Abstract
BACKGROUND:
Electromagnetic fields are associated with production, transmission and use of electricity. In this study we have investigated the effects of short and long time alternative magnetic fields’ (AMF, 50 Hz) exposure on the secretion of hypothalamic-pituitary-gonadal axis in the male rats.
METHODS:
Forty eight Wistar male rats, same range of age and weight were divided into four groups and each group contained 12 rats. After one-week adaptation each group were exposed to AMF (0, 25, 50 and 100 μT respectively) for 17 days, 5 hours a day. In the second protocol the time of exposure extended to 34 days. After experiments rats’ blood serums were removed from their blood samples and kept frozen for usage. The results were analyzed by one way-ANOVA statistical method (p < 0.05).
RESULTS:
Chronic exposures (5h/day for 34 days) to AMFs had no effect on serum's testosterone and LH. But, AMF at 100 μT induced an increase of serum's FSH level in comparison with 25 μT, 50 μT and control groups. In contrast, sub-chronic AMFs (5 h/day for 17 days) induced a decrease of serum's testosterone in control group in comparison with 25, 50 and 100 μT groups. But these AMFs had no effect on serum's LH and FSH levels.
CONCLUSIONS:
Increased level of FSH suggests damage to the seminiferous tubules. Our results suggest that AMFs probably causes dysfunction in gonadal axis at the hypothalamic-pituitary level in male rats in different protocols.
Keywords: Sex Hormones, Male Rats, Magnetic Field
Exposure of extremely low frequency (ELF, 50 and 60 Hz) electromagnetic fields (EMF) to human environment in the modern societies due to power stations, power cable transporting and many electrical appliances are very obvious, therefore the potential effects of ELF-EMF on human organs are very high and effective.1 ELF-EMF has possible adverse effects on reproductive and developmental outcomes which have studied extensively in animals in the past.2 Despite of the advantages of electricity there are some possible adverse effects too like being harmful for tissues, hormonal secretions, sperms process, development of proliferation and other biological cells.3–8 One area that has been studied by a number of researchers is the potential of EMFs to adversely affect reproduction.9 Studies have been, in most cases, evaluated the effects on the female, included effects on infertility, placental resorption, miscarriages, or embryo malformation.10 The possible effects of EMF exposure on male reproductive processes are few and experimental outcomes are quite different: reversible changes in spermatogenesis epithelium, aberrations in rat spermatozoa, and no effects on human fertility.1 Forgacs et al11 indicated a presumably direct effect of whole body magnetic fields (MF) exposure on the chorionic gonadotropin-stimulated steroidogenic response of mice's leydig cells. MF exposure can make changes in neuroendocrine system's function in a mammalian laboratory model.12 Suppression of melatonin in the blood and pineal of rats and hamsters as a consequence of EMF exposure has been reported by several laboratories. Reduction in activity of N-acetyl transferase and the rate-limiting enzyme in the production of melatonin have been reported in a number of these studies too. Melatonin has been demonstrated to inhibit the hypothalamic-pituitary-gonadal (HPG) axis in certain species of mammals.12,13 There is also increasing concern that chronic or long-term exposure to low EMFs from many appliances, in the home or workplace, may cause adverse reproductive effects. Various researchers using mice as experimental models have attempted to elucidate the reproductive toxic effects of exposure to weak MFs and the results have been found to be rather contradictory.11 A significant increase in malformations was found after gestational exposure to a pulsed MF (20 KHz) in C3H mice. Al-Akhras et al have applied 25 μT sinusoidal MF for 90 days on adult male and female rats and have found some adverse effects on fertility of them.5
Also a lot of studies have been conducted on useful biological effects of EMF exposure on mammals, for example, Okano et al14 found that whole body exposure to static MF (SMF) at 10 and 25 mT for 2-9 weeks suppressed and delayed blood pressure (BP) elevation in young, stroke resistant, spontaneously hypertensive rats (SHR). They suggested in another study that SMF at 5 mT may suppress and delay BP elevation via the nitric oxide pathways and hormonal regulatory systems.15 But data about the effects on the male reproductive system are limited. There would seem to be a lack of agreement between scientists on the possible negative effects of EMFs on male reproductive parameters. Thus, there is an obvious need for additional information about the effects of exposure on HPG axis.
