Abstract
Abstract Chloroplast transit peptides have been proposed to function as substrates for Hsp70 molecular chaperones. Many models of chloroplast protein import depict Hsp70s as the translocation motors that drive protein import into the organelle, but to our knowledge, no direct evidence has demonstrated that transit peptides function either in vivo or in vitro as substrates for the chaperone. In this report, we demonstrate that DnaK binds SStp (the full-length transit peptide for the precursor to the small subunit of Rubisco) in vivo when fused to either glutathione-S-transferase (GST) or to an His6-S-peptide tag (His-S) via an ATP-dependent mechanism. Three independent biophysical and biochemical assays confirm the ability of DnaK and SStp to interact in vitro. The cochaperones, DnaJ and GrpE, were also associated with the DnaK/SStp complex. Therefore, both GST-SStp and His-S–SStp can be used as affinity-tagged substrates to study prokaryotic chaperone/transit peptide interactions as well as to provide a novel functional probe to study the dynamics of DnaK/DnaJ/GrpE interactions in vivo. The combination of these results provides the first experimental support for a transit peptide–dependent interaction between a chloroplast precursor and Hsp70. These results are discussed in light of a general mechanism for protein translocation into chloroplasts and mitochondria.
INTRODUCTION
The chloroplast contains its own genome, but most chloroplast-localized proteins are nuclear encoded and synthesized as larger molecular weight precursors. These precursors contain an amino-terminal extension known as a transit peptide that is both necessary and sufficient to direct the targeting and translocation of precursors into the chloroplast with high fidelity (Bruce and Keegstra 1994). This transit peptide enables the productive interaction of precursors with the translocation apparatus of the chloroplast envelope. Early sequence analysis of transit peptides suggested the existence of 3 loosely conserved domains (Karlin Neumann and Tobin 1986). Only recent works, however, combining both in vitro (Pilon et al 1995; Pinnaduwage and Bruce 1996; Bruce 1998) and in vivo (Kindle 1998; Rensink et al 1998) approaches have demonstrated that different regions of the transit peptide perform different functions during the import process. A persistent problem with this quasimodular organization is that little to no homology exists between different transit peptides. This degeneracy is particularly difficult to explain, and it suggests either some unknown common secondary structure, involvement of an interaction that intrinsically requires low amino acid–sequence specificity, or both.
Precursors targeted for different organelles lack homology in their targeting sequences, but most current models of protein translocation across a membrane include a peripherally attached Hsp70 that functions as an ATP-dependent molecular motor (Craig et al 1989; Ostermann et al 1990; Scherer et al 1990; Brodsky et al 1995; Schatz and Dobberstein 1996). Because Hsp70s have degenerate peptide substrate specificities, they can bind a diverse set of precursor proteins during import into these organelles. Though not supported by direct evidence, current models of chloroplast protein transport assume a direct interaction between the transit peptide of the incoming precursor and the peptide-binding domain of the molecular chaperone (Gray and Row 1995; Keegstra et al 1995; Heins et al 1998). These proposals are supported by a statistical analysis indicating that transit peptides are flexible structures enriched in sequences that are predicted to exist as random coils (von Heijne and Nishikawa 1991). To date, however, and to our knowledge, there has been no evidence for a direct interaction of transit peptides with Hsp70 or clear agreement on the involvement of Hsp70 in the chloroplast protein import process (Soll and Waegemann 1992; Nielsen et al 1997).
In this study, we demonstrate the first direct interaction between a chloroplast transit peptide and the prokaryotic Hsp70 homologue, DnaK. Though not the physiologic chaperone involved in chloroplast protein import, DnaK is highly homologous (similarity, >74%) to the major chloroplast Hsp70 CSS1 (Marshall and Keegstra 1992), and it is a valid model chaperone for studying interactions between mitochondrial precursors and the mitochondrial Hsp70 SSC1 (Zhang et al 1999). Both in vivo and in vitro evidence establish that SStp contains one or more sequences that function as high-affinity, ATP-dependent substrates for DnaK. We also discuss the implications of these findings on the interactions between precursors and molecular chaperones for chloroplast and mitochondrial protein import.
MATERIALS AND METHODS
Construction of vectors for expression of SStp
The pea prSSU gene in pSP65 (Promega, Madison, WI, USA) was digested with BamHI/SphI to release a 220-bp fragment that lacked the codons for the first 5 amino acids, MASMI, and the last 2 amino acids at the C-terminus, QY. This fragment was ligated into a BamHI/SphI-digested pGEM-7Zf (Promega) to yield pGEM-7Zf-SStp1. To rebuild the C-terminus of SStp, pGEM-7Zf-SStp1 was digested with SphI/ApaI, and a linker was inserted that added codons encoding QY at the C-terminus. This linker also engineered a SmaI site to enable cloning into pGEX-2T (Pharmacia, Piscataway, NJ, USA). This construct, pGEM-7Zf-SStp2, was then digested with BamHI/EcoRV, and a second linker was inserted that rebuilt the N-terminal MASMI sequence. This construct, pGEM-7Zf-SStp3, was then digested with BamHI/SmaI to liberate a 188-bp fragment that was inserted into a BamHI/SmaI-digested pGEX-2T vector to form pGEX-2T-SStp. Primers corresponding to regions flanking the SStp-encoding region were used to amplify the insert via standard polymerase chain reaction protocols, which engineered BamHI and EcoRI restriction sites at the 5′ and 3′ ends of the amplified product, respectively. The amplified product was digested and ligated into pET30a vector (Novagen, Madison, WI, USA) to yield pET30a-SStp. Both constructs were confirmed by DNA sequencing.
Escherichia coli protein expression
The first construct (pGEX-2T-SStp), which placed the sequence encoding SStp in frame at the C-terminus of glutathione-S-transferase (GST), was transformed into E coli [BL(21)]. The cells were grown to a cell density corresponding to an OD600 of 0.6, induced with 100 μM isopropylthio-β-d-galactoside (IPTG) for 1 hour, pelleted, resuspended, and lysed by sonication on ice in phosphate-buffered saline (PBS) at a pH of 8.0 and containing 1% (v/v) Triton X-100, 1 mM 1,4-dithiothreitol, 5 mM ethylenediaminetetraacetic acid, 2 mM leupeptin, 2 mM pepstatin, 1 mM PMSF, 5 mM 1,10-phenanthroline, and 3 mM ADP. The second construct (pET30a-SStp), which placed the SStp sequence at the C-terminus of the dual His6-S-peptide (His-S) tag, was transformed into E coli [BL(21)(DE3)]. The cells were grown to a cell density corresponding to an OD600 of 0.6, induced with 1 mM IPTG for 3 hours, pelleted, resuspended, and lysed by sonication on ice according to a pET manual protocol (Novagen).
