Abstract
BACKGROUND:
E-cadherin/catenin complexes exert a role in cell adhesion. β-catenin is a key player in Wnt signaling pathway in gastric cancer. P53 is a tumor suppressor gene which also regulates apoptosis. We assessed the expression of E-cadherin, β-catenin and p53 in gastric adenocarcinoma, and their correlations with clinicopathological features.
METHODS:
Fifty six formalin-fixed, paraffin-embedded archival specimens of gastric adenocarcinoma were randomly included as cases. Adjacent tumor-free gastric mucosa of different premalignant stages was obtained from the cases. Immunohistochemical staining was performed to assess E-cadherin, β-catenin and p53 expression.
RESULTS:
All chronic atrophic gastritis and intestinal metaplasia revealed normal membranous staining. Only one patient with dysplasia had abnormal expression of E-cadherin and β-Catenin. Abnormal E-cadherin, β-catenin and p53 expression was found in 50%, 48.2% and 76.8% of cancer specimens respectively. Abnormal expression of E-cadherin was significantly correlated with aberrant β-catenin expression. Abnormal E-cadherin and β-catenin expression were significantly correlated with depth of tumor invasion and advanced gastric cancer (p < 0.05), lower degree of differentiation and diffused tumor type (p < 0.001). Node metastasis was not influenced by abnormal expression of E-cadherin and β-catenin. P53 was not associated with clinicopathological variables.
CONCLUSIONS:
Abnormal expression of the E-cadherin and β-catenin were associated with each other and influenced by histogenesis of gastric cancer and malignant behavior of tumor but not significant in premalignant lesions. They are more frequent in diffuse type and associated with advanced gastric cancer. P53 alterations are more frequent in the Iranian population compared with others.
Keywords: Gastric Cancer, E-cadherin, β-catenin, p53, Immunohistochemistry
Gastric cancer is the second leading cause of cancer related death in the world. In Iran, it is the most frequent cancer and top of the list of cancer mortality.1 There are geographical variations in epidemiology and genetics of gastric adenocarcinoma. Japan and China have highest incidence of gastric cancer with different epidemiologic and genetic patterns as compared with western countries.2 Similarly, Iran stands above the average in the incidence of gastric cancer (ASR: 26.1 in males and 11.1 for females)1 but the genetic of gastric cancer and its pathophysiologic implications in this high incidence region of the world and probable variations has remained to be clarified. E-cadherin, β-catenin and p53 are amongst the most important genes along the gastric carcinogenesis pathways. Several studies have reported controversial results in different pathophysiological aspects.
Cell adhesion mechanisms have a crucial role in processes such as embryonic development, tissue and organ pattern formation, induction and maintenance of normal tissue architecture.3,4 In epithelial cells, intercellular adhesion is mediated by E(epithelial)-cadherin. This 120-kd molecule belongs to a family of transmembrane glycoproteins and has an important role in calcium-dependent homophilic cell-cell adhesive interactions. Members of the catenin family (α-catenin, β-catenin and γ-catenin) are required for the function of E-cadherin. They link the cytoplasmic domain of E-cadherin to the actin cytoskeleton or to other transmembrane and cytoplasmic proteins. β-catenin and γ-catenin bind to E-cadherin, whereas α- catenin forms the link with actin.5,6 The interaction between E-cadherin and β-catenin at the adherence junction provides one obvious mechanism by which E-cadherin silencing can disrupt growth signaling and develop tumorigenesis. In addition to its role in regulating cell adhesion, β-catenin is a transcription co-factor in the Wnt (wingless) signaling pathway, and elevated levels of free β-catenin are associated with tumorigenesis in humans.7 E-cadherin competes with the Adenomatousis Polyposis Coli (APC) gene product for β-catenin binding,8 and APC mutation and methylation is found frequently in gastric adenocarcinomas.9,10 Wild type APC regulates cellular β-catenin levels,11 therefore, APC may modulate the interaction between E-cadherin and the Catenins, and affect the function of this complex.