Some of previous studies have measured rat's mass, which are controversy.16–18 Some of the investigators reported increase,16 some reported decrease 17 and some reported no difference 18 between the weight of the control and electric or magnetic field treated rats, depending on the time of the exposure.
This study evaluated the possible effect of short and long time AMFs of 50 Hz on the secretion of testosterone, FSH and LH in the male rats, or the secretion of hypothalamic-pituitary-gonadal axis.
Methods
Animal Maintenance
Forty eight Wistar male rats, (obtained from Razi institute, Karaj, Iran) with same range of age (4-5 month old) and weight (215-230 g), were divided into four groups, each group contained 12 rats. After one-week for adaptation, each group was exposed to AMF (0, 25, 50 and 100 μT respectively), 5 hours daily for 17 days. In the second protocol the range of age was 2-3 month, weight was 63-70 gr and the time of exposure was extended to 34 days. Experiments were performed using 4 alternative groups. To ignore several unwanted factors experiments were started at 9:00 A.M. Rats were housed; six per cage, with free access to tap water and fed laboratory chow (palletized commercial chow diet purchased from Pars Veterinary Food Company/Iran). They maintained at approximately 25°C and 55% humidity with a dark-light cycle of 12/12 hours. All procedures were performed in accordance with the guidelines of the University and National policies of animal for the care and use of laboratory animals, under an institutionally approved protocol.
Experimental Procedure
Exposure cages were made of transparent Kaolin (resin) and each cage was equipped with top ventilation pores. Due to electrical safety conditions, water was withheld during the exposure period. The transparent cages and the open constructed coil systems allowed the animals to be easily viewed and to experience similar ambient conditions during both normal and exposure periods. In all experiments, the long axis of the cages was aligned with in direction of applied AMFs.
AMFs Generating Device
AMFs were designed and produced using homemade coil (Department of Medical Physics, Jundishapour Medical Sciences University of Ahwaz) which could generate static and pulsative MFs. The apparatus was electrical coil with up to 10000 turns and with nucleus rectangular cubical dimensions of 15×15 cm2, made from pure iron in which the distance between poles could be varied and was adjustable. MFs were distinguished and measured using a gauss meter (Brockhause Messtechnic Company model 410) at different distances between and around the poles. Distances of cages were determined and rats were moved freely in these homogeneous fields. In both sides between the poles, the AMFs intensities were 25 ± 1.25, 50 ± 2.5 and 100 ± 5 μT and 6 rats were in each cage during exposure of AMF. Each field was determined using a gauss meter that we assured constancy of the field. The current could be activated through a switch which was housed in a locked box together with the transformer and resistor. The constancy of the fields could be controlled by the variation of the amperage which achieved by rheostat in the circuit of the system. The generated field did not cause any sound or any other manifestation that could be perceived by the rats or experimenters. Whole magnetic generator fixed to wooden framed boxes and the absence of significant vibrations was confirmed by acceleration measurements. Furthermore, the frame with coils was mechanically separated from the animals’ cages. The coil's design (number of turns, cross-section of conductor and rated current) was optimized in order to reduce joule-heating losses to the level which did not affect the temperature of the exposure area. Control group's rats were held in a similar non-energized system in which the MF was equal to the background level (< 0.05 mT).
Collecting Blood Samples
Prior to exposure, animals were randomly assigned to one of the four exposure groups (n = 12). After 17 or 34 days of AMFs exposure, they anesthetized using ether and decapitated between 09:00 and 11:30 A.M. and their blood were immediately drawn into tubes without anticoagulant, allowed to clot, and then centrifuged to obtain the serum which was kept frozen under -20°C until the time of usage. Hormones were measured using gamma counter equipment (Contron Company of Swiss) with RIA and IRMA methods.
Measurement of Testosterone, LH and FSH
Fifty μl of serum was used for the measurement of testosterone by radioimmunoassay (Biosource Testo-RIA-CT Kit). In this assay, the cross-reactivity with dihydrotestosterone is less than 1% and the minimal detectable concentration (M.D.C.) of testosterone was 0.44 ng/dl. The intra- and interassay variances (CV) were 4% and 8%, respectively.
