Abstract
Hepatitis B virus infection is a major public health problem worldwide. Islamic Republic of Iran is a country in which hepatitis B prevalence is intermediate. The aim of this study is to assess prevalence of chronic hepatitis B infection in Iran according to demographic characteristics. All cross-sectional studies concerning prevalence of chronic hepatitis B infection in Iran were included irrespective of date and language. The outcome of interest was prevalence of chronic hepatitis B infection confirmed by blood specimen positive for HBsAg. The prevalence of chronic hepatitis B infection was estimated about 1.7% or lower in general population; 0.8% (95% CI: 0.6% to 0.9%) in blood donors and 3.2% (95% CI: 2.3% to 4.1%) in intravenous drug users and varied from zero to 1.5% in beta thalassemic patients. Since mass vaccination in 1993, prevalence of chronic hepatitis B infection has being reduced among children and adolescents. This reduction can be attributed to the effectiveness of the national immunization program and it may impact on reduction of prevalence of hepatitis B infection in general population.
Keywords: Hepatitis B, Prevalence, Blood Donors, Thalassemia, Drug Users, Iran
Hepatitis B virus (HBV) infection is one of the major public health problems in the world.1 Approximately 45% of the world population live in hyper-endemic where prevalence of hepatitis B surface antigen (HBsAg) is greater than 8%; 43% live in midendemic areas where HBsAg prevalence is 2% to 7%, and 12% live in hypo-endemic areas where HBsAg prevalence is less than 2%.2,3 According to the report of World Health Organization (WHO) in 2001 and Centers for Disease Control and Prevention (CDC) in 2005, prevalence of chronic hepatitis B infection in Iran is between 2-7%.2,3 In hyper-endemic areas, the lifetime risk of HBV infection is more than 60% and infection occurs mostly through perinatal and child-to-child transmission. In midendemic areas (such as Iran) the lifetime risk of HBV infection is 20-60% and infection involves all age groups. In hypo-endemic areas the lifetime risk of HBV infection is less than 20% and infection occurs mostly in high-risk adults.3 At present, vaccination is the most effective and cost saving means of prevention of HBV infection.4 Hepatitis B vaccine was introduced within National Immunization Program (NIP) in Iran in 19935 and immunization of teenagers under 18 years old integrated into NIP since 2006. In this review, we aimed to assess prevalence of chronic HBV infection in Iran and to determine high-risk populations.
Inclusion and Exclusion Criteria
Types of Studies
We included all cross-sectional, systematic review and meta-analysis studies conducted in Iran concerning HBV infection irrespective of date and publication language.
Types of Participants in The Studies
All participants of the included studies were native of Iran with different ages, sexs and occupations.
Types of Outcome
The primary outcome that we intended to assess was “HBV infection prevalence” confirmed by detection of blood specimen positive for HBsAg.
Search Strategy
The search strategies were developed, then searching national and international databases was conducted using a combination of the word “hepatitis B” with the words “prevalence” or “incidence” and “Iran”. MEDLINE (from 1950 to 2008), EMBASE (from 1966 to 2008), Science Citation Index Expanded (from 1994 to 2008), Ovid (from 1960 to 2008), Google Scholar, IranMedex, MagIran, Scientific Information Database (SID), and Scientific Journal of Iran Blood Transfusion Organization (SJIBTO).
Methods
To assess prevalence of chronic HBV infection in Iran, not only both national and international databases were searched, but reference list were screened to find as much publication as possible.
Data Collection and Extraction
The data was extracted from the included studies, using an electronic data collection and abstraction form developed by Stata 9 computer program. The extracted variables included bibliography, type of studies, total sample size, number of HBsAg positive, demographic characteristics of the participants, date and region in which the studies were conducted.
Methodological Quality
Methodological quality and the strengths and weaknesses of the report of included studies was investigated using a modified STROBE checklist consisted of the following items: title and abstract, introduction, methods, results and conclusion.6 Thirty four cross-sectional studies were found, 4 of which were published as abstracts. The STROBE items reported by included studies varied from 55% to 85%. Items reported by English and Persian articles were nearly the same (69% versus 70% respectively).
