Abstract
The polyisoprenoid compound undecaprenyl phosphate is required for biosynthesis of cell wall peptidoglycans in Gram-positive bacteria, including pathogenic Enterococcus, Streptococcus, and Staphylococcus spp. In these organisms, the mevalonate pathway is used to produce the precursor isoprenoid, isopentenyl 5-diphosphate. Mevalonate diphosphate decarboxylase (MDD) catalyzes formation of isopentenyl 5-diphosphate in an ATP-dependent irreversible reaction and is therefore an attractive target for inhibitor development that could lead to new antimicrobial agents. To facilitate exploration of this possibility, we report the crystal structure of Staphylococcus epidermidis MDD (1.85 Å resolution) and, to the best of our knowledge, the first structures of liganded MDD. These structures include MDD bound to the mevalonate 5-diphosphate analogs diphosphoglycolyl proline (2.05 Å resolution) and 6-fluoromevalonate diphosphate (FMVAPP; 2.2 Å resolution). Comparison of these structures provides a physical basis for the significant differences in Ki values observed for these inhibitors. Inspection of enzyme/inhibitor structures identified the side chain of invariant Ser192 as making potential contributions to catalysis. Significantly, Ser → Ala substitution of this side chain decreases kcat by ∼103-fold, even though binding interactions between FMVAPP and this mutant are similar to those observed with wild type MDD, as judged by the 2.1 Å cocrystal structure of S192A with FMVAPP. Comparison of microbial MDD structures with those of mammalian counterparts reveals potential targets at the active site periphery that may be exploited to selectively target the microbial enzymes. These studies provide a structural basis for previous observations regarding the MDD mechanism and inform future work toward rational inhibitor design.
Keywords: Enzyme Catalysis, Enzyme Inhibitors, Enzyme Mechanisms, Enzyme Mutation, Enzyme Structure, EC 4.1.1.33, GHMP Kinases, Isoprenoid Biosynthesis, Mevalonate 5-Diphosphate Decarboxylase, Mevalonate Pathway
Introduction
The ever-growing trend among many bacterial pathogens toward antibiotic resistance represents one of the single greatest threats to public health in both developing and modern nations. In particular, a growing body of literature from the last decade has demonstrated that many strains of the widespread Gram-positive organisms Staphylococcus aureus and Staphylococcus epidermidis are now insensitive toward an array of the β-lactam class antibiotics that were once considered frontline therapeutics (1, 2). As recently as a few years ago, the problem of antibiotic resistance was associated primarily with those infections arising from within the healthcare setting. However, recent studies have shown that resistant strains are now spreading rapidly within the community, where they may cause potentially life-threatening illness in persons not recently hospitalized or undergoing invasive medical procedures (1, 3). Given the limited nature of effective therapeutic tools to combat these diseases, all such infections must be carefully managed to prevent further spread throughout the population. As a consequence, there is now renewed interest in novel classes of antimicrobials that are effective against sensitive and resistant strains alike.
In all eubacteria, the C55 polyisoprenoid molecule undecaprenyl phosphate is an essential intermediate in the biosynthesis of the peptidoglycan polymer that comprises an integral component of the cell wall. As a polyisoprenoid, undecaprenyl phosphate is synthesized in stepwise fashion from the building block isopentenyl 5-diphosphate (IPP).5 Whereas IPP is generated via the methylerythritol phosphate pathway in Gram-negative organisms (4), many Gram-positive pathogens (including those mentioned above) rely on the mevalonate pathway for its synthesis (5). The mevalonate pathway produces one molecule of IPP from three molecules of acetyl-CoA in six enzymatic steps. Of these, five have been shown to be essential for the growth of various Gram-positive organisms (5), presumably because of their requirement for providing IPP groups needed for synthesis of menaquinone (for ATP production) as well as undecaprenyl phosphate (for peptidoglycan production). Because compounds that selectively inhibit any of the enzymatic reactions required for bacterial cell wall biogenesis have been recognized for their potential value as antimicrobial agents, the enzymes of the mevalonate pathway present new, attractive targets for the identification and development of next-generation antibiotics. Importantly, these targets have not yet been exploited for such purposes, making it very likely that potential drug candidates will have activity against resistant strains of Gram-positive pathogens.