LH and FSH were measured using IRMA method (DSL Inc). In this assay, the cross-reactivity was non-detectable with other glycoprotein hormones (FSH, LH, TSH, HCG and β-hCG). M.D.C. of LH and FSH were 0.12 mIU/ml and 0.11 mIU/ml, respectively. The intra- and inter-assay variances of LH were 8% and 8%, and of FSH were 3% and 7%, respectively. Because rat's specific glycoprotein hormonal kit is expensive, human glycoprotein hormonal kits were used instead of animals’ kits. Routine cross-reactivity test on rat's hypophysis (n = 15) also have been done for majority of experiments, which showed there are no differences between rat's LH and FSH (U.S.A.DRG International RIA catalog No.3732 and 3729, respectively) and human's kit in these circumstances.
Glycoprotein hormones’ measurement must be done using rat's special kits. For this reason cross-reactivity test with human FSH and LH kits, was done on some of the rats which couldn’t use rat's special kits and correlation coefficient (r = 0.89) obtained which showed there are much more similarity between humans’ and rats’ FSH and LH.
Statistical Analysis
Results are expressed as mean ± S.E.M. One way analysis of variance (ANOVA) which in some necessary cases, followed by, Tukey's test was used to determine the statistical significance of differences between means. A p value of less than 0.05 was taken as statistical significance.
Results
Effect of The AMFs 50 Hz on Plasma Testosterone, FSH and LH
There were statistically significant decreases in serum testosterone values in the groups that were exposed to AMFs for 17 days at 25, 50 and 100 μT in comparison with control group (Figure 1). These data showed that no changes in LH and FSH level were found at short time MFs exposure protocols in exposed or control group's animals. Testosterone hormone wasn’t changed significantly (p > 0.05) while exposing to AMFs for 34 days. These data showed that no changes in LH level were found at long time MFs exposure protocols were in exposed or control group's animals. FSH level of rats which exposed to 100 μT for 34 days increased significantly (p < 0.05) in comparison with those that exposed to 25 μT and 50 μT and control group's (Figure 2).
Figure 1.

The level of testosterone concentration of the animals’ group that exposed to 25, 50 and 100 μT AMFs, 5 hours daily for 17 days. Animals that exposed to 25 and 100 μT (p < 0.01) and 50 μT (p < 0.001) decreased significantly in comparisons with shamexposed (n = 12). (Mean ± SE)
Figure 2.

The level of FSH concentration of the animals’ group that exposed to 25, 50 and 100 μT AMFs, 5 hours daily for 34 days. Animals that exposed to 100 μT increased significantly (p < 0.05 ) in comparison with sham-exposed, 25 and 50 μT (n = 12). (Mean ± SE)
Effect of The AMFs 50 Hz on The Weight
Figures 3 and 4 have shown that animal's weight changed related to their age at the end of the exposures. Except those animals which exposed to 25 and 50 μT (17 days) AMFs (p < 0.05) (Figure 3).
Figure 3.

The weight of animals which have exposed to 25, 50 and 100 μT AMFs, 5 hours daily for 17 days. The sham and 100 μT groups have a significantly increase of weight (p < 0.01) in comparisons to the final stage of experiments but the weight of those animals which have been exposed to 25 and 50 μT have not changed significantly (n = 12). (Mean ± SE)
Figure 4.
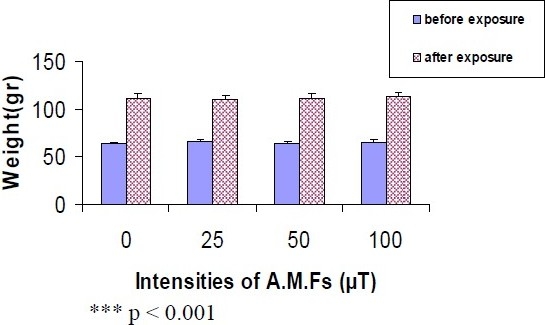
The weight of animals which have been exposed to 25, 50 and 100 μT AMFs, 5 hours daily for 34 days. The weight of animals in sham, 25, 50 and 100 μT groups have been increased significantly (p < 0.001) comparing with their weight at the end (n = 12). (Mean ± SE)
Discussion
Effect of The AMFs 50 Hz on Plasma Testosterone, FSH and LH
We have studied possible effect of an alternative 50 Hz MFs with densities of 25, 50 and 100 μT exposure, 5 hours daily for 17 or 34 days, on hormonal changes of the control and exposed subjects.