Stepwise purification of DnaK, SStpGST, and GST
The lysate was centrifuged at 25 000 × g for 30 minutes at 4°C. The resulting supernatant was then loaded at 4°C onto a 5-mL column of glutathione-Sepharose (Pharmacia). The column was first washed with 10 column volumes of PBS containing 1% Triton, then equilibrated with DnaK elution buffer (PBS) at a pH of 8.0 and containing 2 mM MgCl2 and 50 mM KCl. DnaK was eluted with 3 mM ATP in the same buffer. Next, the column was equilibrated with thrombin buffer (PBS at a pH of 8.0 and containing 2.5 mM CaCl2). Thrombin (5 U) was then added onto the column in the same buffer and allowed to digest the fusion protein for 4–6 hours. After SStpGST elution in thrombin buffer, GST was eluted by equilibrating the column with PBS containing 5 mM reduced glutathione.
Purification of SStpHIS
Unless otherwise noted, purification of SStpHIS was performed under denaturing conditions in urea, imidazole, and 0.5 M NaCl according to a pET manual protocol (Novagen). The fusion protein was digested with enterokinase after removing the urea with a linear gradient until the column was equilibrated with wash buffer (Novagen). Eluted SStpHIS was separated from the protease by ultrafiltration through a 30-kDa molecular weight cut-off (MWCO) membrane and concentrated using a 1-kDa MWCO membrane. The peptide was then dialyzed into buffer A (25 mM Tris, 20 mM HEPES [pH, 7.15], 47.5 mM KCl, 2.25 mM Mg[OAc]2), aliquoted, and frozen at −85°C.
Circular dichroism, Tb+3 luminescence, and tryptophan fluorescence measurements
Circular dichroism (CD) spectrometry was performed on a Jasco J-40A Spectropolarimeter (Jasco, Victoria, BC, Canada) at 20°C using circular quartz cells with pathlengths of 0.1 cm and a sensitivity setting of 1 × 10−5 to 5 × 10−6. On addition of SStp, the complex was allowed to equilibrate at 20°C for 10 minutes. The CD spectra are reported in millidegrees of optical rotation after baseline correction for the buffer (triethanolamine-buffered saline [TBS] plus Mg-ADP). Measurements of the DnaK/SStp complex were also corrected by subtraction of the contribution of the transit peptide alone.
Intrinsic fluorescence measurements of DnaK's single tryptophan were performed on a Perkin-Elmer LS 50 B Luminescence Spectrometer (Norwalk, CT, USA). Samples were excited at 290 nm with a slit with of 1 nm. DnaK (1 μM) was added to TBS in the presence of 3 mM Mg-ADP. Purified SStp was added to yield a molar ratio of SStp:DnaK from 0–10. On addition of SStp, the complex was allowed to equilibrate for 10 minutes at 20°C. Spectra were read at 20°C at a scan rate of 1 nm/s. All spectra were initially corrected by baseline subtraction (TBS plus Mg-ADP).
Sensitized luminescence, arising from the resonance energy transfer from tryptophan to Tb+3, was performed on a Perkin-Elmer LS 50 B Luminescence Spectrometer. For excitation of the tryptophan chromophore, the sample was excited using a pulsed xenon flash lamp with a pulse width at half-peak height of 10 μs. The decay time was 1 ms, and the gate time was 10 ms. Excitation was at 290 nm, and both the excitation and emission slit widths were set at 5 nm. For phosphorescence measurements, equimolar concentrations of terbium chloride and disodium ATP were added to solutions containing either SStp, DnaK, or a mixture of SStp and DnaK. The phosphorescence spectra of these solutions were then collected from 450 to 650 nm.
Native gel shift competition assay with 125I-labeled reduced, carboxymethylated α-lactalbumin
DnaK-binding competition studies with reduced, carboxymethylated α-lactalbumin radiolabeled with iodine (125I-RCMLA), which were similar to those studies performed by Freeman et al (1995), were performed by incubating 125I-RCMLA, DnaK, and the competing peptides for 30 minutes at 37°C in buffer A. Native sample buffer (100 mM Tris-HCl at a pH of 6.8 and containing 10% [v/v] glycerol and 0.04% [w/v] bromphenol blue) was added, and the samples were resolved by electrophoresis on a 6% native acrylamide gel (gel buffer, 400 mM Tris-HCl [pH, 8.6]; stacking gel, 4.5% [w/v] acrylamide with an acrylamide : bis-acrylamide ratio of 30:0.8 in 120 mM Tris-HCl [pH, 6.8]; running buffer, 25 mM Tris-HCl [pH, 8.3] containing 192 mM glycine). The proteins were then fixed with acetic acid, the gels dried, and the autoradiograms developed. Quantitative scanning densitometry was performed with a Molecular Dynamics Model 3000 Series Computing Densitometer.
Glutathione-agarose affinity precipitation of E coli chaperones
Small cell cultures (10 mL) were grown and induced normally but lysed by sonication in buffers containing different detergent and/or salt concentrations. The crude lysates were spun at 16 000 × g. Each of the supernatants was then added to a microfuge tube containing 100 μL of glutathione-Sepharose and mixed gently for 5 minutes at 4°C. After washing the glutathione-Sepharose in the batch 3 times with 1 mL of the same lysis buffer, 100 μL of sodium dodecyl sulfate (SDS) sample buffer was added directly to the glutathione-Sepharose to elute the bound proteins.
Electrophoresis, Western blotting, and far-Western blotting
All appropriate samples were boiled in reducing SDS–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, run on 10%–20% gradient SDS-PAGE, and stained with Coommassie brilliant blue. Appropriate samples were electroblotted to polyvinyl difluoride membrane, and Western blots were performed with α-DnaK, α-DnaJ, α-GrpE, and α-GST antibodies. Secondary antibodies were conjugated to alkaline phosphatase. Far-Western blots were performed on samples containing His-S–SStp with the RNAse S protein conjugated to alkaline phosphatase. All blots were developed with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium.