A variety of human cancers exhibit abnormal expression of E-cadherin that correlates with higher pathologic grades and advanced tumor stage.12–16 Decreased or completely lost E-cadherin expression has been reported in gastric cancer, although results regarding the degree of aberrant E-cadherin expression and its correlations with clinicopathological feature were different and sometimes contradictory.17–19 P53 is a tumor suppressor gene which prevents cells with DNA damage from entering S-phase by G1 arrest so that as a check point regulator facilitates DNA repair system. It also promotes apoptosis in mutated cells by stimulating bax synthesis.20 The wild-type p53 protein has a short half-life and is removed rapidly from the nucleus. On the contrary, mutant p53 with a prolonged half-life accumulates in the nucleus, where it is detectable by immunohistochemistry. Therefore, the accumulation of the p53 protein may be considered abnormal.21,22
Although many studies have been conducted targeting p53 expression and its impact on clinicopathological features some controversial results have been reported.23–25
In this study, we evaluated the immunohistochemical expression of E-cadherin, β-catenin and p53 in gastric adenocarcinoma, and their correlations with clinicopathological features.
Methods
Sample Collection
Archival, formalin-fixed, paraffin-embedded gastric adenocarcinoma tissue samples from 56 gastrectomy tissues were randomly included from 8 high-incidence provinces in Iran. Adjacent tumor-free gastric mucosa of different premalignant steps was obtained from all cases. A section from each specimen block was stained with H&E for histological evaluation and representative blocks were chosen for immunostaining. Quality of preservation was good with no significant autolysis. In addition, normal gastric mucosa adjacent to the tumor was used as an internal positive control. Tumors were classified using the Lauren's classification26 into intestinal and diffuse type. Intestinal type tumors were graded as well, moderate, or poorly differentiated according to the predominant pattern in tumor. Mixed tumors had elements of both intestinal and diffuse patterns. Tumor staging was in accordance with the unified TNM criteria for gastric cancer.
Immunohistochemical Staining
Formalin-fixed, paraffin-embedded tissue blocks were cut to 4-μm-thick sections for immunostaining. A standard Streptavidinbiotin indirect immunoperoxidase technique was used. Sections were dewaxed, rehydrated and incubated with 3% hydrogen peroxide to block endogenous peroxidase activity. Antigen retrieval was performed by microwave pre-treatment in 0.01M citrate buffer (pH 6.0) for 20min at 750 watt. Subsequently, sections were incubated for 2 hours at 37°C with a mouse monoclonal antibody against E-cadherin (Transduction Laboratories, Lexington, KY, USA) diluted at 1:250, Goat Anti-β-Catenin (Research Diagnostic, Inc. NJ, USA) and Antibodies against p53 (clone DO-7, M7001, dilution 1:100) followed by biotinylated link anti-mouse and anti-rabbit immunoglobulin (Dakopatts, Denmark) and Streptavidin-biotin peroxidase complex (Dako LSAB®2system, Peroxidase Kit, Denmark). The peroxidase reaction was visualized with 3,3’-diaminobenzidine tetrahydrochloride, supplemented with hydrogen peroxide. The slides were then counterstained with Mayer's hematoxylin and dehydrated before mounting.
Interpretation of Immunostaining
Staining was scored independently by two observers and a high level of concordance (90%) was achieved. In case of disagreement, the slides were reviewed and a consensus view achieved. A semi quantitative estimation of membranous E-cadherin and β-catenin expression was made, using a composite score obtained by adding the values of the immunoreaction intensity and relative abundance of the E-cadherin and β-catenin immunoreactive cells, as previously reported. Briefly, the intensity was graded from 0 to 3, with 0 for denoting absent staining, 1 for cytoplasmic distribution, 2 for heterogeneous staining (i.e. when tumors were composed of both normal and abnormally staining areas), and 3 for normal membranous pattern of staining. Because staining patterns often varied within the same tumor, particularly when degree of differentiation varied, the score was based on the dominant pattern. Tumors with more than 10% variation were scored as heterogeneous.
The abundance of positive cells was graded from 0 to 4 (0: less than 5% of positive cells, 1: 5-25%, 2: 26-50%, 3: 51-75%, 4: 76-100%).
E-cadherin and β-catenin expression was considered preserved when the composite score was 6 or 7. Cases with scores between 0 and 5 were considered as decreased expression. In order to facilitate statistical analysis, all tumors with loss of membranous expression were categorized as abnormal including those with absent, heterogeneous or cytoplasmic staining patterns. P53 immunoreactivity was defined by observing nuclear staining. P53 expression was graded as negative (< 20%) or positive (> 20% of tumor cells stained).27
Statistical Analysis
Statistical analysis was performed using SPSS software (version 11.5). The correlation between two variables was evaluated using Pearson's chi-square and Fisher's exact test. Statistical significance was defined as p < 0.05.