The enzyme mevalonate diphosphate decarboxylase (MDD; EC 4.1.1.33) catalyzes the final step of the mevalonate pathway, where it produces IPP from mevalonate 5-diphosphate (MVAPP) in an irreversible reaction dependent upon Mg2+-ATP. The reaction proceeds as shown in Reaction 1. As the mevalonate pathway is present in fungi, protists, and higher eukaryotes, the MDD reaction is well established, and MDD enzymes have been extensively characterized from yeast (6, 7), avian (8), and mammalian sources (9–11). Together, these results have elucidated many details of the MDD mechanism.6 In particular, the side chain of Asp283 has been assigned the role of a general base, which serves to deprotonate the MVAPP C3 alcohol and to promote formation of a metastable 3-phospho-MVAPP adduct. Departure of this phosphoryl leaving group allows a carbocation intermediate to develop at C3. It is this, in conjunction with interactions between the C1 carboxylate and the guanidinium group Arg144, that drives subsequent decarboxylation and production of IPP. Indeed, mutation of either of these side chains results in a 3–5 order of magnitude decrease in catalysis (7, 11).
REACTION 1.
MDD proteins are members of the galactokinase, homoserine kinase, mevalonate kinase, and phosphomevalonate kinase (GHMP kinase) family (12). These enzymes are characterized by a cone-shaped fold, where the β-grasp-like N-terminal region packs orthogonally against the relatively planar C-terminal region comprised of five prominent α-helices (13). High resolution crystal structures of MDD proteins have been reported from a number of species across several kingdoms of life, including S. aureus (13), Trypanosoma brucei (13), Saccharomyces cerevisiae (12), as well as Homo sapiens (11). These structures reveal the presence of a deep cleft that forms between the roughly equal halves of the enzyme, which houses the catalytic Asp and Arg residues. Despite the importance of this work, a significant limitation of all MDD structures published to date is the absence of any bound ligands at the active site. Consequently, a detailed and direct identification of substrate-binding residues has remained elusive.
To clarify previous observations regarding the MDD mechanism and to better understand the basis for substrate recognition by MDD proteins, we report here the high resolution x-ray crystal structures of S. epidermidis MDD both free and bound to the inhibitory substrate analogs diphosphoglycolyl proline (DPGP) and 6-fluoromevalonate diphosphate (FMVAPP). In addition to providing a physical basis for differences in inhibitory potency between these two molecules, this work has identified a new residue, Ser192, that contributes to catalysis by MDD. Furthermore, a significant sequence/structure difference between bacterial and eukaryotic MDDs at position 193 has also been identified. These studies inform structure/function relationships and highlight subtle yet noteworthy differences within diverse MDD proteins. The results will inform future work on developing selective MDD inhibitors that may be evaluated for their antimicrobial properties.
EXPERIMENTAL PROCEDURES
Cloning, Overexpression, and Purification of Recombinant Forms of MDD
A gene fragment encoding the entire open reading frame (residues 1–327) of MDD was amplified from S. epidermidis genomic DNA via PCR and subcloned into the expression plasmid pT7HMT (14). Upon confirmation of its DNA sequence, this plasmid was transformed into BL21 (DE3) Escherichia coli cells, and the resulting strain was cultured in Terrific Broth supplemented with kanamycin (50 μg/ml) at 37 °C to an A600 nm of 0.8. Protein expression was induced overnight at 18 °C by adding isopropyl 1-thio-β-d-galactopyranoside to a 1 mm final concentration. Bacterial cells were harvested by centrifugation, resuspended in lysis buffer (20 mm Tris-HCl (pH 8.0), 500 mm NaCl, and 10 mm imidazole), and then lysed by microfluidization. The soluble tagged protein was collected in the supernatant following centrifugation of the cell homogenate and purified on a Ni2+-NTA-Sepharose column according to published protocols (14). Recombinant tobacco etch virus (TEV) protease was used to digest the fusion affinity tag from the target protein; however, following removal of the affinity tag, the sequence GSTGS remains at the enzyme N terminus as an artifact of the subcloning procedure. After desalting into 20 mm Tris-HCl (pH 8.0), final purification was achieved by ResourceQ anion-exchange chromatography (GE Healthcare). Following this, the purified protein was concentrated to 5 mg/ml and buffer exchanged by ultrafiltration into 10 mm Tris-HCl (pH 7.5), 50 mm NaCl, and stored at 4 °C for further use. An analogous expression vector for the S192A mutant was constructed using standard molecular biology techniques (15). The mutant enzyme was purified and stored in an identical manner to the wild type protein.