RESULTS
Association of DnaK with SStp fusion proteins in E coli
The full-length transit peptide for the pea small subunit of RuBisCo was cloned into pGEX-2T to generate a GST-SStp fusion (34.6 kDa) and into pET-30a to produce a His-S–SStp fusion (11.5 kDa). The organization and sequence of the resulting SStp from both these vectors is shown in Figure 1. Both fusion peptides contain protease cleavage sites that allow the separation of SStp from the respective fusion partner. Routinely, 1–2 mg of the purified peptide was recovered per liter of E coli culture using the pGEX expression system, whereas the pET system yielded 60–80 mg of purified peptide per liter of E coli culture. Part of this discrepancy regarding intact SStp yield may result from the difference in solubility of the GST-SStp (highly soluble) and the His-S–SStp (majority forms inclusion bodies) fusion proteins in vivo. GST-SStp is highly susceptible to cellular proteases in as little as 40 minutes after induction (Fig 2A), whereas His-S–SStp, which is a much smaller peptide with less overall structure, remains intact as long as after 3 hours of induction (Fig 2B).
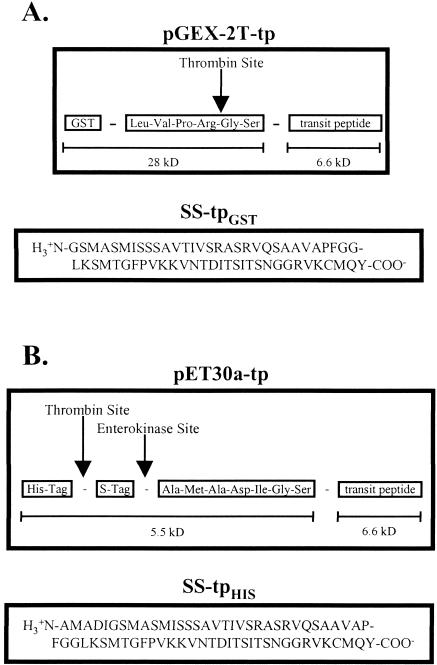
Fig 1.
Design of SStp fusion constructs. (A) Expression of SStp in pGEX-2T yields a 34.6-kDa fusion product with SStp attached to the C-terminus of glutathione-S-transferase (GST). On cleavage with thrombin, the resulting SStpGST contains 2 additional N-terminal amino acids, glycine and serine. Also, both fusion proteins contain the first 2 amino acids (MQ) of the mature domain of the small subunit and a tyrosine at the C-terminus of SStp. (B) Expression of SStp in pET-30a yields a 12.1-kDa fusion product with SStp attached to the C-terminus of the S-tag epitope sequence. On cleavage with enterokinase, the resulting SStpHIS contains 7 additional N-terminal amino acids (AMADIGS)
Fig 2.
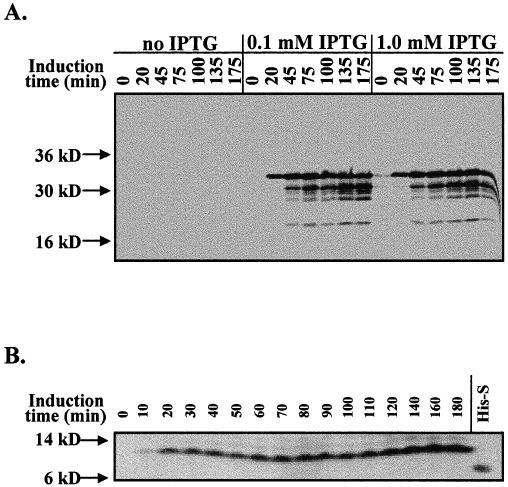
Glutathione-S-transferase (GST)–SStp and His6-S-peptide tag (His-S)–SStp expression. (A) Western blot of Escherichia coli whole-cell extract with α-GST antibody at various stages of induction of GST-SStp expression. (B) Far-Western blot of E coli whole-cell extract with S-protein alkaline phosphatase conjugate during induction of His-S–SStp expression. His-S refers to the peptide expressed by induction of the pET30a vector in BL21(DE3) cells
When the GST-SStp fusion protein was eluted from the glutathione-Sepharose column, 2 major bands were routinely observed: GST-SStp with a molecular weight of approximately 34 kDa, and a second protein with an apparent molecular weight of 70 kDa. The 70-kDa protein, though appearing in varying amounts depending on the induction, lysis, and chromatography conditions, was strongly associated with GST-SStp, because extensive washing with 1% Triton and 1 M NaCl did not disrupt the complex (data not shown). Thrombin cleavage of the bound GST-SStp released SStpGST from GST, and the 70-kDa protein coeluted with SStpGST from the glutathione-Sepharose column (Fig 3A). Attempts to separate SStpGST from this 70-kDa protein via ultrafiltration through a 30-kDa MWCO membrane yielded no protein in the flowthrough (data not shown), further indicating the existence of a high-molecular-weight complex. Western blotting identified the 70-kDa protein as DnaK, the prokaryotic Hsp70 homologue (Fig 3B). This interaction was mediated through SStpGST, because isolation of GST from cells containing the control pGEX-2T plasmid did not contain DnaK.
Fig 3.