Results
Study population was consisted of 40 male and 16 female patients (median age: 60, ranged from 26 to 76) from 8 high-incidence provinces in Iran. In normal histology and pre-cancerous gastric mucosa including atrophic gastritis, intestinal metaplasia E-cadherin was expressed uniformly at the cell membrane. One patient with dysplasia had abnormal expression of both E-cadherin and β-catenin.
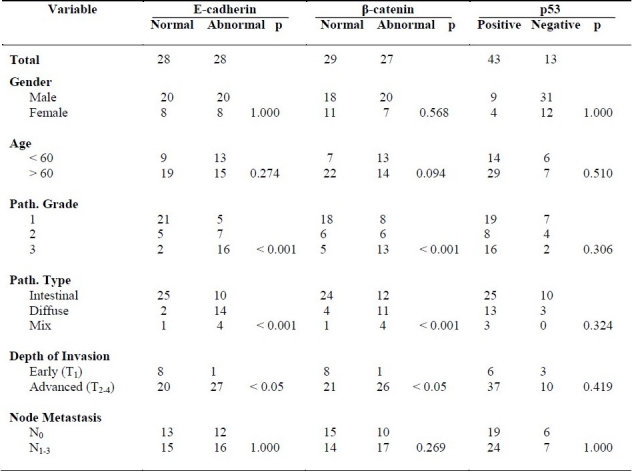
Figure 1 demonstrates different patterns of staining. Abnormal E-cadherin, β-catenin and p53 expression was found in 50%, 48.2% and 76.8% of cancer specimens, respectively. Abnormal expression of E-cadherin and β-catenin were significantly correlated with each other with 85.7% of cases showing same pattern of expression. Abnormal E-cadherin and β-catenin expression was associated with depth of tumor and advanced stages (p < 0.05) while node metastasis was not influenced by abnormal expression of E-cadherin and β-Catenin. Abnormal E-cadherin and β-catenin expression was significantly correlated with lower degree of differentiation (p < 0.001). It was also significantly more frequent in diffused type of tumor. In mixed-type carcinomas with elements of both intestinal and diffuse patterns, intense staining was seen in the intestinal component whereas areas with a diffuse pattern showed more heterogeneous and abnormal or diminished patterns of staining. P53 was not associated with clinicopathological variables. Expression in neither of genes was affected by gender and age. Table 1 shows clinicopathological features of patients and their correlation to E-cadherin, β-catenin and p53 expression.
Figure 1.

Immunohistochemical staining of tumoral tissues. Normal gastric glands are served as internal control. A) Complete loss of E-cadherin expression in diffuse type gastric adenocarcinoma. B) Complete loss of E cadherin expression in intestinal type gastric adenocarcinoma, grade 2. C) Heterogeneous membranous and cytoplasmic E-cadherin expression in intestinal type gastric adenocarcinoma, grade 3. D) Heterogeneous membranous E-cadherin expression in intestinal type gastric adenocarcinoma, grade 2. E) Heterogeneous membranous β-catenin expression in intestinal type gastric adenocarcinoma, grade 2. F) Heterogeneous membranous and cytoplasmic β-catenin expression in intestinal type gastric adenocarcinoma G) Intense nuclear staining for p53 in intestinal type.
Table 1.