Statistical Methods
The measure of interest was “prevalence of HBV infection” with 95% confidence interval (CI). Statistical heterogeneity was explored using Cochran's Q-test and Higgins’ I2 statistic and the publication bias was addressed using funnel plot. The analysis was performed in Stata 9 statistical software. The random effects model was reported with 95% CI.
Characteristics of Studies
One hundred and two publications were found by searching electronic databases as well as screening the references list. After reading the titles and abstracts, 61 of these publications were excluded because they were duplicates or had objectives different from this review. The remaining 41 publications were retrieved for further assessment. Of these, 5 publications were excluded because their full texts were not available and their abstracts were not as complete as to provide necessary data for this review. Two publications were excluded after reviewing the full texts; one publication did not meet the inclusion criteria and the other one for duplication (Diagram 1). Of the remaining 34 publications, 30 studies were published as full paper articles and 4 studies as abstracts. Twenty studies were published in English and the remaining 14 studies in Persian.
Diagram 1.
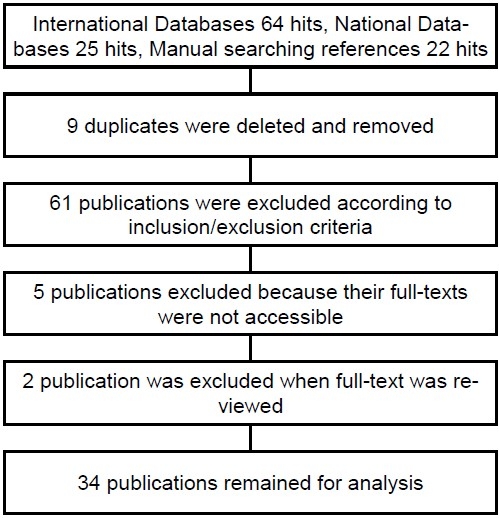
Inclusion and exclusion flowchart
We did not find any meta-analysis related to HBV infection in Iran. All included publications were cross-sectional method. Also 5 narrative reviews were identified that the references of which were checked for additional studies.7–11
Thirty one studies had been conducted after introduction of hepatitis B vaccine within NIP12–41 and the remaining 3 studies were conducted theretofore.42–44
Four studies had been conducted at national level23,41,43,44; 2 studies had been carried out as multi-center25,32 and the remaining 28 studies had been conducted in different provinces of Iran.12–22,24,26–31,33–40,42
Characteristics of The Participants in The Studies
The 34 included studies had been conducted on the following populations: 6 studies13,18,35,41,42,44 on general population; 15 studies on blood donors12,14–17,19,20,24,28,29,31,33,34,40,43; 5 studies on beta thalassemic patients21,26,32,36,38; 3 studies were conducted in hemophilic patients21,30,39, 4 studies on intravenous drug abusers22,25,27,37 and one study on long vehicle drivers.
The sex of the participants was not specified in one study,25 two studies were restricted to males,23,27 one study was restricted to females22 and the remaining 30 studies included both males and females.12–21,24,26,28–44 Proportion of male participant's to female participants was 53% to 93%, while this proportion was opposite in one study. There was a remarkable diversity in the participants’ age. The studies related to general population included all age groups. All blood donors were more than 18 years old i.e. not including children and teenagers while studies conducted on beta thalassemic patients composed mostly of children and teenagers.
Results
We were not able to pool all data to conduct meta-analyses due to the lack of homogeneity between studies’ participants. Therefore, we performed a stratified meta-analysis according to the characteristics of the participants to obtain a summary measure.