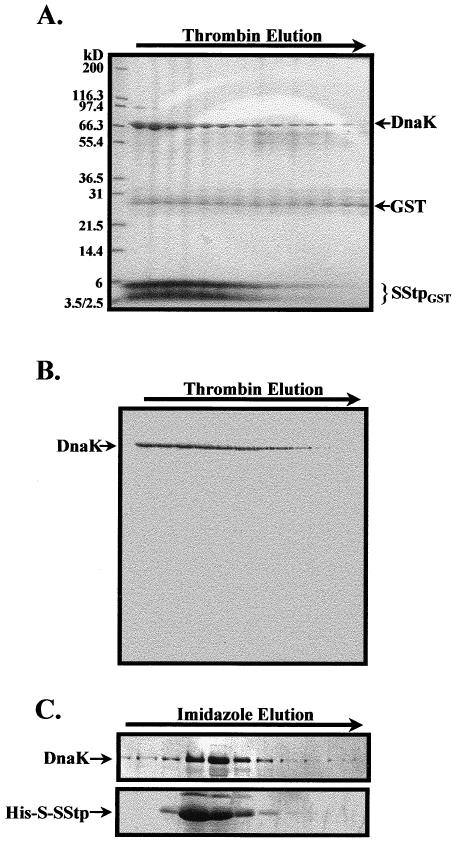
Copurification of DnaK and SStp. (A) Coommassie brilliant blue–stained sodium dodecyl sulfate gel of the elution profile from a glutathione-Sepharose column after thrombin treatment. A 70-kDa contaminant protein (DnaK) coelutes with SStpGST. The minor band at 26 kDa, which is constant in every fraction, shows column leakage of cleaved glutathione-S-transferse (GST). The 4-kDa band is an in vivo degradation product of SStp after 16 hours of induction. (B) Western blot of the fractions in panel A probed with α-DnaK confirmed that the contaminating protein was the molecular chaperone DnaK interacting in vivo with SStpGST. (C) The imidazole elution profile from a Ni-Sepharose column was probed with α-DnaK (upper panel) in a Western blot and with the S-protein–alkaline phosphatase conjugate (lower panel) in a far-Western blot. The cells were lysed under native conditions (without denaturant)
To verify that the sequences responsible for DnaK interaction were localized in SStp, a second fusion protein, His-S–SStp, was expressed in E coli. When “native” lysate from these cells was applied to a Ni-Sepharose column, His-S–SStp and DnaK copurified in the same fractions. This fusion protein allows the S-peptide epitope to be detected via its interaction with an alkaline phosphatase conjugate of S protein (Kim and Raines 1993). Figure 3C shows a duplicate Western and a far-Western analysis of imidazole elution fractions using an α-DnaK antibody in the former and an S protein/alkaline phosphatase conjugate in the latter. Again, DnaK coeluted with His-S–SStp. DnaK did not coelute with the vector-encoded control peptide containing both the His-tag and the S-tag epitopes (data not shown). Considered together, these data strongly indicate that SStp contains sequences recognized by DnaK when expressed in E coli. This DnaK/SStp association is independent of SStp's fusion partner and is seen for both moderate and strong levels of expression.
Purification of DnaK, SStpGST, and GST
Binding and/or hydrolysis of ATP induces Hsp70s to undergo a conformation change that results in the release of their peptide substrates (Liberek et al 1991; Szabo et al 1994). Therefore, after sample loading and column washing, 3 mM ATP was used to elute pure DnaK from the glutathione-Sepharose column (Fig 4A). Subsequent thrombin digestion of the remaining protein on the column yielded pure SStpGST (Fig 4B). Finally, GST was also eluted with 5 mM glutathione (Fig 4C). Thus, 3 proteins were sequentially purified from a total E coli lysate using a single chromatography step. That SStpGST dissociated from DnaK in an ATP-dependent fashion indicates that it was specifically bound via the peptide-binding domain of DnaK (similar to other DnaK substrates).
Fig 4.
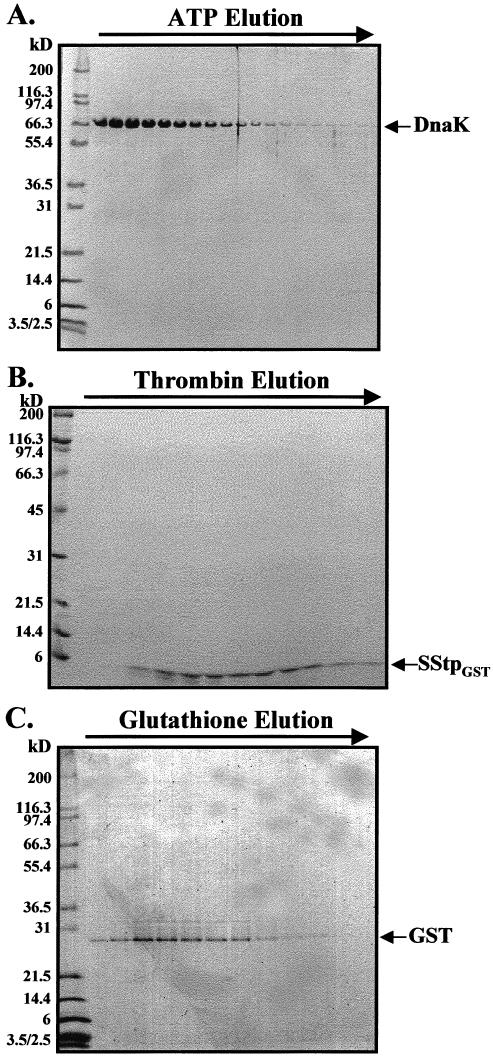
Purification of SStpGST. (A) Coommassie brilliant blue (CBB)–stained sodium dodecyl sulfate (SDS) gel of the elution profile from a glutathione-Sepharose column after incubation with 3 mM ATP. DnaK elutes alone, leaving glutathione-S-transferase (GST)–SStp bound to the matrix. (B) CBB-stained SDS gel of the elution profile from the same glutathione-Sepharose column in panel A after subsequent thrombin treatment. Pure SStpGST elutes from the column. (C) GST elutes after subsequent equilibration with 5 mM glutathione
In vitro analyses of DnaK/SStp interaction
To explore the ability of DnaK and SStp to interact in vitro, we conducted several biophysical and biochemical analyses to detect conformational changes in DnaK on addition of SStp. Initially, for a global average of possible peptide binding–induced secondary changes in DnaK, circular dichroism was performed both in the presence and in the absence of SStp (Fig 5A). The increase in magnitude of the negative peak at 211 nm on addition of SStp is similar to spectra previously observed for Hsc70/peptide complexes in which β-turn content increased (ie, stabilized) but β-sheet content decreased (Park et al 1993). Because the change in the CD spectra has already been corrected for the contributions of the transit peptide, the spectra we observed indicate that the presence of a substrate altered the secondary structure of the chaperone. The details of DnaK/SStp interaction are not known, but the observed changes in the CD spectra are entirely consistent with the recently described DnaK crystal structure, which demonstrates several direct interactions between the extended peptide substrate and the β-turns in DnaK's substrate binding domain (Zhu et al 1996). These results are also consistent with: 1) predictions that transit peptides exist in extended conformations with little secondary structure (von Heijne and Nishikawa 1991), 2) proteolytic studies with preplastocyanin that demonstrate the accessibility of transit peptides to in vivo processing by stromal endopeptidases (Musgrove et al 1989), and 3) our own data that describe partial degradation of GST-SStp during expression in E coli (Fig 2A).
Fig 5.