Clinicopathological features of patients and their correlation with E-cadherin, β-catenin and p53 expression
Discussion
Abnormalities in expression and function of E-cadherin, β-catenin and p53 have been shown in various cancers. The aim of this study was to clarify the clinicopathological significance of expression of some important genes in gastric carcinogenesis in the Iranian population. For this purpose, expression of E-cadherin, β-catenin and p53 were assessed. Previous studies have shown variable abnormal E-cadherin expression ranging from 23% up to 92% of tumors.17,28,29 However different antibodies and different scoring systems of previous studies make the comparison of the overall expression of E-cadherin-catenin complex difficult. Our results showed abnormal E-cadherin and β-catenin expression in 50% and 48% of the gastric carcinomas respectively, consistent with the study performed by Zhou YN et al.30
Eighty four percent of tumors exhibited abnormal pattern of expression for E-cadherin and β-catenin simultaneously, significantly correlated with each other, consistent with results of Huiping et al.31 E-cadherin interacts with either β- or γ-catenins which bridge E-cadherin to the cytoskeleton through α-catenin.32 Abnormal expression of α- or β-catenin due to mutation, deletion, or post-translational modification (e.g. by phosphorylation) may account for loss of cell adhesion in the tumors retaining normal expression of E-cadherin.33 β-catenin exerts a dual role as an essential cytoplasmic-interaction counterpart of cadherins, essential for cell-cell-adhesion, and as a nuclear partner of the T-cell factor (TCF)/lymphocyte-enhancer factor (Lef) family of transcription factors regulating genes of the canonical Wnt signaling pathway.34 Switching from one role to another takes advantage from distinct molecular forms of β-catenin that have different binding properties.35 The manifestation of cancer by aberrant Wnt signaling pathway most likely results from inappropriate gene activation mediated by stabilized β-catenin. APC mutations and β- catenin gene mutations increase the association of β-catenin with the TCF-4 and Lef-1,36,37 which are involved in the transcriptional regulation of E-cadherin 38 and possibly, of other target genes and provide evidence of functional interaction between signaling pathways and cell adhesion molecules during tumorigenesis. As an important molecule in cell adhesion, loss of E-cadherin will disrupt proliferation inhibition and lead to more scattered types of tumor like diffuse type which have more malignant behavior with poorly differentiated cells. We demonstrated a significant correlation between E-cadherin expression and tumor histology. Aberrant E-cadherin expression is associated with both poorly differentiated and diffuse-type adenocarcinomas, in accordance with the results of Gabbert et al and Bolk et al.29,39 Abnormal expression patterns of β- catenin were also more frequent in poorly differentiated and diffuse-type tumors.
Gastric cancer has one of the most complex genetic pathways with lots of questions still remained to be clarified. Controversial conclusions have been reported about the role of E-cadherin in the gastric carcinogenesis. Correa et al have proposed a pathway of carcinogenesis starting with atrophic gastritis, followed by intestinal metaplasia and dysplasia, leading to gastric adenocarcinoma.40 Although intestinal types are best described by this cascade, diffused types are not consistent with this proposed pathway.39 Some previous studies reported E-cadherin alterations as a crucial early events significantly rising in dysplasia.30 Our result does not support this conclusion in our population. Almost all of the preneoplastic lesions exhibited normal expression, in accordance with the results of Chen HC et al.41 It was also significantly more frequent in advanced gastric cancer and the majority of early gastric cancers preserved E-cadherin expression. We conclude that loss of normal E-cadherin expression affects invasion of tumor to the muscularis propria resulting in advanced gastric cancer. In this study E-cadherin influenced tumor depth of invasion but it was not significantly associated with lymph node metastasis. This reveals that preserved E-cadherin expression does not necessarily lead to intact cell adhesion mechanisms. It might be a result of malfunctioned E-cadherin protein despite normal staining in some cases. Point mutations or partial methylation might affect the normal function of E-cadherin gene product while the immunostaining is preserved normally.42
Varied range of results has been reported for p53 expression in gastric cancer and its correlations with clinicopathological features in several studies. We demonstrated p53 expression in 76.8% of cases, which is more frequent than previous studies ranging from 25.2% to 57.5%.43–47 Correlation with intestinal type was reported by Hurlimann et al and Fukomaga et al48,49 while many studies demonstrated no preference of tumor histological type. Joypaul et al and Strazynska et al reported impact of p53 overexpression on staging22,50 ; Kim et al and Roviella et al revealed its association with tumor depth of invasion and lymph node metastasis.51,52 Lee et al also showed lymph node metastasis is influenced by p53 overexpression53 while Deveci et al reported less frequency of p53 expression in the presence of more than five metastatic lymph nodes.54 We demonstrated that p53 overexpression was not associated with clinicopathological features, in consistent with the results of Sasao et al and Gamboa-Dominguez et al.55,56
Conclusions
In conclusion, loss of normal E-cadherin and β-catenin occurs in a considerable proportion of gastric carcinomas while it is not significant in the precancerous lesions and p53 is over expressed more frequently in Iranian population. E-cadherin and β-catenin are associated with more malignant behavior of tumor and might be served as a marker of differentiation. Loss of these cell adhesion molecules is associated with diffuse type. It is more common in advance gastric cancer. Clinicopathological features were not influenced by p53 overexpression.
Authors’ Contributions
MRA and MRZ have designed the study. MRA, HAA, KGh, KhJA, MF, AA and OM have performed the study. KGh, MRA and OM have analyzed the results. OM and KJA have written the manuscript. MRA and MRZ scientifically revised the manuscript.