Our data were shown that no changes of LH and FSH at any MFs protocols were found in exposed or control animals except FSH levels of rats which exposed to 100 μT (34 days) increased significantly in comparison with control group and 25 and 50 μT exposed. Also there was statistically significant decrease in serum testosterone values in those exposed to AMFs for 17 days, in comparison with control group.
However there are some controversial studies about the hypophysis-gonadal axis hormones which have been reported by different laboratories. Free et al (1981) have found alterations in the secretion pattern of FSH in rats exposed to an 80 KV/m electric field for 20 to 56 days. They have also observed a reduction in plasma's testosterone level after 120 days of electric field exposure.19 Al-Akhras et al (2006) reported that there were no significant effects on the serum's level of male follicle stimulating hormone (FSH) during the 18 weeks of exposure period. On the other hand, there was a significant increase in the serum's level of male luteinizing hormone (LH) after 18 weeks of exposing male rats to intensity magnetic field of 25 μT, while testosterone's level were significantly decreased only after 6 and 12 weeks of exposure. These results suggest that long term exposure to ELF could have adverse effects on mammalian fertility and reproduction.20 Al-Akhras (2008) reported that serum's level of luteinizing hormone (LH), follicle stimulating hormone (FSH), progesterone, and estrogen were measured before, after, and during the 18 weeks of exposing female rats to intensity magnetic field of 25 μT. Body and uterine weights were not affected by the field. The reduction in the levels of gonadotropins (FSH and LH) was significant after six weeks of exposure. FSH level was affected only on the sixth week of exposure while LH was still being affected after 12 and 18 weeks. Interestingly, no significant effects were found in 6 and 12 weeks after removing the field. The level of progesterone and estrogen was significantly decreased after 12 weeks of exposure, while no other effects on progesterone level was observed during exposure or after removing the field. The level of estrogen was also significantly reduced in 12 weeks after removing the field. These results suggest possible adverse effect on mammalian fertility and reproduction. The effects of ELF-MF on sex hormones were shown to be partly reversible.21 These are similar to the data that is being presented in this result. But Forgacs et al (1998) have demonstrated that exposure to sinusoidal, 50 Hz and 100μT MF increased the basal testosterone production in the primary 48 hours that mouse cultured leydig cell, whereas the steroidogenic capacity response to hCG remained unchanged.22
These results suggest the probable causes of dysfunction in gonadal axis at the hypothalamic-pituitary level in male rats. So it can be concluded that AMFs were decreased the secretion of hypothalamic-pituitary-gonadal axis in male rats, and origin of disturbed testis in this protocol at least was related to hypothalamic-pituitary level.
Although our data show that there were no changes in serum's testosterone value in AMFs exposed groups for 34 days, in comparison with control group. This is similar to McGivern et al (1990) research that have reported pregnant Sprague-Dawley dams were exposed to a low-level, low-frequency pulsed EMF (15 Hz, 0.3 milli second duration, peak intensity 8 gauss) for 15 min twice a day from 15th day to 20th day of gestation (a period in development that is critical for sexual differentiation of the male rat brain). At the age of 120 days, field-exposed male offspring had normal circulating levels of the testosterone, LH and FSH hormones, as well as epididymal sperm counts.23 Also, Margonato et al (1993) who had done three years of investigation on the biological effects of high intensity electric field exposure to rats for up to 18% of their life span, but they did not find any differences on LH, FSH and testosterone between exposed animals and control group's animals.24 Kato et al (1994) reported that 6 weeks of nearly continuous exposure to circularly polarized 50 Hz magnetic fields did not change plasma's testosterone level in rats.25 This is similar to the data that is being presented in this paper. Margonato et al (1995) did not find any magnetic field-induced morphologic and histological changes in tested rats after prolonged exposure to a 50 Hz magnetic field at 5μT.26 Selmaoui et al (1997) in the same research direction reported that LH and FSH secretion was unaffected by the acute exposure to a 50 Hz linearly polarized magnetic fields of 10μT on 32 young men of 20-30 years old age.27 Zecca et al (1998) have chosen two groups of adult male Sprague-Dawley rats and exposed them to EMF of two different field strength combinations: 5μT-1KV/m and 100 μT-5KV/m for 8 months. They did not find any changes in serum's LH concentration.28 The latter finding is compatible with the absence of plasma's testosterone changes described by Kato et al (1994) in rats exposed to MFs of similar strength. Indeed, testosterone's release is mainly controlled by LH.25 These are similar to the data that is being presented in this study.