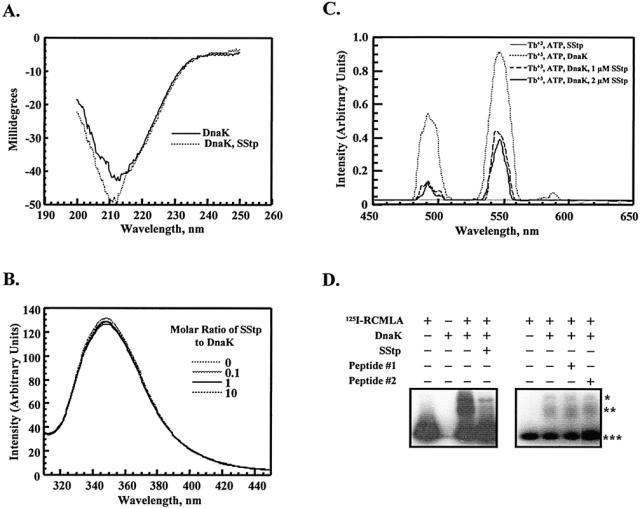
In vitro analyses of DnaK/SStp interaction. (A) Circular dichroism spectra of DnaK (500 nM) in the presence and absence of SStp (500 nM). (B) Tryptophan fluorescence emission spectra of DnaK (1 μM) in the presence and absence of SStp. (C) Stimulated luminescence spectra of Tb+3 (300 μM) complexed to ATP (300 μM) in the presence and absence of DnaK (1 μM) and/or SStp (1 or 2 μM as indicated). (D) Native gel shift competition assay with reduced, carboxymethylated α-lactalbumin radiolabeled with iodine (125I-RCMLA; 7 μM), DnaK (0.7 μM), and SStp (70 μM). *Putative DnaKdimer:125I-RCMLA complex; **Putative DnaKmonomer:125I-RCMLA complex; ***unbound 125I-RCMLA. Peptide 1, DGNDIITDSDGNDKLSFS; peptide 2, GKGDDIFVHRKGDGND.
Next, the fluorescence spectrum of DnaK's only tryptophan was analyzed to determine if a secondary structural change at position 102 occurred on SStp binding. On excitation at 290 nm, purified DnaK exhibited an emission peak at approximately 347 nm (Fig 5B), indicating that W102 resides in a relatively hydrophilic region of the native protein. Addition of SStp (even to as much as 10 times the molar ratio of DnaK) did not significantly affect the quantum yield or the emission wavelength maximum; therefore, W102 remains in a hydrophilic environment when DnaK is bound to SStp. Given that W102 is positioned at the surface of DnaK, and specifically at the “tip” of subdomain 2 of the ATPase domain (Harrison et al 1997), these findings are not surprising.
To demonstrate that SStp binding in vitro also affects DnaK's ATPase domain, energy-transfer experiments were conducted to observe any distance changes between W102 and the ATP-binding site at the opposite end of DnaK's subdomain 2 (Harrison et al 1997) on addition of SStp. Terbium(III) complexed to ATP (TbATP) at DnaK's ATP-binding site allowed a specific luminescence-labeling strategy to observe this phenomenon in a controlled experiment rather than in a copurification from a variable, crude cellular extract. On addition of TbATP to purified DnaK and initial excitation of W102 at 290 nm, Tb+3 exhibited characteristic, sensitized luminescence dependent on excitation by W102 emission at 320 nm (Fig 5C). When an equimolar amount of SStp was added, the luminescence intensity decreased, indicating an increased distance between W102 and Tb+3 on SStp binding. The effect was also saturatable, because doubling the amount of SStp did little to change the luminescence signal. Therefore, SStp interacts with DnaK in a 1:1 stoichiometric ratio, which is consistent with current evidence and models for other peptides. SStp alone did not stimulate Tb+3 luminescence. Because the R0 value for tryptophan (donor) and Terbium (acceptor) (Kwok and Churchich 1994) is many times less than the distance between W102 and the ATP-binding site (Harrison et al 1997), no specific distance calculations were attempted.
Finally, to show that SStp can competitively bind DnaK, native gel shift assays were performed with 125I-RCMLA and purified DnaK. Several studies have previously shown that the permanently unfolded nature of RCMLA allows it to function as a model substrate for DnaK and other Hsp70 homologues (Cyr et al 1992; Freeman et al 1995). By coincubating DnaK with both 125I-RCMLA and a competing, nonlabeled substrate, the relative affinities for the 2 substrates can be evaluated. The first autoradiograph in Figure 5D of 125I-RCMLA incubated with DnaK shows 2 new bands of lower electrophoretic mobility (lane 3). These 2 bands probably reflect the association of 125I-RCMLA with a monomer and dimer form of DnaK, similar to what has been observed in other reports (King et al 1995; Schonfeld et al 1995). When purified SStp was added to the incubation at a 3.6-fold mass excess relative to 125I-RCMLA, the 2 bands with lower mobility were disrupted, and RCMLA regained its original mobility. Scanning densitometry of these bands indicated that less than 10% of the original radiolabeled complex persisted in the presence of SStp, which in turn indicated that SStp successfully competes as an alternative substrate for DnaK. In fact, SStp prevents the formation of both DnaKmonomer:125I-RCMLA and DnaKdimer:125I-RCMLA complexes. As shown in the second autoradiograph of Figure 5D, 2 unrelated peptides, one of 16 and the other of 18 amino acids in length, failed to compete with RCMLA for DnaK binding, indicating that DnaK's affinity for SStp is specific. Furthermore, synthetic peptides corresponding to different regions of SStp tested in similar assay indicated that sequences at the N-terminus were most effective in competing with RCMLA, and the C-terminal region is inactive in competing with RCMLA for DnaK binding (Ivey et al, in preparation).
Purification of a cellular DnaK/DnaJ/GrpE chaperone complex
Glutathione-Sepharose affinity precipitation was used to evaluate the ability of SStpGST to associate simultaneously with DnaK and other members of the E coli DnaK chaperone machine. When the cells were lysed under mild conditions (Fig 6; no detergent and NaCl at low, medium, and high concentrations), DnaK, DnaJ, and GrpE all coprecipitated with GST-SStp. Obviously, DnaJ and GrpE were present in substoichiometric amounts, because similar lysis conditions showed neither cochaperone in CBB-stained gels (Figs 3 and 4). Also, neither DnaK, DnaJ, nor GrpE were coprecipitated from lysates of cells transformed with the control plasmid pGEX-2T (Fig 6). Consistent with results described earlier, these findings indicate that sequences contained within SStpGST mediate the interaction with chaperones. Interestingly, the interaction between DnaK and GrpE is detected under all the lysis conditions except high concentrations of the zwitterionic detergent Deriphat-160 and the anionic detergent SDS. DnaJ, however, coprecipitated only with low concentrations of Deriphat-160 and SDS or with no detergent (NaCl only). Whether DnaJ's association with the complex was mediated via direct interaction with SStpGST or, alternatively, by its association with the other chaperones has not been determined. Nonetheless, copurification of the entire DnaK chaperone complex provides further direct evidence that SStpGST serves as an Hsp70 substrate in a cellular environment.