All authors have read and approved the content of the manuscript.
Acknowledgments
This study was supported by a grant awarded from Mashhad University of Medical Sciences.
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Sadjadi A, Nouraie M, Mohagheghi MA, Mousavi-Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6(3):359–63. [PubMed] [Google Scholar]
- 2.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56(1):1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 3.Demetter P, De Vos M, Van Damme N, Baeten D, Elewaut D, Vermeulen S, et al. Focal up-regulation of E-cadherin-catenin complex in inflamed bowel mucosa but reduced expression in ulcer-associated cell lineage. Am J Clin Pathol. 2000;114(3):364–70. doi: 10.1093/ajcp/114.3.364. [DOI] [PubMed] [Google Scholar]
- 4.Karayiannakis AJ, Syrigos KN, Chatzigianni E, Papanikolaou S, Karatzas G. E-cadherin expression as a differentiation marker in gastric cancer. Hepatogastroenterology. 1998;45(24):2437–42. [PubMed] [Google Scholar]
- 5.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherence junctions. Annu Rev Cell Dev Biol. 1997;13:119–46. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 6.Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996;77(8 Suppl):1605–13. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1605::AID-CNCR28>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–51. [PubMed] [Google Scholar]
- 8.Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with α-catenin, β-catenin, and plakoglobin. J Biol Chem. 1995;270(10):5549–55. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- 9.Nakatsuru Sh, Yanagisawa A, Ichii Sh, Tahara E, Kato Y, Nakamura Y, et al. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1(8):559–63. doi: 10.1093/hmg/1.8.559. [DOI] [PubMed] [Google Scholar]
- 10.Roa JC, Anabalón L, Roa I, Tapia O, Melo A, Villaseca M, et al. Promoter methylation profile in gastric cancer. Rev Med Chil. 2005;133(8):874–80. [PubMed] [Google Scholar]
- 11.Ilyas M, Tomlinson IPM. The interactions of APC, E-cadherin and β-catenin in tumor development and progression. J Pathol. 1997;182(2):128–37. doi: 10.1002/(SICI)1096-9896(199706)182:2<128::AID-PATH839>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, et al. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991;139(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Shiozaki H, Iihara K, Oka H, Kadowaki T, Matsui S, Gofuku J, et al. Immunohistochemical detection of alpha-catenin expression in human cancers. Am J Pathol. 1994;144(4):667–74. [PMC free article] [PubMed] [Google Scholar]
- 14.Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, et al. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142(4):987–93. [PMC free article] [PubMed] [Google Scholar]
- 15.Inada S, Koto T, Futami K, Arima S, Iwashita A. Evaluation of malignancy and the prognosis of esophageal cancer based on an immunohistochemical study (p53, E-cadherin, epidermal growth factor receptor) Surg Today. 1999;29(6):493–503. doi: 10.1007/BF02482343. [DOI] [PubMed] [Google Scholar]
- 16.Lou P, Chen W, Sheen T, Ko J, Hsu M, Wu J. Expression of E-cadherin/catenin complex in nasopharyngeal carcinoma: correlation with clinicopathological parameters. Oncol Rep. 1999;6(5):1065–71. doi: 10.3892/or.6.5.1065. [DOI] [PubMed] [Google Scholar]
- 17.Jawhari A, Jordan S, Poole S, Browne P, Pignatelli M, Farthing MJ. Abnormal immunoreactivity of the E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterol. 1997;112(1):46–54. doi: 10.1016/s0016-5085(97)70218-x. [DOI] [PubMed] [Google Scholar]
- 18.Yonemura Y, Endou Y, Kimura K, Fushida S, Bandou E, Taniguchi K, et al. Inverse expression of S100A4 and E-cadherin is associated with metastatic potential in gastric cancer. Clin Cancer Res. 2000;6(11):4234–42. [PubMed] [Google Scholar]
- 19.Tanaka M, Kitajima Y, Edakuni G, Sato S, Miyazaki K. Abnormal expression of E-cadherin and β-catenin may be a molecular marker of submucosal invasion and lymph node metastasis in early gastric cancer. Br J Surg. 2002;89(2):236–44. doi: 10.1046/j.0007-1323.2001.01985.x. [DOI] [PubMed] [Google Scholar]