Prevalence of Chronic HBV Infection in General Population
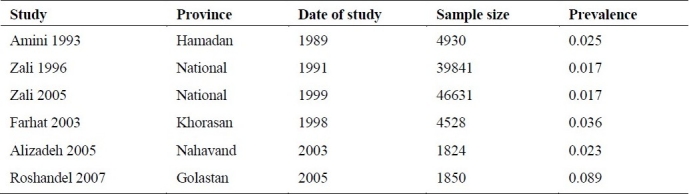
Six studies had been conducted on general population from 1989 to 2005 (Table 1) 2 of which41,44 were national and 4 of which13,18,35,42 were regional. These studies included all age groups. The sample sizes of these studies varied form 1824 to 46631. The lowest prevalence of 1.7%41,44 was reported by national studies and the highest prevalence of 8.9% was reported by Golestan province study.35 Because of unbalanced among sample sizes (1824 to 46631), there was a prominent significant heterogeneity across these six studies (p value < 0.001). Thus we analyzed high sample size (national studies) and low sample size (regional studies) separately as well as together. The prevalence of HBV infection in general population according to the national studies was 1.7% [95% CI: 1.6% to 1.8%], according to regional studies was 3.6% [95% CI: 2.3% to 4.9%] and according to both national and regional studies was 2.7% [95% CI: 2.2% to 3.1%] using the random effect model.
Table 1.
Prevalence of chronic HBV infection in general population
Prevalence of Chronic HBV Infection in Blood Donors
Fourteen studies had been conducted to assess prevalence of HBV infection in blood donors in different provinces of Iran from 1998 to 2005.12,14–17,19,20,24,28,29,31,33,34,40 A national study was conducted with same purposes in 1979.43 All blood donors aged 18 years or older. The proportion of male blood donors (82% to 93%) were much more than female blood donors (7% to 18%). Majority of blood donors (35% to 84%) mentioned a history of previous blood donation. There was a remarkable diversity among sample sizes (441 to 221508). The lowest and highest reported prevalence of HBV infection in blood donors was 0.4% 17 and 3.4% 43 respectively. The later study was much older than the other studies (1979 versus 2000-2007) and looked like an outlier. We excluded this study and re-performed meta-analysis using the random effects model with 95% confidence interval (Figure 1). According to the results obtained from the meta-analysis, prevalence of HBV infection in blood donors of Iran is 0.8% [95% CI: 0.6% to 0.9%].
Figure 1.
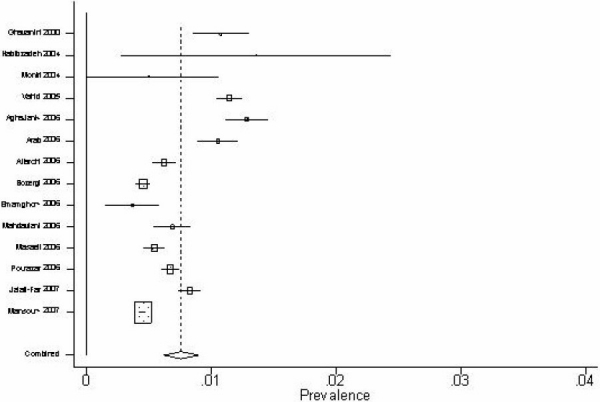
Forest plot for estimating of HBV prevalence in blood donors
Prevalence of Chronic HBV Infection in Beta Thalassemic Patients
There were five studies21,26,32,36,39 related to prevalence of HBV infection in beta thalassemic patients which were conducted from 2000 to 2002 in Iran (Table 2). The sample sizes of these studies were unbalanced (84 to 755). There were no HBsAg positive cases in two studies.22,39 In the remaining 3 studies, the lowest and highest reported prevalence were 0.3% and 1.5% respectively.32,36 Because of standard error (SE) of zero in two studies, we were unable to run meta-analysis. According to the results of these studies, it seems that prevalence of HBV infection in beta thalassemic patents of Iran is lower than general population.
Table 2.
Prevalence of chronic HBV infection in beta thalassemic patients
Prevalence of Chronic HBV Infection in Hemophilic Patients
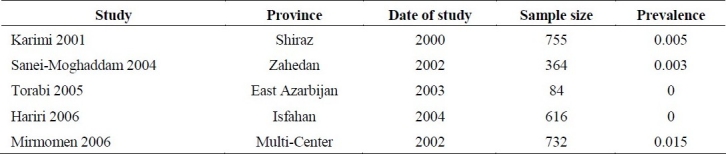
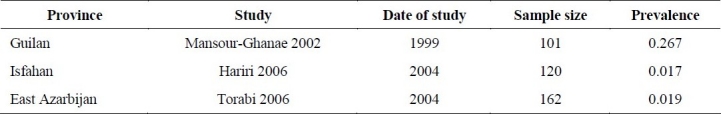
We identified only 3 studies21,30,38 concerning prevalence of HBV infection in hemophilic patients (Table 3). The sample sizes of these studies were small (101 to 162) and the results were very diverse (2% to 27%) due to random error. We were not able to pull the data and perform a meta-analysis due to lack of reliability and strong heterogeneity between studies’ results.