Fig 6.
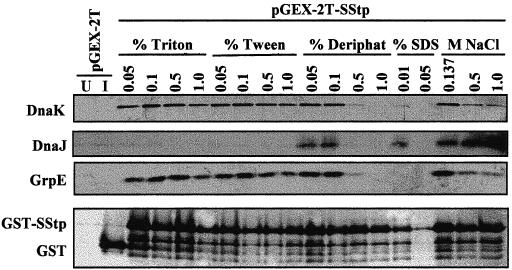
Cochaperones affinity-precipitated with DnaK and GST-SStp and Western blots of purified complexes using various cell lysis conditions (see text) with 10 μL of each sample loaded onto the gel. U indicates uninduced cells (no isopropylthio-β-d-galactoside [IPTG]), and I indicates induced cells (100 μM IPTG). The blot in the top panel was probed with α-DnaK, in the second panel with α-DnaJ, in the third panel with α-GrpE, and in the bottom panel with α–glutathione-S-transferse (GST). Note the in vivo proteolytic degradation of GST-SStp under all lysis conditions
DISCUSSION
A direct interaction between the Hsp70 class of molecular chaperones and chloroplast transit peptides has been proposed previously (von Heijne and Nishikawa 1991). Several recent studies have attempted to identify the linear peptide sequences recognized by Hsp70s (Blond-Elguindi et al 1993; Gragerov et al 1994; Rudiger et al 1997), but to our knowledge, none have directly analyzed chloroplast precursor proteins or their targeting sequences. Application of the peptide-binding preferences for DnaK to a group of mitochondrial presequences, however, predicts that as a rule, these presequences contain sequences that function as good substrates for DnaK (Zhang et al 1999). In this report, we demonstrate the first direct interaction between the Hsp70 class of molecular chaperones and a full-length targeting sequence. Specifically, the chloroplast transit peptide SStp was observed to copurify with DnaK under all but the harshest buffer conditions, indicating a tightly bound complex. This DnaK/SStp interaction is independent of SStp's fusion partner and is specific for sequences in the transit peptide, because DnaK failed to associate with either GST or the dual epitope tag His-S alone. This interaction is mediated through the DnaK peptide-binding domain, and like that of other high-affinity substrates, SStp's association is modulated by the status of nucleotide binding.
The stability of the DnaK/SStp interaction was sufficient to allow purification of the entire DnaK/DnaJ/GrpE chaperone complex. To our knowledge, this represents the first demonstration of an affinity-tagged substrate that can recover the in vivo assembled chaperone complex. An artificial substrate, cro repressor-protein A-β-galactosidase (CRAG), was recognized by DnaK when expressed in E. coli (Sherman and Goldberg 1991). This substrate also bound large quantities of GroEL, however, as well as lower amounts DnaK. In addition, the La protease and several other unidentified proteins also bound CRAG. These additional interactions, along with the fact that a significant amount of DnaK (≈30%) could not be released from the CRAG protein with ATP, argue for more than one mode of chaperone interaction and suggest possible nonspecific interactions. In contrast, our work shows that SStp is specific for DnaK and can be readily removed by ATP treatment. Therefore, we propose that SStp is an ideal DnaK substrate that can be used to explore the reaction dynamics of the DnaK chaperone cycle both in vivo and in vitro. Expression of SStp could also be used to monitor the effects of cellular and environmental stress on prokaryotic chaperone expression.
Finally, a productive Hsp70/transit peptide interaction might play an important role during chloroplast protein import. In mitochondria, a precursor's targeting sequence has been proposed to interact with Hsp70 not only to facilitate translocation but also to present the site of proteolytic cleavage to the matrix-processing protease (Klaus et al 1996). This chaperone/precursor interaction is supported by an earlier observation that the precursor for mitochondrial aspartate aminotransferase binds DnaK (Schmid et al 1992). Unlike SStp, however, this interaction did not include the cochaperones DnaJ or GrpE and was easily disrupted by low-salt treatment. In addition, that no complex was found with the mature protein suggests that DnaK recognition may occur via the presequence, as predicted recently (Zhang et al 1999). Conversely, in chloroplast protein transport, an Hsp70/transit peptide interaction may occur at more than one site during protein import. In fact, 4 different Hsp70 homologues are associated with the chloroplast: 1) Com70, which binds to the outer surface of the outer envelope (Ko et al 1992); IAP70, which resides within the intermembrane space (Marshall et al 1990), 3) CSS1, which is the major stromal Hsp70 (Marshall et al 1990), and 4) a thylakoid lumen homologue that has been most recently discovered (Schlicher and Soll 1996). The possible involvement of multiple Hsp70s in the chloroplast protein import pathway may constitute a significant difference between the protein translocation apparatus of the chloroplast, which contains a robust “unfoldase” activity that can overcome the folded status of a precursor (Guera et al 1993; America et al 1994; Clark and Theg 1997), and that of mitochondria, which cannot drive the import of large, “tightly folded” precursors (Eilers and Schatz 1986; Vestweber and Schatz 1988). The placement of Com70 at the outer envelope and IAP70 in the intermembrane space would provide 2 additional ATP-dependent sites for protein unfolding (Schnell et al 1994; Kourtz and Ko 1997). The ability of IAP70 to function as a “molecular motor” in the intermembrane space would also allow precursors to cross the chloroplast envelope one membrane at a time, as recently reported (Scott and Theg 1996).
Additional work in our laboratory has analyzed the transit peptides in the chloroplast transit peptide (CHLPEP) database (von Heijne et al 1991) using the data from 2 DnaK-affinity studies (Gragerov et al 1994; Rudiger et al 1997). It was found that most transit peptides are predicted to contain at least one Hsp70-binding site (Ivey et al, in preparation). Statistical analysis of the placement of these Hsp70-interacting regions suggests that sequences at the N-terminus are primarily responsible for this interaction. This observation is intriguing, because a similar analysis of mitochondrial presequences showed a similar N-terminal placement of Hsp70-interacting domains (Zhang et al 1999). The commonality of these 2 observations argues for a universal placement and involvement of Hsp70-interacting domains in the posttranslational transport of proteins into both the chloroplast and the mitochondria. Perhaps this observation is not surprising considering the common prokaryotic origins and evolution via endosymbiosis of the organelles. It will be interesting to see if chloroplast and mitochondrial Hsp70 homologues bind transit peptides in vivo and where within the targeting sequences these interactions occur.
