berry MJ, Cefalo RC. The effect of pregnancy on metabolic responses during rest, immersion, and aerobic exercise in the water. Am J Obstet Gynecol. 1988;158:481–6. doi: 10.1016/0002-9378(88)90009-9. [DOI] [PubMed] [Google Scholar]
- 3.Katz VL. Water exercise in pregnancy. Seminars in Perinatology. 1996;20(4):285–91. doi: 10.1016/s0146-0005(96)80021-8. [DOI] [PubMed] [Google Scholar]
- 4.Avery ND, Wolfe LA, Amara CE, Davies GAL, McGrath MJ. Effects of human pregnancy on cardiac autonomic function above and below the ventilatory threshold. J Appl Physiol. 2001;90(1):321–8. doi: 10.1152/jappl.2001.90.1.321. [DOI] [PubMed] [Google Scholar]
- 5.Hartman S, Bung P. Physical training during pregnancy-physiological considerations and recommendations. J Perinat Med. 1999;27(3):204–15. doi: 10.1515/JPM.1999.029. [DOI] [PubMed] [Google Scholar]
- 6.Lotgering FK, Gilbert RD, Longo LD. Exercise responses in pregnant sheep: oxygen consumption, uterine blood flow and blood volume. J Appl Physiol. 1983;55(3):834–41. doi: 10.1152/jappl.1983.55.3.834. [DOI] [PubMed] [Google Scholar]
- 7.Lotgering FK, Gilbert RD, Longo LD. Exercise responses in pregnant sheep: blood gases, temperatures, and fetal cardiovascular system. J Appl Physiol. 1983;55(3):842–50. doi: 10.1152/jappl.1983.55.3.842. [DOI] [PubMed] [Google Scholar]
- 8.Osorio RAL, Silveira VLE, Maldjian S, Morales A, Christofani JS, Russo AK, et al. Swimming of pregnant rats at different water temperatures. Comp Biochem Physiol. 2003;135(4):605–11. doi: 10.1016/s1095-6433(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 9.Nesbitt AE, Murphy RJ, O’Hagan KP. Effect of gestational stage on uttering artery blood flow during exercise in rabbits. J Apple Physiol. 2005;99(6):2159–65. doi: 10.1152/japplphysiol.00236.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lang U, Scott Baker R, Braems G, Zygmunt M, Künzel W, Clark KE. Uterine blood flow—a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol. 2003;110 (1 Suppl):S55–61. doi: 10.1016/s0301-2115(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 11.Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144(8):3575–85. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- 12.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clinical Nutrition. 2000;71:1256S–61S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 13.King JC. Physiology of pregnancy and nutrient metabolism. Am J Clinical Nutrition. 2000;71(5 Suppl):1218S–25S. doi: 10.1093/ajcn/71.5.1218s. [DOI] [PubMed] [Google Scholar]
- 14.Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985;17(1):22–31. [PubMed] [Google Scholar]
- 15.Brooks GA. Current concepts in lactate exchange. Med Sci Sports Exerc. 1991;23(8):895–906. [PubMed] [Google Scholar]
- 16.Kreisman SH, Halter JB, Vranic M, Marliss EB. Combined infusion of epinephrine and norepinephrine during moderate exercise reproduces the glucoregulatory response of intense exercise. Diabetes. 2003;52(6):1347–54. doi: 10.2337/diabetes.52.6.1347. [DOI] [PubMed] [Google Scholar]
- 17.Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. Journal of Clinical Endocrinology & Metabolism. 2004;89(9):4656–64. doi: 10.1210/jc.2004-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86(2):435–64. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 19.Haussmann MF, Vleck CM, Farrar ES. A laboratory exercise to illustrate increased salivary cortisol in response to three stressful conditions using competitive ELISA. Adv Physiol Educ. 2007;31(1):110–5. doi: 10.1152/advan.00058.2006. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi R, Ishihara H, Tamura A, Yamaguchi S, Yamada T, Takei D, et al. Cell type-specific activation of metabolism reveals that P-cell secretion suppresses glucagon release from Q-cells in rat pancreatic islets. Am J Physiol Endocrinol Metab. 2006;290(2):E308–16. doi: 10.1152/ajpendo.00131.2005. [DOI] [PubMed] [Google Scholar]
- 21.Perreault L, Lavely JM, Bergman BC, Horton TJ. Gender differences in insulin action after a single bout of exercise. J Appl Physiol. 2004;97(3):1013–21. doi: 10.1152/japplphysiol.00186.2004. [DOI] [PubMed] [Google Scholar]
- 22.Horton TJ, Grunwald GK, Lavely JM, Donahoo WT. Glucose kinetics differ between women and men, during and after exercise. J Appl Physiol. 2006;100(6):1883–94. doi: 10.1152/japplphysiol.01431.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann V, Regnat K. Study of resistance to physical stress in pregnant women: influence of standardized work on cardiovascular system, ventilation, gaseous interchange, carbohydrate metabolism and acid-base balance. Z Geburtshilfe Perinatol. 1976;180(4):279–89. [PubMed] [Google Scholar]
- 24.Philp A, Macdonald AL, Watt PW. Lactate–a signal coordinating cell and systemic function. Journal of Experimental Biology. 2005;208(24):4561–75. doi: 10.1242/jeb.01961. [DOI] [PubMed] [Google Scholar]