Table 3.
Prevalence of chronic HBV infection in hemophilic patients
Prevalence of Chronic HBV Infection in Intravenous Drug abusers
We identified 4 studies 22,25,27,37 concerning prevalence of HBV infection in Iranian intravenous drug abusers from 2001 to 2003. The sample sizes of the studieswere diverse (196 to 1431). The lowest and highest reported prevalence was 1.5% and 3.7% respectively. We performed a meta-analysis using the random effects model with 95% confidence interval (Figure 2). According to the results obtained from the meta-analysis, prevalence of HBV infection in Iranian intravenous drug abusers was 3.2% [95% CI: 2.3% to 4.1%].
Figure 2.
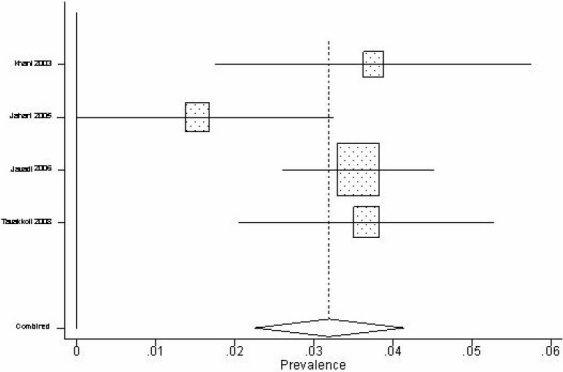
Forest plot for estimating of HBV prevalence in intravenous drug abusers
Prevalence of Chronic HBV Infection Related to Age and Sex
There was remarkable diversity in participants’ age groups. The most prevalent age groups were not specified in 21 studies.13,16,17,19–22,24,26–29,31,33–36,38,39,43 Eight studies 14,15,18,37,40–42,44 reported the most prevalence of HBV infection in participants aged over 30 while 6 studies15,18,23,41,42,44 reported in those aged over 40. The gender of the participants was not specified in 12 studies.19–21,25,26,30,31,34,36,38,39 Two studies 23,27 restricted to males and one study 22 restricted to females. Nine studies 14–16,18,24,28,33,37,42 reported the same prevalence rate for both males and females. Infection rate was reported more prevalent in males in comparison with females in 9 studies12,13,17,29,32,35,41,43,44 while opposite results were reported in one study.40 However, two national studies 41,44 justified that infection rate in males is significantly more than females (1.9% versus 1.5% respectively).
Discussion
Hepatitis B virus (HBV) infection is a major problem of public health in the world particularly in developing countries. According to WHO report in 2001 3 and CDC report 2 in 2005, considered Iran as a mid-endemic region with a prevalence of 2% to 7%. A review article conducted by Merat et al 10 in the 1980s indicated that 3% of Iranian population were chronic carriers of HBV infection. While two big national studies conducted by Zali et al in 1990 44 and 1999 41 revealed that the overall prevalence of chronic HBV infection was 1.7% in the 1990s. Furthermore, these two consecutive studies which were carried out before and after mass vaccination of hepatitis B in 1993 indicated a significant decline in infection rate (from 1.3% to 0.8%) in children of 2-14 years old and may be attributed to hepatitis B vaccine effectiveness. Nevertheless, other regional studies in deferent provinces reported HBV prevalence more than 1.7% in last decade.13,18,35 However, 14 years is passed since integration of hepatitis B vaccine within NIP and now the immunization program have covered a large population of children and teenagers under 18 years old with the coverage rate of 62% in 1993 to 94% in 2005.8 Thus, it seems that prevalence of HBV infection is now about 1.7% or lower in general population of Iran.