Acknowledgments
We would like to thank Drs K. Keegstra and S. Perry for the gift of the pGEX-2T-SStp construct, Drs C. Georgopoulous and W. Wicks for the gifts of antibodies, and Drs J. Churchich and R. Miltenburger for many helpful discussions during the preparation of this manuscript. This work was supported by a grant to B.D.B. from the Cell Biology Program of the National Science Foundation.
Footnotes
This manuscript is dedicated to the memory of Professor Jorge E. Churchich—Friend, Colleague, and Mentor
REFERENCES
- America T, Hageman J, Guera A, Rook F, Archer K, Keegstra K, Weisbeek P. Methotrexate does not block import of a DHFR fusion protein into chloroplasts. Plant Mol Biol. 1994;24:283–294. doi: 10.1007/BF00020168.0167-4412(1994)024<0283:MDNBIO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJ. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9.0092-8674(1993)075<0717:APOALO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brodsky JL, Goeckeler J, Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995;92:9643–9646. doi: 10.1073/pnas.92.21.9643.0027-8424(1995)092<9643:BASARF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce BD. The role of lipids in plastid protein transport. Plant Mol Biol. 1998;38:223–246.0167-4412(1998)038<0223:TROLIP>2.0.CO;2 [PubMed] [Google Scholar]
- Bruce BD, Keegstra K 1994 Translocation of proteins across chloroplast membranes. In: Advances in Molecular and Cell Biology: Molecular Processes of Photosynthesis, ed. Barber J. Jai Press, Greenwich, CT, 389–430. [Google Scholar]
- Clark SA, Theg SM. A folded protein can be transported across the chloroplast envelope and thylakoid membranes. Mol Biol Cell. 1997;8:923–934. doi: 10.1091/mbc.8.5.923.1059-1524(1997)008<0923:AFPCBT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Kramer J, Shilling J, Werner-Washburne M, Holmes S, Kosic-Smithers J, Nicolet CM. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989;9:3000–3008. doi: 10.1128/mcb.9.7.3000.0270-7306(1989)009<3000:SAEMOT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DM, Lu X, Douglas MG. Regulation of Hsp70 function by a eukaryotic DnaJ homolog. J Biol Chem. 1992;267:20927–20931.0021-9258(1992)267<20927:ROHFBA>2.0.CO;2 [PubMed] [Google Scholar]
- Eilers M, Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986;322:228–232. doi: 10.1038/322228a0.0028-0836(1986)322<0228:BOASLI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x.0261-4189(1995)014<2281:IOARMI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragerov A, Zeng L, Zhao X, Burkholder W, Gottesman ME. Specificity of DnaK-peptide binding. J Mol Biol. 1994;235:848–854. doi: 10.1006/jmbi.1994.1043.0022-2836(1994)235<0848:SODPB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gray J, Row P. Protein translocation across chloroplast envelope membranes. Trends Cell Biol. 1995;5:243–247. doi: 10.1016/s0962-8924(00)89018-2.0962-8924(1995)005<0243:PTACEM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Guera A, America T, van Waas M, Weisbeek PJ. A strong protein unfolding activity is associated with the binding of precursor chloroplast proteins to chloroplast envelopes. Plant Mol Biol. 1993;23:309–324. doi: 10.1007/BF00029007.0167-4412(1993)023<0309:ASPUAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl FU, Kuriyan J. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science. 1997;276:431–435. doi: 10.1126/science.276.5311.431.0036-8075(1997)276<0431:CSOTNE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heins L, Collinson I, Soll J. The protein translocation apparatus of chloroplast envelopes. Trends Plant Sci. 1998;3:56–61.1360-1385(1998)003<0056:TPTAOC>2.0.CO;2 [Google Scholar]
- Karlin Neumann GA, Tobin EM. Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J. 1986;5:9–13. doi: 10.1002/j.1460-2075.1986.tb04170.x.0261-4189(1986)005<0009:TPONEC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K, Bruce BD, Hurley M, Li H-M, Perry S. Targeting of proteins into chloroplasts. Physiol Plant. 1995;93:157–162.0031-9317(1995)093<0157:TOPIC>2.0.CO;2 [Google Scholar]
- Kim JS, Raines RT. Ribonuclease S-peptide as a carrier in fusion proteins. Protein Sci. 1993;2:348–356. doi: 10.1002/pro.5560020307.0961-8368(1993)002<0348:RSPAAC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle KL. Amino-terminal and hydrophobic regions of the Chlamydomonas reinhardtii plastocyanin transit peptide are required for efficient protein accumulation in vivo. Plant Mol Biol. 1998;38:365–377. doi: 10.1023/a:1006025606330.0167-4412(1998)038<0365:ATAHRO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- King C, Eisenberg E, Greene L. Polymerization of 70-kDa heat shock protein by yeast DnaJ in ATP. J Biol Chem. 1995;270:22535–22540. doi: 10.1074/jbc.270.38.22535.0021-9258(1995)270<22535:POKHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Klaus C, Guiard B, Neupert W, Brunner M. Determinants in the presequence of cytochrome b2 for import into mitochondria and for proteolytic processing. Eur J Biochem. 1996;236:856–861. doi: 10.1111/j.1432-1033.1996.00856.x.0014-2956(1996)236<0856:DITPOC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ko K, Bornemisza O, Kourtz L, Ko ZW, Plaxton WC, Cashmore AR. Isolation and characterization of a cDNA clone encoding a cognate 70-kDa heat shock protein of the chloroplast envelope. J Biol Chem. 1992;267:2986–2993.0021-9258(1992)267<2986:IACOAC>2.0.CO;2 [PubMed] [Google Scholar]
- Kourtz L, Ko K. The early stage of chloroplast protein import involves Com70. J Biol Chem. 1997;272:2808–2813. doi: 10.1074/jbc.272.5.2808.0021-9258(1997)272<2808:TESOCP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kwok F, Churchich JE. The binding of substrates and inhibitors to the metal center of myoinositol monophosphatase. FEBS Lett. 1994;346:304–306. doi: 10.1016/0014-5793(94)00506-0.0014-5793(1994)346<0304:TBOSAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liberek K, Skowyra D, Zylicz M, Johnson C, Georgopoulos C. The Escherichia coli DnaK chaperone, the 70-kDa heat shock protein eukaryotic equivalent, changes conformation upon ATP hydrolysis, thus triggering its dissociation from a bound target protein. J Biol Chem. 1991;266:14491–14496.0021-9258(1991)266<14491:TECDCT>2.0.CO;2 [PubMed] [Google Scholar]