Substrates and Analogs
The synthesis of MVAPP has been previously reported (16) and is briefly summarized. Methyl 3-hydroxy-3-methyl-5-iodopentanoate was synthesized by reacting mevalonolactone with trimethylsilyl iodide, followed by diazomethane derivatization to form the methyl ester. The product was subsequently purified by silica gel chromatography. Methyl 5-diphosphomevalonate was synthesized by reacting the purified methyl 3-hydroxy-3-methyl-5-iodopentanoate with an excess of tetrabutylammonium diphosphate. This material was purified using a DEAE-Sephadex A-25 (bicarbonate form) column. The purified methyl 5-diphosphomevalonate was converted to the lithium salt using Dowex 50 (lithium form). Deesterification was accomplished by alkaline hydrolysis in 0.5 n LiOH for 20 h at 4 °C. The pH was adjusted to ∼8.0 with cold HCl, and the concentration of the physiologically active R isomer was determined using MDD for an enzymatic end point assay.
Preparation of FMVAPP employed the method described by Voynova et al. (11). Five milligrams (33.8 μmol) of (RS)-6-fluoromevalonolactone was delactonized in 0.1 n KOH by incubation for 1 h at 37 °C prior to neutralization. The reaction mixture for the formation of FMVAPP included the following in 1.5 ml: 30 mm Tris-HCl (pH 7.5), 22.5 mm (RS)-6-fluoromevalonate, 5 mm ATP, 6 mm MgCl2, 0.7 mm DTT, 50 mm phosphoenolpyruvate, 6 units of pyruvate kinase, and 2.7 units of mevalonate kinase. The reaction was incubated at 30 °C. 16.9 μmol of (R)-6-fluoromevalonate were converted to the monophosphate in 1 h as determined by a phosphomevalonate kinase end point assay. Phosphomevalonate kinase (4 units of human enzyme) was then added, and the incubation was continued at 25 °C for 12 h. 10 μmol (∼60%) of the (R)-6-fluoromevalonate 5-phosphate was converted to the diphosphate as determined with phosphomevalonate kinase (reverse reaction). The reaction mixture was deproteinized (95 °C for 3 min), cooled to 0 °C, and centrifuged for 15 min at 16,100 × g, and the supernatant was adjusted to pH ∼5.0. Nucleotides were precipitated with cold ethanol (65% v/v for 30 min). Ethanol was removed from the supernatant by evaporation, and the sample was adjusted to pH ∼8.0 prior to dilution and chromatographic purification (DEAE Sephadex A-25).
The competitive inhibitor DPGP was synthesized by the strategy of Vlattas et al. (18). Detailed methodology has been described by Krepkiy and Miziorko (19).
Kinetic Characterization of Wild Type and Mutant S. epidermidis MDD
Enzymatic activity was determined using a spectrophotometric assay coupling product ADP formation to pyruvate kinase/lactate dehydrogenase oxidation of NADH at 340 nm using a PerkinElmer Life Sciences 35 spectrophotometer. Assays were performed at 30 °C in 100 mm HEPES-NaOH (pH 7.0) containing 100 mm KCl, 10 mm MgCl2, 0.2 mm NADH, 0.4 mm phosphoenolpyruvate, and 4 units each of pyruvate kinase and lactate dehydrogenase. All assays were initiated by addition of MVAPP. Vmax and Km values (Table 1) were determined by fitting data to the Michaelis-Menten equation. For inhibition kinetics, substrate (MVAPP) was varied versus inhibitor concentrations for FMVAPP or DPGP. Optimal data fits (R2 >0.97) were obtained using either competitive (FMVAPP) or mixed (DPGP) inhibition models. All kinetic data analysis was performed using SigmaPlot 10.0/Enzyme Kinetics Module 1.3 (Systat Software, Inc.). FMVAPP concentrations varied from 40 to 320 nm. DPGP concentrations varied from 3 to 10 μm.
TABLE 1.