Effect of The AMFs 50 Hz on The Weight
Weight's changes experiments show different patterns according to various protocols. Our data about the weight's changes in those exposed for 34 days 5 hours a day, is the same as the report of Hilton and Phillips.18 There were no differences between effects of MFs on weight of the control group and electric or magnetic field treated rats. In this, protocol we have not seen any significant changes in the weight due to AMFs exposure which can be speculated according to no change in the testosterone concentration.
In contrast to the groups which were exposed to the AMFs of 25 and 50 μT intensity for 17 days 5 hours a day, which the effect on their weight was observed, unchanged animal's weight can be due to decreased level of testosterone. It was expected that the weight of animals would be decreased similar to the report of Sandrey et al.17
In conclusion the results of this study show the effects of AMFs on the secretion of hypothalamic-pituitary-gonadal axis in male rats. Present results show that there was statistically significant decrease in HPG axis at short duration (17 days). In our views the effects of EMFs on the biological systems depend on the intensity and time of exposure. We have reported that short duration (17 days) of exposing to SMFs have no effects on hypothalamic-pituitary-gonadal and thyroidal hormones.29–31 In contrast we have also reported that long term (34 days) and increased daily time exposure to SMFs can alter hypothalamic-pituitary-gonadal and thyroidal hormones.31,32
Conclusions
Therefore we can conclude that MFs have widespread biological effects especially on sex organ and CNS. The melatonin can be involved to affect on dysfunction in gonadal axis at the hypothalamic-pituitary level in rats exposed to 50 Hz electromagnetic fields. The mechanism of action is unknown.
Authors’ Contributions
AA and HFM designed the study, collected and analyzed the data and wrote the manuscript.
MJTB operated and controlled magnetic fields, HS endocrinology consultant and MB helped in statistics analysis.
All authors have read and approved the content of manuscript.
Acknowledgments
This research was financially supported by the research affairs Jundishapour Medical Sciences University of Ahwaz, Ahwaz, Iran (N.389).
Footnotes
Conflict of Interests
Authors have no conflict of interests regarding this paper.
References
- 1.Chung MK, Lee SJ, Kim YB, Park SC, Shin DH, Kim SH, et al. Evaluation of spermatogenesis and fertility in F1 male rats after in utero and neonatal exposure to extremely low frequency electromagnetic fields. Asian J Androl. 2005;7(2):189–94. doi: 10.1111/j.1745-7262.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Brent RL. Reproductive and teratologic effects of low-frequency electromagnetic fields: a review of in vivo and in vitro studies using animal models. Teratology. 1999;59(4):261–86. doi: 10.1002/(SICI)1096-9926(199904)59:4<261::AID-TERA12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Lundsberg LS, Bracken MB, Belanger K. Occupationally related magnetic field exposure and male subfertility. Fertil Steril. 1995;63(2):384–91. doi: 10.1016/s0015-0282(16)57373-7. [DOI] [PubMed] [Google Scholar]
- 4.Heredia-Rojas JA, Caballero-Hernandez DE, Rodriguez-de la Fuente AO, Ramos-Alfano G, Rodriguez-Flores LE. Lack of alterations on meiotic chromosomes and morphological characteristics of male germ cells in mice exposed to a 60 Hz and 2.0 mT magnetic field. Bioelectromagnetics. 2004;25(1):63–8. doi: 10.1002/bem.10184. [DOI] [PubMed] [Google Scholar]