- Marshall JS, DeRocher AE, Keegstra K, Vierling E. Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci U S A. 1990;87:374–378. doi: 10.1073/pnas.87.1.374.0027-8424(1990)087<0374:IOHSPH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, Keegstra K. Isolation and characterization of a cDNA clone encoding the major Hsp70 of the pea chloroplastic stroma. Plant Physiol. 1992;100:1048–1054. doi: 10.1104/pp.100.2.1048.0032-0889(1992)100<1048:IACOAC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove JE, Elderfield PD, Robinson C. Endopeptidases in the stroma and thylakoids of pea chloroplasts. Plant Physiol. 1989;90:1616–1621. doi: 10.1104/pp.90.4.1616.0032-0889(1989)090<1616:EITSAT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Akita M, Davila-Aponte J, Keegstra K. Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J. 1997;16:935–946. doi: 10.1093/emboj/16.5.935.0261-4189(1997)016<0935:SAOCPW>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Voos W, Kang PJ, Craig EA, Neupert W, Pfanner N. Precursor proteins in transit through mitochondrial contact sites interact with hsp70 in the matrix. FEBS Lett. 1990;277:281–284. doi: 10.1016/0014-5793(90)80865-g.0014-5793(1990)277<0281:PPITTM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Park K, Flynn GC, Rothman JE, Fasman GD. Conformational change of chaperone Hsc70 upon binding to a decapeptide: a circular dichroism study. Protein Sci. 1993;2:325–330. doi: 10.1002/pro.5560020304.0961-8368(1993)002<0325:CCOCHU>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon M, Wienk H, Sips W, et al. Functional domains of the ferredoxin transit sequence involved in chloroplast import. J Biol Chem. 1995;270:3882–3893. doi: 10.1074/jbc.270.8.3882.0021-9258(1995)270<3882:FDOTFT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pinnaduwage P, Bruce BD. In vitro interaction between a chloroplast transit peptide and chloroplast outer envelope lipids is sequence-specific and lipid class-dependent. J Biol Chem. 1996;271:32907–32915. doi: 10.1074/jbc.271.51.32907.0021-9258(1996)271<32907:IVIBAC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rensink WA, Pilon M, Weisbeek P. Domains of a transit sequence required for in vivo import in Arabidopsis chloroplasts. Plant Physiol. 1998;118:691–699. doi: 10.1104/pp.118.2.691.0032-0889(1998)118<0691:DOATSR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501.0261-4189(1997)016<1501:SSOTDC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519.0036-8075(1996)271<1519:CPOPTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scherer PE, Krieg UC, Hwang ST, Vestweber D, Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990;9:4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x.0261-4189(1990)009<4315:APPPTI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicher T, Soll J. Molecular chaperones are present in the thylakoid lumen of pea chloroplasts. FEBS Lett. 1996;379:302–304. doi: 10.1016/0014-5793(95)01534-5.0014-5793(1996)379<0302:MCAPIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schmid D, Jaussi R, Christen P. Precursor of mitochondrial aspartate aminotransferase synthesized in Escherichia coli is complexed with heat-shock protein DnaK. Eur J Biochem. 1992;208:699–704. doi: 10.1111/j.1432-1033.1992.tb17237.x.0014-2956(1992)208<0699:POMAAS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schnell DJ, Kessler F, Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994;266:1007–1012. doi: 10.1126/science.7973649.0036-8075(1994)266<1007:IOCOTC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schonfeld HJ, Schmidt D, Schroder H, Bukau B. The DnaK chaperone system of Escherichia coli: quaternary structures and interactions of the DnaK and GrpE components. J Biol Chem. 1995;270:2183–2189. doi: 10.1074/jbc.270.5.2183.0021-9258(1995)270<2183:TDCSOE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scott SV, Theg SM. A new chloroplast protein import intermediate reveals distinct translocation machineries in the two envelope membranes: energetics and mechanistic implications. J Cell Biol. 1996;132:63–75. doi: 10.1083/jcb.132.1.63.0021-9525(1996)132<0063:ANCPII>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MY, Goldberg AL. Formation in vitro of complexes between an abnormal fusion protein and the heat shock proteins from Escherichia coli and yeast mitochondria. J Bacteriol. 1991;173:7249–7256. doi: 10.1128/jb.173.22.7249-7256.1991.0021-9193(1991)173<7249:FIVOCB>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J, Waegemann K. A functionally active protein import complex from chloroplasts. Plant J Oxford. 1992;2:253–256. [Google Scholar]
- Szabo A, Langer T, Schroder H, Flanagan J, Bukau B, Hartl FU. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system DnaK, DnaJ, and GrpE. Proc Natl Acad Sci U S A. 1994;91:10345–10349. doi: 10.1073/pnas.91.22.10345.0027-8424(1994)091<10345:TAHDRC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D, Schatz G. A chimeric mitochondrial precursor protein with internal disulfide bridges blocks import of authentic precursors into mitochondria and allows quantitation of import sites. J Cell Biol. 1988;107:2037–2043. doi: 10.1083/jcb.107.6.2037.0021-9525(1988)107<2037:ACMPPW>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Hirai T, Klösgen RB, Steppuhn J, Bruce BD, Keegstra K, Herrmann R. CHLPEP—a database of chloroplast transit peptides. Plant Mol Biol Rep. 1991;9:104–126.0735-9640(1991)009<0104:CADOCT>2.0.CO;2 [Google Scholar]
- von Heijne G, Nishikawa K. Chloroplast transit peptides. The perfect random coil? FEBS Lett. 1991;278:1–3. doi: 10.1016/0014-5793(91)80069-f.0014-5793(1991)278<0001:CTPTPR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang XP, Elofsson A, Andreu D, Glaser E. Interaction of mitochondrial presequences with DnaK and mitochondrial hsp70. J Mol Biol. 1999;288:177–190. doi: 10.1006/jmbi.1999.2669.0022-2836(1999)288<0177:IOMPWD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606.0036-8075(1996)272<1606:SAOSBB>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]