Comparison of kinetic parameters for MDD from S. epidermidis and other sources
| Parametera | S. epidermidis MDD | S. epidermidis S192A | S. aureus MDD | T. brucei MDD | H. sapiens MDDb | Gallus gallus MDDc |
|---|---|---|---|---|---|---|
| Vmax (units/mg) | 9.8 ± 0.3 | 0.0096 ± 0.0003 | 9.8 ± 0.2 | 8.8 ± 0.2 | 6.1 ± 0.5 | 6.3 |
| kcat (s−1) | 5.9 ± 0.2 | 0.0058 ± 0.0002 | 6.0 ± 0.1 | 6.1 ± 0.1 | 4.4 ± 0.4 | 4.5 |
| Km((RS)-MVAPP) (μm) | 9.1 ± 0.9 | 5.0 ± 1.0 | 22.3 ± 1.6 | 6.0 ± 0.4 | 28.9 ± 3.3 | 14.1 |
| Km(ATP) (mm) | 0.027 ± 0.003 | 0.07 ± 0.01 | 0.013 ± 0.001 | 0.30 ± 0.02 | 0.69 ± 0.07 | 0.50 |
| Ki((R)-FMVAPP) (nm) | 49 ± 5 | ND | ND | 22.8 ± 3.8 | 62 ± 5 | ND |
| Ki((RS)-DPGP) (μm) | 4.3 ± 1.0d | ND | ND | ND | 2.3 ± 0.3 | ND |
Crystallization
Recombinant S. epidermidis MDD was crystallized by vapor diffusion of hanging drops at 20 °C. Specifically, 1 μl of protein solution (5 mg/ml in 10 mm Tris-HCl (pH 7.5), 50 mm NaCl) was mixed with 1 μl of reservoir solution containing 0.25 m sodium formate and 16% (w/v) PEG 3350 that had been previously diluted in an equal volume of double distilled H2O and equilibrated over 500 μl of reservoir solution. Single block-shaped crystals appeared after 2 days and continued to grow in size for ∼3 days. Crystals were flash-cooled in a cryoprotectant solution consisting of reservoir buffer with an additional 15% (v/v) glycerol. Cocrystallization of MDD with a molar excess (∼0.5 mm) of both DPGP and FMVAPP was achieved in the same manner as described above. Cocrystals of the S192A mutant MDD bound to FMVAPP were obtained in the same manner as for wild type MDD.
Diffraction Data Collection, Structure Determination, Refinement, and Analysis
Monochromatic x-ray diffraction data were collected from single crystals at 100 K using beamline 22-BM of the Advanced Photon Source, Argonne National Laboratory (Table 2). Following data collection, individual reflections were indexed, integrated, merged, and scaled using HKL2000 (20). Initial phase information was obtained for the unbound MDD structure by maximum-likelihood molecular replacement using PHASER (21). Specifically, chain A of PDB entry 2HK2 (S. aureus MDD) was altered using the SWISS-MODEL server to reflect the sequence of S. epidermidis MDD (22, 23), and the resulting hypothetical structure was used as a search model. The single most highly scored solution contained an MDD dimer in the asymmetric unit; this arrangement corresponded to a Matthews coefficient of 2.23 Å3/Da and a solvent content of 45.0%. The refined structure of unliganded MDD (as obtained below) was used as a search model for all other structures reported here.
TABLE 2.
Diffraction data collection and structure refinement statistics
| Data collectiona | |||||
| Crystal | MDD | MDD + DPGP | MDD + FMVAPP | S192A + FMVAPP | |
| Beamline | APS 22-BM | APS 22-BM | APS 22-BM | APS 22-BM | |
| Wavelength | 1.000 Å | 1.000 Å | 0.9724 Å | 1.000 Å | |
| Space group | C2221 | C2221 | C2221 | C2221 | |
| Cell dimensions | a = 82.98 | a = 82.67 | a = 82.80 | a = 82.50 | |
| b = 101.99 | b = 101.44 | b = 101.49 | b = 101.37 | ||
| c = 155.77 | c = 154.64 | c = 155.56 | c = 155.26 | ||
| Resolution | 26.91-1.85 Å | 29.60-2.05 Å | 30.70-2.20 Å | 29.00-2.10 Å | |
| Reflections (unique) | 307, 824 (52,638) | 573,361 (41,249) | 486,156 (33,608) | 211,067 (34,043) | |
| Completeness | 92.7% (81.5%) | 100.0% (100.0%) | 100.0% (99.9%) | 88.8% (87.5%) | |
| Redundancy | 5.8-Fold | 13.9-Fold | 14.5-Fold | 6.2-Fold | |
| 〈I〉/〈σI〉 | 25.2 (2.5) | 26.6 (5.1) | 20.0 (5.0) | 19.5 (2.9) | |
| Rmergeb | 5.8% (60.3%) | 9.1% (50.5%) | 10.9% (46.0%) | 7.9% (53.2%) | |
| PDB code | 3QT5 | 3QT6 | 3QT7 | 3QT8 | |
| Protein molecules/asymmetric unit | 2 | 2 | 2 | 2 | |
| Rwork/Rfreec | 19.5/22.7% | 18.3/22.6% | 17.8/22.5% | 19.0/24.1% | |
| No. of atoms | |||||
| Protein | 5078 | 5078 | 5038 | 5065 | |
| Ligand | NAd | 40 | 38 | 38 | |
| Solvent | 389 | 312 | 243 | 284 | |
| Ramachandran plot | |||||
| Favored | 98.1% | 98.1% | 98.1% | 98.4% | |
| Allowed | 1.7% | 1.6% | 1.4% | 1.6% | |