- 20.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13(3):332–7. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 21.Okusa Y, Ichikura T, Tamakuma S. Immunohistochemical staining for the p53 protein and proliferating cell nuclear antigen in familial clustering of gastric cancer. J Surg Oncol. 1996;62(4):253–7. doi: 10.1002/(SICI)1096-9098(199608)62:4<253::AID-JSO5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Starzynska T, Bromley M, Ghosh A, Stern PL. Prognostic significance of p53 overexpression in gastric and colorectal carcinoma. Br J Cancer. 1992;66(3):558–62. doi: 10.1038/bjc.1992.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomyo Y, Ikeda M, Osaki M, Tatebe S, Tsujitani S, Ikeguchi M, et al. Expression of p21 (waf1/cip1/sdi1), but not p53 protein, is a factor in the survival of patients with advanced gastric carcinoma. Cancer. 1997;79(11):2067–72. doi: 10.1002/(sici)1097-0142(19970601)79:11<2067::aid-cncr3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi T, Watanabe A, Sawada H, Yamada Y, Yamashita J, Matsuda M, et al. Prognostic value of cyclin E and p53 expression in gastric carcinoma. Cancer. 1998;82(7):1238–43. doi: 10.1002/(sici)1097-0142(19980401)82:7<1238::aid-cncr5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Okuyama T, Maehara Y, Kabashima A, Takahashi I, Kakeji Y, Sugimachi K. Combined evaluation of expressions of p53 and p21 proteins as prognostic factors for patients with gastric carcinoma. Oncology. 2002;63(4):353–61. doi: 10.1159/000066223. [DOI] [PubMed] [Google Scholar]
- 26.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Liu XP, Kawauchi S, Oga A, Suehiro Y, Tsushimi K, Tsushimi M, et al. Combined examination of p27(Kip1), p21(Waf1/Cip1) and p53 expression allows precise estimation of prognosis in patients with gastric carcinoma. Histopathology. 2001;39(6):603–10. doi: 10.1046/j.1365-2559.2001.01283.x. [DOI] [PubMed] [Google Scholar]
- 28.Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53(7):1690–5. [PubMed] [Google Scholar]
- 29.Gabbert HE, Mueller W, Schneiders A, Meier S, Moll R, Birchmeier W, et al. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer. 1996;69(3):184–9. doi: 10.1002/(SICI)1097-0215(19960621)69:3<184::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, et al. Expression of E-cadherin and β-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8(6):987–93. doi: 10.3748/wjg.v8.i6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huiping Ch, Kristjansdottir S, Jonasson JG, Magnusson J, Egilsson V, Ingvarsson S. Alterations of E-cadherin and β-catenin in gastric cancer. BMC Cancer. 2001;1:16. doi: 10.1186/1471-2407-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125(6):1327–40. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimoyama Y, Nagafuchi A, Fujita S, Gotoh M, Takeichi M, Tsukita S, et al. Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alpha-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 1992;52(20):5770–4. [PubMed] [Google Scholar]
- 34.Birchmeier W. Cell adhesion and signal transduction in cancer. Conference on cadherins, catenins and cancer. EMBO Reports. 2005;6(5):413–7. doi: 10.1038/sj.embor.7400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between β-catenin's adhesive and transcriptional functions. Genes Dev. 2004;18(18):2225–30. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275(5307):1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 37.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275(5307):1790–2. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 38.Behrens J, Von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 39.Blok P, Craanen ME, Dekker W, Tytgat GN. Loss of E-cadherin expression in early gastric cancer. Histopathology. 1999;34(5):410–5. doi: 10.1046/j.1365-2559.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 40.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--first American Cancer Society Award Lecture on cancer epidemiology and prevention. Cancer Res. 1992;52(24):6735–40. [PubMed] [Google Scholar]