- 5.Al-Akhras MA, Elbetieha A, Hasan MK, Al-Omari I, Darmani H, Albiss B. Effects of extremely low frequency magnetic field on fertility of adult male and female rats. Bioelectromagnetics. 2001;22(5):340–4. doi: 10.1002/bem.59. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan LA, Abd-Allah AR, Aly HA, Saad-el-Din AA. Testicular toxicity effects of magnetic field exposure and prophylactic role of coenzyme Q10 and L-carnitine in mice. Pharmacol Res. 2002;46(4):363–70. doi: 10.1016/s1043661802001718. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Ahn SS, Jung KC, Kim YW, Lee SK. Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J Androl. 2004;6(1):29–34. [PubMed] [Google Scholar]
- 8.Chung MK, Kim JC, Myung SH, Lee DI. Developmental toxicity evaluation of ELF magnetic fields in Sprague-Dawley rats. Bioelectromagnetics. 2003;24(4):231–40. doi: 10.1002/bem.10114. [DOI] [PubMed] [Google Scholar]
- 9.Tablado L, Perez-Sanchez F, Nunez J, Nunez M, Soler C. Effects of exposure to static magnetic fields on the morphology and morphometry of mouse epididymal sperm. Bioelectromagnetics. 1998;19(6):377–83. doi: 10.1002/(sici)1521-186x(1998)19:6<377::aid-bem5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Chernoff N, Rogers JM, Kavet R. A review of the literature on potential reproductive and development toxicity of electric and magnetic fields. Toxicology. 1992;74(2-3):91–126. doi: 10.1016/0300-483x(92)90132-x. [DOI] [PubMed] [Google Scholar]
- 11.Forgacs Z, Somosy Z, Kubinyi G, Sinay H, Bakos J, Thuroczy G, et al. Effects of whole-body 50 Hz magnetic field exposure on mouse leydig cells. Scientific World Journal. 2004;4(Suppl 2):83–90. doi: 10.1100/tsw.2004.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson BW, Matt KS, Morris JE, Sasser LB, Miller DL, Anderson LE. Effects of 60 Hz magnetic field exposure on the pineal and hypothalamic-pituitary-gonadal axis in the Siberian hamster (Phodopus sungorus) Bioelectromagnetics. 1999;20(4):224–32. [PubMed] [Google Scholar]
- 13.Yellon SM. Acute 60 Hz magnetic field exposure effects on the melatonin rhythm in the pineal gland and circulation of the adult Djungarian hamster. J Pineal Res. 1994;16(3):136–44. doi: 10.1111/j.1600-079x.1994.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 14.Okano H, Ohkubo C. Effects of static magnetic fields on plasma levels of angiotensin II and aldosterone associated with arterial blood pressure in genetically hypertensive rats. Bioelectromagnetics. 2003;24(6):403–12. doi: 10.1002/bem.10139. [DOI] [PubMed] [Google Scholar]
- 15.Okano H, Masuda H, Ohkubo C. Decreased plasma levels of nitric oxide metabolites, plasma angiotensin II and aldosterone in spontaneously hypertensive rats exposed to 5 mT static magnetic fields. Bioelectromagnetics. 2005;26(3):161–72. doi: 10.1002/bem.20055. [DOI] [PubMed] [Google Scholar]
- 16.Marino AA. Different outcomes in biological experiments involving weak EMFs: is chaos a possible explanation? Am J Physiol. 1995;268(4 Pt 2):R1013–8. doi: 10.1152/ajpregu.1995.268.4.R1013. [DOI] [PubMed] [Google Scholar]
- 17.Sandrey MA, Vesper DN, Johnson MT, Nindl G, Swez JA, Chamberiain J, et al. Effect of short duration electromagnetic field exposures on rat mass. Bioelectromagnetics. 2002;23(1):2–6. doi: 10.1002/bem.92. [DOI] [PubMed] [Google Scholar]
- 18.Hilton DI, Phillips RD. Growth and metabolism of rodent exposed to 60 Hz electric fields. Bioelectromagnetics. 1981;2(4):381–90. doi: 10.1002/bem.2250020409. [DOI] [PubMed] [Google Scholar]
- 19.Free MJ, Kaune WT, Phillips RD, Cheng HC. Endocrinological effects of strong 60-Hz electric fields on rats. Bioelectromagnetics. 1981;2(2):105–21. doi: 10.1002/bem.2250020203. [DOI] [PubMed] [Google Scholar]