| Outliers | 0.2% | 0.3% | 0.5% | 0% | |
| Root mean square deviation | |||||
| Bond length | 0.007 Å | 0.008 Å | 0.007 Å | 0.008 Å | |
| Bond angle | 0.99° | 1.03° | 1.07° | 1.09° | |
| B factor | |||||
| Protein | 27.1 Å2 | 31.2 Å2 | 33.0 Å2 | 32.0 Å2 | |
| Ligand | NA | 30.0 Å2 | 25.1 Å2 | 26.2 Å2 | |
| Solvent | 30.2 Å2 | 33.5 Å2 | 33 Å2 | 32.0 Å2 | |
| Ligand occupancy | |||||
| Chain A | NA | 80% | 100% | 100% | |
| Chain B | NA | 92% | 100% | 100% | |
a Numbers in parentheses are for the highest resolution shell.
b Rmerge = ΣhΣi|Ii(h) − 〈I(h)〉|/ΣhΣiIi(h), where Ii (h) is the ith measurement of reflection h, and 〈I(h)〉 is a weighted mean of all measurements of h.
c R = Σh|Fobs(h) − Fcalc(h)|/Σh|Fobs|. Rcryst and Rfree were calculated from the working and test reflection sets, respectively. The test set constituted 5% of the total reflections not used in refinement.
d NA means not applicable.
Structure refinement was carried out using phenix.refine (24). One round of simulated annealing, individual coordinates, and isotropic atomic displacement factor refinement were conducted, and the refined model was used to calculate both 2Fo − Fc and Fo − Fc difference maps. These maps were used to iteratively improve the model by manual building in Coot (25, 26), followed by additional coordinate and atom displacement factor refinement. A final cycle of TLS refinement was also used to complete the structure of wild type MDD bound to FMVAPP. Ordered solvent molecules were added to all structures according to the default criteria of phenix.refine and inspected manually using Coot prior to model completion. Additional information and refinement statistics for all four structures are presented in Table 2.
With the lone exception of chain A in the DPGP-bound structure, a region of poor map quality exists between residues 184 and 194 in all MDD structures reported here. The exact number of residues that could not be modeled ranged between two and six, varying among the chains and structures. Further details regarding the residues that could not be modeled accurately may be found in the corresponding PDB files.
Ligand Fitting
Models for each ligand were generated using the PRODRG server (27), and restraint files were generated using phenix.elbow (24). In all cases reported here, inspection of the initial Fo − Fc maps described above revealed unmodeled contiguous density that corresponded to ordered ligand in the active site of both copies of MDD found within the asymmetric unit. phenix.ligandfit (24) was subsequently used to fit and model an inhibitor molecule in each active site. Refinement of inhibitor-bound MDD structures was carried out as described above, with the exception that constrained group occupancy refinement was used to estimate the fraction of ligand bound at each site independently.
Miscellaneous
Multiple sequence alignments were carried out using ClustalW (28) and aligned with secondary structure elements using ESPRIPT (29). MDD sequences used in alignments, along with their respective GenBankTM accession numbers, were as follows: S. epidermidis, 27467280; S. aureus, 14246359; Legionella pneumophila subsp. pneumophila, 52842257; Streptococcus pyogenes, 5093120; H. sapiens, 4505289; S. cerevisiae, 1706682; T. brucei, 149241992; Mus musculus, 13539580; Listeria monocytogenes, 217965923; Enterococcus faecalis, 315577315; Xenopus (Silurana) tropicalis, 39645379; Bos taurus, 115495513; Arabidopsis thaliana, 18410026. Three-dimensional structures were superimposed using the Local-Global Alignment method (LGA) (30). MDD structures were obtained from the PDB (31) and are as follows: S. cerevisiae (1FI4); S. pyogenes (2GS8); S. aureus (2HK2 and 2HK3); T. brucei (2HKE); H. sapiens (3DJ4); M. musculus (3F0N); L. pneumophila (3LTO). Representations of all structures were generated using PyMol (32). Calculations of electrostatic potentials at the molecular surface were carried out using DELPHI (33, 34). The program CONSURF was used to plot levels of amino acid sequence conservation on the molecular surface (35).