According to the WHO guideline,3 serological surveys of first-time unpaid blood donors generally offer the most useful means of estimating the prevalence of HBV infection among adults in the general population. While, repeat and paid blood donors usually have a higher prevalence of HBV infection and are not representative of the general population.3 However, the serological surveys of blood donors carried out in Iran are not good representatives for assessing prevalence of HBV infection among adults in the general population due to following reasons: first, proportion of male blood donors is much more than female blood donors (82% to 93% versus 7% to 18% respectively). Second, serological findings of first-time unpaid blood donors are not separated from repeated blood donors so that majority of blood donors in these surveys are repeated donors (35% to 84% of whom had reported a history of previous blood donation).15,16,20,28,31,34 Indeed, repeated blood donors must be uninfected to be allowed for repeated blood donation. Thus, it is wise to expect that prevalence of HBV infection in these surveys be reported lower than general population (prevalence rate of 0.8% in blood donors versus 1.7% in general population).
In large studies a negligible amount of heterogeneity may be statistically significant, whereas in small studies a strong heterogeneity may not be statistically significant.45 To obtain a summary measure of prevalence of HBV infection in blood donors, we pooled the data from fourteen regional and national studies and performed a random effects meta-analysis. However, because of very large sample sizes (from 3000 to 200,000) in some studies, there was a strong and statistically significant heterogeneity across studies (p < 0.001), whereas demographic characteristics of the blood donors such as age, sex and date of studies were nearly the same in all studies. Therefore, we performed meta-analysis and reported summary measure not withstanding significant heterogeneity was present.
Although, thalassemic patients receive repeated blood transfusion, the infection rate in these patients was significantly lower than general population (0.5% versus 1.7% respectively). The studies were large enough (included 2551 patients) that we could not attribute such a result to random error. We concluded that it may be due to the immunization of these patients at the beginning of disease before receiving any blood transfusion.
Intravenous drug users are at higher risk for HBV infection primarily through high-risk sexual activity and sharing unsterile needles.2 According to an unpublished data in a review article, Merat et al claimed that over 25% of intravenous drug userswere HBV carriers in a prison in south of Iran in 1980s.10 Nonetheless, there are 4 surveys investigated the prevalence of HBV infection in intravenous drug abusers with total sample size of 2491 during 2001 to 2003.22,25,27,37 These studies reported the prevalence of HBV infection from 1.5% to 3.7% with an estimated summary measure of 3.2%. Thus, very high prevalence of HBV infection in intravenous drug users reported by Merat et al seems to be over estimated.
Conclusions
According to the results of this review we drew the following conclusions. Prevalence of chronic HBV infection in general population of Iran is about 1.7% or lower. Prevalence of chronic HBV infection in Blood donors is 0.8% [95% CI: 0.6% to 0.9%]. Serological surveys of blood donors are not a good representative for estimating the prevalence of HBV infection among adults in the general population. Prevalence of chronic HBV infection in beta thalassemic patients varies from zero to 1.5%. Prevalence of chronic HBV infection in intravenous drug abusers is 3.2% [95% CI: 2.3% to 4.1%]. Prevalence of chronic HBV infection is higher in middle aged and elders than children, teenagers and youth. Prevalence of chronic HBV infection is 25% higher in males than females. Prevalence of chronic HBV infection has reduced after mass vaccination in children and teenagers.
Limitation of the Review
Iranian national databases are not useful enough due to lack of actual search tools. These databases do not encompass all national scientific journals as well. Lack of advanced search tools and impossibility of saving search results are additional insufficiencies of national databases. Many national surveys conducted as dissertations that are not registered completely in databases. Thus, in such situation, it is not possible to conduct a national comprehensive meta-analysis.
Authors’ Contributions
JP developed and wrote the protocol and was responsible for the reference searching, article retrieval, study inclusion and exclusion, data extraction, assessment of risk of bias in included studies, analysis and interpretation of results and writing of the review. RM edited the protocol and was responsible for the analysis, interpretation of results and writing of the review.
All authors have read and approved the final manuscript.