- 41.Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY, Lai KH, et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Letters. 2003;201(1):97–106. doi: 10.1016/j.canlet.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Zhang F, Wu PP, Jiang XC, Zheng L, Yu YY. Disordered beta-catenin expression and E-cadherin/CDH1 promoter methylation in gastric carcinoma. World J Gastroenterol. 2006;12(26):4228–31. doi: 10.3748/wjg.v12.i26.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto-de-Sousa J, Silva F, David L, Leitao D, Seixas M, Pimenta A, et al. Clinicopathological significance and survival influence of p53 protein expression in gastric carcinoma. Histopathology. 2004;44(4):323–31. doi: 10.1111/j.1365-2559.2004.01852.x. [DOI] [PubMed] [Google Scholar]
- 44.Xiangming C, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S, et al. Cooccurrence of reduced expression of alpha-catenin and overexpression of p53 is a predictor of lymph node metastasis in early gastric cancer. Oncology. 1999;57(2):131–7. doi: 10.1159/000012020. [DOI] [PubMed] [Google Scholar]
- 45.Mattioli E, Vogiatzi P, Sun A, Abbadessa G, Angeloni G, D’Ugo D, et al. Immunohistochemical analysis of pRb2/p130, VEGF, EZH2, p53, p16(INK4A), p27(KIP1), p21(WAF1), Ki-67 expression patterns in gastric cancer. J Cell Physiol. 2007;210(1):183–91. doi: 10.1002/jcp.20833. [DOI] [PubMed] [Google Scholar]
- 46.Schneider BG, Hilsenbeck SG, Hensel CH, Pekkel V, Shelton CH, Rodriguez-Martinez HA, et al. P53 mutations in gastric and colorectal cancers in Texas Hispanics versus Anglos. Virchows Arch. 1994;424(2):187–93. doi: 10.1007/BF00193499. [DOI] [PubMed] [Google Scholar]
- 47.Gabbert HE, Müller W, Schneiders A, Meier S, Hommel G. The relationship of p53 expression to the prognosis of 418 patients with gastric carcinoma. Cancer. 1995;76(5):720–6. doi: 10.1002/1097-0142(19950901)76:5<720::aid-cncr2820760503>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Hurlimann J, Saraga EP. Expression of p53 protein in gastric carcinomas.Association with histologic type and prognosis. Am J Surg Pathol. 1994;18(12):1247–53. doi: 10.1097/00000478-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Fukunaga M, Monden T, Nakanishi H, Ohue M, Fukuda K, Tomita N, et al. Immunohistochemical study of p53 in gastric carcinoma. Am J Clin Pathol. 1994;101(2):177–80. doi: 10.1093/ajcp/101.2.177. [DOI] [PubMed] [Google Scholar]
- 50.Joypaul BV, Hopwood D, Newman EL, Qureshi S, Grant A, Ogston SA, et al. The prognostic significance of the accumulation of p53 tumour-suppressor gene protein in gastric adenocarcinoma. Br J Cancer. 1994;69(5):943–6. doi: 10.1038/bjc.1994.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim KM, Lee A, Chae HS, Shim SI. Expression of p53 and NDP-K/nm23 in gastric carcinomas--association with metastasis and clinicopathologic parameters. J Korean Med Sci. 1995;10(6):406–13. doi: 10.3346/jkms.1995.10.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roviello F, Marrelli D, Vindigni C, De Stefano A, Spina D, Pinto E. P53 accumulation is a prognostic factor in intestinal-type gastric carcinoma but not in the diffuse type. Ann Surg Oncol. 1999;6(8):739–45. doi: 10.1007/s10434-999-0739-3. [DOI] [PubMed] [Google Scholar]
- 53.Lee DY, Park CS, Kim HS, Kim JY, Kim YC, Lee S. Maspin and p53 protein expression in gastric adenocarcinoma and its clinical applications. Appl Immunohistochem Mol Morphol. 2008;16(1):13–8. doi: 10.1097/PAI.0b013e31802c4f21. [DOI] [PubMed] [Google Scholar]
- 54.Deveci MS, Deveci G. Prognostic value of p53 protein and MK-1 (a tumor-associated antigen) expression in gastric carcinoma. Gastric Cancer. 2007;10(2):112–6. doi: 10.1007/s10120-007-0418-7. [DOI] [PubMed] [Google Scholar]
- 55.Sasano H, Date F, Imatani A, Asaki S, Nagura H. Double immunostaining for c-erbB-2 and p53 in human stomach cancer cells. Hum Pathol. 1993;24(6):584–9. doi: 10.1016/0046-8177(93)90236-a. [DOI] [PubMed] [Google Scholar]
- 56.Gamboa-Dominguez A, Seidl S, Reyes-Gutierrez E, Hermannstadter Ch, Quintanilla-Martinez L, Busch R, et al. Prognostic significance of p21WAF1/CIP1, p27Kip1, p53 and E-cadherin expression in gastric cancer. J Clin Pathol. 2007;60:756–61. doi: 10.1136/jcp.2006.038976. [DOI] [PMC free article] [PubMed] [Google Scholar]