RESULTS
Kinetic Characterization and Crystal Structure of MDD from S. epidermidis
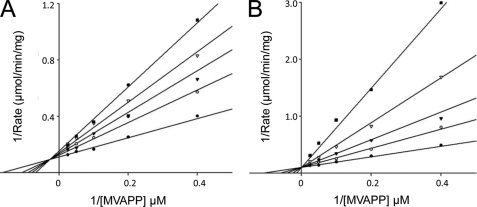
Whereas MDD proteins have been previously described from a variety of organisms, the nature of this enzyme in the pathogen S. epidermidis has so far been undetermined. To this end, a BLAST search was conducted using S. aureus MDD as a query sequence (36), which identified an as yet uncharacterized protein that shared 73% identity across the entire 327-residue polypeptide. A cDNA corresponding to this open reading frame was amplified by S. epidermidis genomic DNA by PCR and subcloned into an expression vector, and the resulting protein was expressed recombinantly and isolated from E. coli. The purified protein was characterized using the assay described under “Experimental Procedures.” The results of this analysis demonstrated that this S. epidermidis protein is a bona fide MDD that exhibits kinetic constants of Vmax = 9.8 units/mg, Km, (RS)-MVAPP = 9.1 μm, and Km (ATP) = 27 μm (Table 1). These values are comparable to corresponding parameters reported for most other MDD enzymes, although the prokaryotic MDD enzymes exhibit lower Km values for ATP than reported for eukaryotic enzymes. In all cases, Ki (FMVAPP) (<100 nm) indicates high affinity binding, whereas the analog DPGP inhibits 40–90-fold more weakly to human and S. epidermidis MDD, respectively (Fig. 1 and Table 1). Table 1 also includes kinetic parameter data for previously uncharacterized S. aureus and T. brucei MDD proteins (for which unliganded structures have been determined (13)), demonstrating that these proteins efficiently catalyze the MDD reaction.
FIGURE 1.
Inhibition of S. epidermidis MDD by MVAPP analogs. Double-reciprocal plots of MDD activity versus substrate MVAPP concentration are shown as measured in the presence of either DPGP or FMVAPP. A, inhibition of MDD by DPGP. Concentrations of DPGP are as follows: 0 μm (●), 3 μm (○), 5 μm (▾), 7 μm (▿), and 10 μm (■). B, inhibition of MDD by FMVAPP. Concentrations of FMVAPP are as follows: 0 nm (●), 40 nm (○), 80 nm (▾), 160 nm (▿), and 320 nm (■). Concentrations of substrate (MVAPP) were 2.5, 5, 10, 20, and 40 μm in both panels. Optimal fits to the data were obtained using a mixed inhibition model in A and a competitive inhibition model in B.
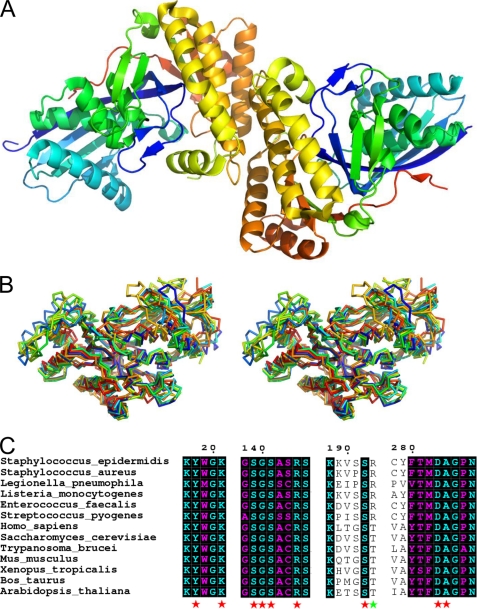
To better understand the nature of this enzyme, purified S. epidermidis was entered into crystallization trials using a hanging drop vapor diffusion approach. Following optimization of initial crystals, the final samples diffracted synchrotron x-rays to 1.85 Å resolution (Table 2). The S. epidermidis MDD structure was solved by molecular replacement and subsequently refined to final Rwork and Rfree values of 19.5 and 22.7%, respectively (Fig. 2A). This structure adopts a canonical GHMP kinase fold and displays a high degree of homology to those of related MDD proteins (Fig. 2B), even though these enzymes collectively share a rather limited sequence identity with one another. For example, S. epidermidis MDD is only 34% identical with the human enzyme, yet 300 of its 327 backbone atoms superimpose at less than 4.0 Å distance of their corresponding positions with a root mean square deviation of 1.54 Å (30).