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.WHO. Hepatitis B immunization. WHO. 2001. WHO/V&B/01.28 Available at URL: http://www.who.int/vaccinesdocuments/
- 2.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States; recommendations of ACIP part 1: immunization of infants, children, and adolescents. MMWR. 2005;54(RR16):1–23. [PubMed] [Google Scholar]
- 3.WHO. Introduction of hepatitis B vaccine into childhood immunization services: management guidelines, including information for health workers and parents. WHO. 2001. WHO/V&B/01.31 Available at URL: http://www.who.int/vaccines-documents/
- 4.WHO. Hepatitis B. WHO. 2002. WHO/CDS/CSR/LYO/2002.2. Available at URL: http://www.who.int/emc .
- 5.Vaccination and immunity guideline. Ministry of Health and Medical Education Publishing. 1998 [Google Scholar]
- 6.Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Medicine. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alavian SM. Ministry of health in Iran is serious about controlling hepatitis B. Hepatitis Monthly. 2007;7(1):3–5. [Google Scholar]
- 8.Alavian SM, Fallahian F, Lankarani KB. The changing epidemiology of viral hepatitis B in Iran. Journal of Gastrointestinal and Liver Diseases. 2007;16(4):403–6. [PubMed] [Google Scholar]
- 9.Hekmatdoost A, Shalmani Mohaghegh H, Zali MR. A review of hepatitis B transmission in an organized registry center, Tehran, Iran. Clinical Microbiology & Infection Supplement. 2003;9(1 Suppl):84. [Google Scholar]
- 10.Merat S, Malekzadeh R, Rezvan H, Khatibian M. Hepatitis B in Iran. Arch Iran Med. 2000;3(4):192–201. [Google Scholar]
- 11.Rezvan H, Abolghassemi H, Amini Kafiabad S. Transfusion-transmitted infections among multitransfused patients in Iran: a review. Transfusion Medicine. 2007;17(6):425–33. doi: 10.1111/j.1365-3148.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 12.AghaJaniPoor K, Zandieh T. Seroepidemiological investigation of hepatitis B,C and HIV virus in safe blood donors of Babol Blood Transfusion Center in 2002. SJIBT. 2006;2(7):339–41. (Persian) [Google Scholar]
- 13.Alizadeh AH, Ranjbar M, Ansari S, Alavian SM, Shalmani HM, Hekmat L, et al. Intra-familial prevalence of hepatitis B virologic markers in HBsAg positive family members in Nahavand, Iran. World J Gastroenterol. 2005;11(31):4857–60. doi: 10.3748/wjg.v11.i31.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arab M, Abas Zadeh A, Pour Aboli B, Soleimani Zadeh L, Shahsavari M, Javadi M. Prevalence of HBsAg positivity in blood donors in Bam, 1999-2002. SJIBTO. 2006;3(3):277–80. (Persian) [Google Scholar]
- 15.Attarchi Z, Ghafouri M, Hajibaygi B, Assari SH, Alavian SM. Donor deferral and blood-borne infections in blood donors of Tehran. SJIBTO. 2006;2(7):353–64. (Persian) [Google Scholar]
- 16.Bozorgi SH, Ahmadzad Asl M, Ramazani H, Kargarfard H, Alavian SM. Study of viral infections prevalence in blood donors of Qazvin province in different time intervals and during Bam earthquake. Govaresh. 2007;11(4):242–8. (Persian) [Google Scholar]
- 17.Emamghorashi F, Fathi GH, Mohtashami A. Evaluation of demographic characteristics and hepatitis B,C and HIV prevalence among blood donors in Jahrom, 2001-2003. SJIBTO. 2006;2(7):373–8. (Persian) [Google Scholar]