- 25.Denadai BS, Piçarro Ida C, Madjian S, Bergamaschi CT, Santos VC, da Silva AC, et al. High-intensity exercise during pregnancy of rats.Effects on mother and offspring. Comp Biochem Physiol A physiol. 1994;109(3):727–40. doi: 10.1016/0300-9629(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 26.Djurhuus CB, Gravholt CH, Nielsen S, Pedersen SB, Møller N, Schmitz O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab. 2004;286(3):E488–94. doi: 10.1152/ajpendo.00199.2003. [DOI] [PubMed] [Google Scholar]
- 27.Knopp RH, Montes A, Childs M, Li JR, Mabuchi H. Metabolic adjustments in normal and diabetic pregnancy. Clin Obstet Gynecol. 1981;24(1):21–49. doi: 10.1097/00003081-198103000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Terada M. Effect of physical activity before pregnancy on fetuses of mice exercised forcibly during pregnancy. Teratology. 1974;10(2):141–4. doi: 10.1002/tera.1420100208. [DOI] [PubMed] [Google Scholar]
- 29.Jenkin RR, Ciconne C. Exercise effect during pregnancy on brain nucleic acids of pregnant in rats. Arch Phys Med Rehabil. 1980;61(3):124–7. [PubMed] [Google Scholar]
- 30.Wilson NC, Gisolfi CV. Effects of exercising rats during pregnancy. J Appl Physiol. 1980;48(1):34–40. doi: 10.1152/jappl.1980.48.1.34. [DOI] [PubMed] [Google Scholar]
- 31.Mottola MF, Bagnall KM, Belcastro AN, Foster J, Secord D. The effects of strenuous maternal exercise during gestation on maternal body components in rats. J Anat. 1986;148:65–75. [PMC free article] [PubMed] [Google Scholar]
- 32.Piçarro IC, Barros Neto TL, Carrero de Teves D, Silva AC, Denadai DS, Tarasantchi J, et al. Effect of exercise during pregnancy graded as a percentage of aerobic capacity: maternal and fetal response of the rat. Comp Biochem Physiol A Physiol. 1991;100(4):795–9. doi: 10.1016/0300-9629(91)90294-m. [DOI] [PubMed] [Google Scholar]
- 33.Clapp JF, 3rd, Little KD. Effect of recreational exercise on pregnancy weight gain and subcutaneous fat deposition. Med Sci Sports Exerc. 1995;27(2):170–7. [PubMed] [Google Scholar]
- 34.Parizkova J. Impact of daily work load during pregnancy on the microstructure of rat heart in male offspring. Europ J Appl Physiol. 1975;34(1):323–6. doi: 10.1007/BF00999945. [DOI] [PubMed] [Google Scholar]
- 35.Parizkova J. The impact of daily work load during pregnancy and/or post-natal life on the heart microstructure of rat male offspring. Basic Res Cardiol. 1978;73(5):433–41. doi: 10.1007/BF01906524. [DOI] [PubMed] [Google Scholar]
- 36.Treadway JL, Lederman SA. The effects of exercise on milk yield, milk composition and offspring growth in rats. Am J Clin Nutr. 1986;44(4):481–8. doi: 10.1093/ajcn/44.4.481. [DOI] [PubMed] [Google Scholar]
- 37.Mottola MF, Fitzgerald HM, Wilson NC, Taylor AW. Effect of water temperature on exercise-induced maternal hyperthermia on fetal development in rats. Int J Sports Med. 1993;14(5):248–51. doi: 10.1055/s-2007-1021172. [DOI] [PubMed] [Google Scholar]
- 38.Edwards MJ. Congenital defects on guinea pigs following induced hyperthermia during gestation. Arch Pathol. 1967;84(1):42–8. [PubMed] [Google Scholar]
- 39.Edwards MJ. Congenital defects in guinea pigs: fetal resorptions, abortions, and malformations following induced hyperthermia during early gestation. Teratology. 1969;2(4):313–28. doi: 10.1002/tera.1420020406. [DOI] [PubMed] [Google Scholar]
- 40.Edwards MJ. Congenital defects in guinea pigs: prenatal retardation of brain growth of guinea pigs following hyperthermia during gestation. Teratology. 1969;2(4):329–36. doi: 10.1002/tera.1420020407. [DOI] [PubMed] [Google Scholar]
- 41.Barter RH, Albert SN, Winshel AW. The use of Hypothermic-Hypotensive technique in the fulminant toxemia of pregnancy. Am J Obstet Gynecol. 1958;76(5):1062–70. doi: 10.1016/0002-9378(58)90187-x. [DOI] [PubMed] [Google Scholar]
- 42.Mottola M, Bagnall KM, McFadden KD. The effects of maternal exercise on developing rat fetuses. Br J Sports Med. 1983;17(2):117–21. doi: 10.1136/bjsm.17.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMurray RG, Katz VL. Thermorregulation in pregnancy.Implications for exercise. Sports Med. 1990;10(3):146–58. doi: 10.2165/00007256-199010030-00002. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki J, Yamaguchi A, Nabeshima Y, Shigemitsu S, Mesaki N, Kubo T. Exercise at high temperature causes maternal hyperthermia and fetal anomalies in rats. Teratology. 1995;51(4):233–6. doi: 10.1002/tera.1420510407. [DOI] [PubMed] [Google Scholar]
- 45.Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6–12. doi: 10.1136/bjsm.37.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barakat R, Stirling JR, Lucia A. Does exercise training during pregnancy affect gestational age. A randomised controlled trial? Br J Sports Med. 2008;42(8):674–8. doi: 10.1136/bjsm.2008.047837. [DOI] [PubMed] [Google Scholar]