FIGURE 2.
1.85 Å crystal structure of apo-MDD from S. epidermidis. A, crystal structure of S. epidermidis MDD shown in ribbon format. Two copies of MDD are found within the asymmetric unit and are colored blue (N terminus) and red (C terminus). B, stereo view of MDD structures in ribbon format deposited within the PDB. Structures correspond to the following organisms: S. epidermidis (red-orange); S. aureus (cyan); L. pneumophila (orange); S. pyogenes (red); H. sapiens (yellow-green); S. cerevisiae (blue); T. brucei (green); and M. musculus (yellow). C, limited structure-based sequence alignment of prokaryotic and eukaryotic MDD proteins. Alignment was generated using ClustalW and rendered with ESPRIPT. Numbers above the sequences correspond to S. epidermidis MDD. Red stars below the sequences correspond to invariant amino acid side chains involved in DPGP and FMVAPP interaction, and the green star represents the single variable active site residue. Sequences from B as well as the following were used in alignment: L. monocytogenes; E. faecalis; Xenopus. tropicalis; B. taurus; A. thaliana.
S. epidermidis MDD behaves as a dimer of 36-kDa subunits in analytical gel filtration chromatography experiments (supplemental Fig. S2). Consistent with this, the MDD polypeptide is found as a dimer within the asymmetric unit of its centered orthorhombic crystal. This quaternary structure buries 1950 Å2 or nearly 7% of the available surface area when both protein chains are considered together. This is comparable to the observation of a dimer interface of 7.7% (2250 Å2) for rat mevalonate kinase (37), which also exhibits the GHMP kinase fold. Although examination of neighboring asymmetric units reveals the presence of alternative modes for dimerization, only this interface is predicted to be significantly thermodynamically favored in solution, as judged by the protein interface surfaces and assemblies (PISA) server (38). These considerations aside, the entrance to the active site cavity is unhindered by any of the potential dimerization contacts described here. Thus, although observation of a dimeric species for MDD is typical, it is unclear that a dimeric enzyme is required for catalysis.
Structural Basis for Substrate Recognition and Competitive Inhibition of MDD
Prior crystallographic studies on MDD proteins have been limited to the unliganded form of the enzyme, which has precluded detailed analysis of both their structure/function relationships and modes of substrate binding. In two cases in particular (i.e. S. aureus and T. brucei (13)), attempts to obtain an informative ligand-bound structure were unsuccessful, presumably because of the presence of well ordered sulfate ions found within the purported active site cleft of these enzymes (13). Examination of additional MDD structures available in the PDB suggests that moderate to high concentrations of sulfate anions indeed promote crystallization of these enzymes, as five of the seven structures resulted from crystals harvested from sulfate-containing mother liquids. In this regard, the fact the crystals of S. epidermidis MDD grow readily in the absence of sulfate raised the possibility that this specific enzyme may be more amenable to determination of a structure with bound ligand.
Previous investigation into the MDD mechanism has identified two separate diphosphorylated compounds, DPGP (11) and FMVAPP (16, 39), that mimic the MVAPP substrate and competitively inhibit MDD activity (11). Despite these similarities, characterization of their effects on S. epidermidis MDD revealed markedly different Ki values of 4 μm for DPGP and 49 nm for FMVAPP (Table 1). A similar trend was also observed for the same inhibitors and human MDD, where the observed Ki values were 2 μm and 62 nm, respectively. Because comparison of Ki values for competitive inhibitors and Ki slope values for mixed inhibitors primarily reflects differences in binding affinity, these data suggested that meaningful insight into both the catalytic mechanism and mode of substrate binding by MDD proteins could be revealed by determining the cocrystal structures of S. epidermidis MDD bound to DPGP and FMVAPP.