- 18.Farhat A, Khademi Gh, Mazlouman ShJ. The prevalence of hepatitis B carrier state in Khorassan province of Iran. Saudi Medical Journal. 2003;24(5):549–51. [PubMed] [Google Scholar]
- 19.Ghavanini AA, Sabri MR. Hepatitis B surface antigen and anti-hepatitis C antibodies among blood donors in the Islamic Republic of Iran. Eastern Mediterranean Health Journal. 2000;6(5-6):1114–6. [PubMed] [Google Scholar]
- 20.Habibzadeh S, Davarnia B, Bazazataei A, Bagherzadeh S, Kholgh GRH. Epidemiological evaluation of transfusion transmitted diseases in Ardabil in Tasoua and Ashoura 1381(2003) SJIBTO. 2004;1(2):55–60. (Persian) [Google Scholar]
- 21.Hariri MM, Akbari N, Yavari F, Javadi E, Javer SH. Prevalence of hepatitis B, C and HIV markers in thalassemic and haemophilic patients in Isfahan, Iran, 2004. SJIBTO. 2006;2(3):201–4. (Persian) [Google Scholar]
- 22.Jahani MR, Alavian SM, Shirzad H, Kabir A, Hajarizadeh B. Distribution and risk factors of hepatitis B, hepatitis C, and HIV infection in a female population with “illegal social behavior”. Sexually Transmitted Infections. 2005;81(2):185. doi: 10.1136/sti.2004.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahani MR, Motevalian SA, Mahmoodi M. Hepatitis B carriers in large vehicle drivers of Iran. Vaccine. 2003;21(17-18):1948–51. doi: 10.1016/s0264-410x(02)00805-8. [DOI] [PubMed] [Google Scholar]
- 24.Jalali Far M, Torabi Zadeh Maatoghi J, Sajadi SM, Paridar M, Ghasem Zadeh A, Nasimian A. 17th Europian Congress of Clinical Microbiology and Infectious Diseases. Munich, Germany. Hoboken: Wiley; 2007. Significant reduce in hepatitis B prevalence among blood donors admitted to an Ahwaz blood transfusion service due to educational and vaccination programmes. Mar 31-Apr04. [Google Scholar]
- 25.Javadi AA, Avijgan M, Hafizi M. Prevalence of HBV and HCV infections and associated risk factors in addict prisoners. Iranian Journal of Public Health. 2006;35(4):33–6. [Google Scholar]
- 26.Karimi M, Ghavanin AA. Seroprevalence of hepatitis B, hepatitis C and human immunodeficiency virus antibodies among multitransfused thalassaemic children in Shiraz, Iran. Journal of Pediatric and Child Health. 2001;37(6):564–6. doi: 10.1046/j.1440-1754.2001.00709.x. [DOI] [PubMed] [Google Scholar]
- 27.Khani M, Vakili MM. Prevalence and risk factors of HIV, hepatitis B virus and hepatitis C virus infections in drug addicts among Zanjan prisoners. Archive of Iranian Medicine. 2003;6(1):1–4. [Google Scholar]
- 28.Mahdaviani FS, Saremi S, Maghsoudlu M, Pour Fathollah AA. Prevalence of blood transmitted viral infections in regular and non-regular donors of Arak blood center. SJIBTO. 2006;2(7):343–51. (Persian) [Google Scholar]
- 29.Mansour-Ghanaei F, Fallah MS, Jafarshad R, Joukar F, Salari A, Tavafzadeh R. Prevalence of hepatitis B surface antigen and hepatitis C virus antibody and their risk factors among Guilan's volunteer blood donors (1998-2003) Hepatitis Monthly. 2007;7(4):239–41. [Google Scholar]
- 30.Mansour-Ghanaei F, Fallah MS, Shafaghi A, Yousefi-Mashhoor M, Ramezani N, Farzaneh F, et al. Prevalence of hepatitis B and C seromarkers and abnormal liver function tests among hemophiliacs in Guilan. Med Sci Monit. 2002;8(12):CR797–800. [PubMed] [Google Scholar]
- 31.Masaeli Z, Jaberi MR, Magsudlu M. A comparison of seroprevalence of blood-borne infections among regular, sporadic, and first-time blood donors in Isfahan. SJIBTO. 2006;2(7 Suppl):301–7. (Persian) [Google Scholar]