- 20.Al-Akhras MA, Darmani H, Elbetieha A. Influence of 50 Hz magnetic field on sex hormones and other fertility parameters of adult male rats. Bioelectromagnetics. 2006;27(2):127–31. doi: 10.1002/bem.20186. [DOI] [PubMed] [Google Scholar]
- 21.Al-Akhras MA. Influence of 50 Hz magnetic field on sex hormones and body, uterine, and ovarian weights of adult female rats. Electromagn Biol Med. 2008;27(2):155–63. doi: 10.1080/15368370802072125. [DOI] [PubMed] [Google Scholar]
- 22.Forgacs Z, Thuroczy G, Paksy K, Szabo LD. Effect of sinusoidal 50 Hz magnetic field on the testosterone production of mouse primary leydig cell culture. Bioelectromagnetics. 1998;19(7):429–31. [PubMed] [Google Scholar]
- 23.McGivern RF, Sokol RZ, Adey WR. Prenatal exposure to a low-frequency electromagnetic field demasculinizes adult scent marking behavior and increases accessory sex organ weights in rats. Teratology. 1990;41(1):1–8. doi: 10.1002/tera.1420410102. [DOI] [PubMed] [Google Scholar]
- 24.Margonato V, Veicsteinas A, Conti R, Nicolini P, Cerretelli P. Biologic effects of prolonged exposure to ELF electromagnetic field in rats: I. 50 Hz electric fields. Bioelectromagnetics. 1993;14(5):479–93. doi: 10.1002/bem.2250140508. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Honma K, Shigemitsu T, Shiga Y. Circularly polarized, sinusoidal, 50 Hz magnetic field exposure does not influence plasma testosterone levels of rats. Bioelectromagnetics. 1994;15(6):513–8. doi: 10.1002/bem.2250150604. [DOI] [PubMed] [Google Scholar]
- 26.Margonato V, Nicolini P, Conti R, Zecca L, Veicsteinas A, Cerretelli P. Biologic effects of prolonged exposure to ELF electromagnetic field in rats: II.50 Hz magnetic fields. Bioelectromagnetics. 1995;16(6):343–55. doi: 10.1002/bem.2250160602. [DOI] [PubMed] [Google Scholar]
- 27.Selmaoui B, Lambrozo J, Touitou Y. Endocrine functions in young men exposed for one night to a 50-Hz magnetic field. A circadian study of pituitary, thyroid and adrenocortical hormones. Life Sci. 1997;61(5):473–86. doi: 10.1016/s0024-3205(97)00407-4. [DOI] [PubMed] [Google Scholar]
- 28.Zecca L, Mantegazza C, Margonato V, Cerretelli P, Caniatti M, Piva F, et al. Biologic effects of prolonged exposure to ELF electromagnetic field in rats: III. 50 Hz electromagnetic fields. Bioelectromagnetics. 1998;19(1):57–66. doi: 10.1002/(sici)1521-186x(1998)19:1<57::aid-bem7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Fathi Moghaddam H, Ahangarpour A, Tahmasebi Birgani MJ, Shahbazian H, Badavi M. The effect of 17 days exposure to static magnetic fields on the hypothalamic-pituitary-gonadal axis in the male rat. Iranian Journal of Pharmaceutical Research. 2005;4(3):161–8. [Google Scholar]
- 30.Fathi Moghaddam H, Ahangarpour A, Tahmasebi Birgani MJ, Shahbazian H, Badavi M. Proceedings of Physiological Society. Cork, Irland. Cork: University College Cork; 2004. The effect of exposure to different durations of static magnetic fields on the secretion of testosterone, FSH and LH in the male rat; p. 560. [Google Scholar]
- 31.Ahangarpour A, Fathi Moghaddam H, Tahmasebi Birgani MJ, Shahbazian H, Badavi M. Effects of different duration and intensities exposure of static magnetic fields on the hypothalamic-pituitary-thyroid axis in the male rats. Ahwaz Sci Med J. 2008;7(3):337–45. (Persian) [Google Scholar]
- 32.Ahangarpour A, Fathi Moghaddam H, Tahmasebi Birgani MJ, Shahbazian H, Badavi M. Effects of constant static magnetic fields exposure on testosterone, FSH and LH secretion in male rat. Rafsanjan Med Sci J. 2008;7(3):147–56. (Persian) [Google Scholar]