- 32.Mirmomen SH, Alavian SM, Hajari Zadeh B, Kafaee J, Yektaparast B, Zahedi MJ, et al. Epidemiology of hepatitis B, hepatitis C, and human immunodeficiency virus infections in patients with beta-thalassemia in Iran: a multicenter study. Archives of Iranian Medicine. 2006;9(4):319–23. [PubMed] [Google Scholar]
- 33.Moniri R, Mosayebii Z, Mossavi G. Seroprevalence of cytomegalovirus, hepatitis B, hepatitis C and human immunodeficiency virus antibodies among volunteer blood donors. Iranian J Publ Health. 2004;33(4):38–42. [Google Scholar]
- 34.Pourazar A, Akbari N, Hariri M, Yavari F, Akbari Sh. Evaluation of demographic profiles and prevalence of major viral markers in first time vs repeat blood donors in Esfahan. SJIBTO. 2006;2(7 Suppl):323–9. (Persian) [Google Scholar]
- 35.Roshandel Gh, Semnani Sh, Keshtkar A, Joshaghani HR, Moradi A, Kalavi Kh, et al. Seroprevalence of hepatitis B virus and its co-infection with hepatitis D virus and hepatitis C virus in Iranian adult population. Indian Journal of Medical Sciences. 2007;61(5):263–8. [PubMed] [Google Scholar]
- 36.Sanei-Moghaddam E, Savadkoohi S, Rakhshani F. Prevalence of hepatitis B and C in patients with major thalassaemia referred to Ali-Asghar hospital in Zahedan in 2002. SJIBTO. 2004;1(1):19–26. [Google Scholar]
- 37.Tavakkoli H, Mir-Nasseri MM, Poustchi H, Afshar P, Motalebi MN, Mohammadkhani A, et al. Prevalence and risk factors of hepatitis B infection in injection drug users, Tehran (2001-2002) Hepatitis Monthly. 2008;8(1):29–33. [Google Scholar]
- 38.Torabi SA, Abed-Ashtiani K, Dehkada R, Moghadam AN, Bahram MKh, Dolatkhah R, et al. Prevalence of hepatitis B, C and HIV in hemophiliac patients of East Azarbaijan in 2004. SJIBTO. 2006;2(7 Suppl):291–9. (Persian) [Google Scholar]
- 39.Torabi SE, Abed Ashtiani K, Dehkhoda R, Moghadam AN, Sorkhabi RA, Bahram MK, et al. Prevalence of hepatitis B and C in thalassemic patients of East Azarbaijan in 2003. SJIBTO. 2005;2(4):115–22. (Persian) [Google Scholar]
- 40.Vahid T, Alavian SM, Kabir A, Kafaee J, Yektaparast B. Hepatitis B prevalence and risk factors in blood donors in Ghazvin, IR. Iran. Hepatitis Monthly. 2005;5(4):117–22. [Google Scholar]
- 41.Zali MR, Mohammad K, Noorbala AA, Noorimayer B, Shahraz S. Rate of hepatitis B seropositivity following mass vaccination in Islamic Republic of Iran. East Mediterranean Health Journal. 2005;11(1-2):62–7. [PubMed] [Google Scholar]
- 42.Amini S, Mahmoodi M, Andalibi S, Solati AA. Seroepidemiology of hepatitis B, delta and human immunodeficiency virus infections in Hamadan province, Iran: a population based study. J Trop Med Hyg. 1993;96(5):277–87. [PubMed] [Google Scholar]
- 43.Farzadegan H, Harbour C, Ala F. The prevalence of hepatitis B surface antigen and its antibody in blood donors and high-risk groups in Iran. Vox Sang. 1979;37(3):182–6. doi: 10.1111/j.1423-0410.1979.tb02289.x. [DOI] [PubMed] [Google Scholar]
- 44.Zali MR, Mohammad K, Farhadi A, Masjedi MR, Zargar A, Nowroozi A. Epidemiology of hepatitis B in the Islamic Republic of Iran. Eastern Mediterranean Health Journal. 1996;2(2):290–8. [Google Scholar]
- 45.Whitehead A. 1st ed. New Jersey: John Wiley & Sons Ltd; 2002. Meta-analysis of controlled clinical trials. [Google Scholar]