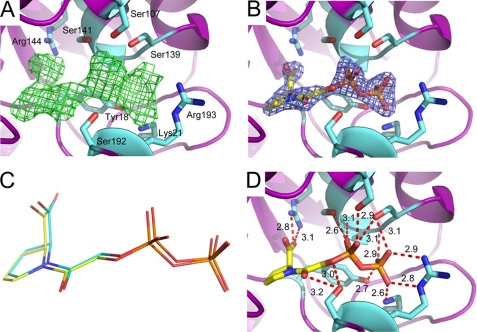
Crystals of MDD grown in the presence of (RS)-DPGP diffracted x-rays to 2.05 Å limiting resolution (Table 2). Following modeling and refinement of two copies of MDD, analysis of an Fo − Fc difference map revealed a region of strong contiguous density within the MDD active site cleft (Fig. 3A) (11). Placement and refinement of a single (R)-DPGP molecule per enzyme monomer (occupancy of 0.80 and 0.92 in chain A and B, respectively) are in good agreement with the observed diffraction data (Table 2 and Fig. 3B). Significantly, the conformations of both (R)-DPGP molecules, bound selectively from the R,S mixture, are nearly indistinguishable from one another, as all 24 atoms modeled superimpose with root mean square deviation values of 0.75 Å (Fig. 3C).
FIGURE 3.
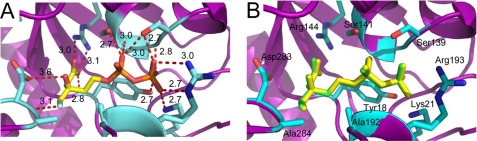
2.05 Å cocrystal structure of MDD bound to the inhibitor DPGP. A, Fo − Fc map (green mesh at 2.0σ contour) of the refined structure in the absence of modeled ligand. Active site side chains within interaction distance are depicted in ball and stick format (cyan). MDD backbone is depicted in ribbon format (purple). B, 2Fo − Fc map (blue mesh at 2.0σ contour) of the refined structure with one molecule DPGP modeled per enzyme. Color scheme is the same as A, with DPGP colored yellow. C, overlay of DPGP molecules from the active sites of chains A (yellow) and B (cyan). D, active site side chains within 2.6–3.4 Å of DPGP. Further information on these distances can be found in supplemental Table S1.
Numerous invariant amino acid side chain and backbone atoms contribute to the strong interaction of MDD with DPGP (Figs. 2C and 3D and supplemental Fig. S1). Several of these, including four serines (Ser107, Ser 139, Ser141, and Ser192), two residues from the N-terminal most β-strand in the enzyme (Tyr18 and Lys21), as well as Gly140 and Arg193 lie within a 3.5 Å distance of the atoms that comprise the α- and β-phosphoryl groups of DPGP. Separately, both nitrogen atoms of the catalytic Arg144 guanidinium group hydrogen bond with a single oxygen atom from the DPGP carboxylate (2.8 and 3.1 Å, respectively). Finally, while the available carbonyl oxygen of DPGP is also close enough to hydrogen bond with the side chain of Ser192 (3.2 Å), the general base Asp283 is notable in that it is too distant to interact with any atoms of the inhibitor.
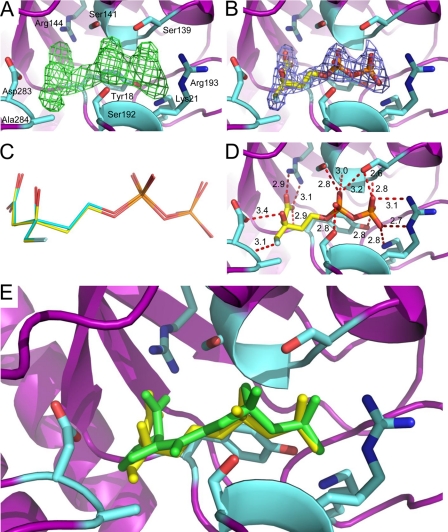
In a similar manner, MDD crystals were grown in the presence of (R)-FMVAPP. These crystals diffracted x-rays to 2.20 Å limiting resolution (Table 2). Well defined electron density was likewise apparent upon inspection of the Fo − Fc difference map obtained following initial refinement (Fig. 4A). A single copy of FMVAPP was placed in each MDD active site and refined at 100% occupancy (Table 2 and Fig. 4B). As was seen for DPGP, both FMVAPP molecules are bound in identical conformations, as all 19 atoms modeled superimpose with a root mean square deviation of 0.72 Å (Fig. 4C). Although several recurring side chain and backbone contacts from the DPGP-bound enzyme are present in the FMVAPP-inhibited structure (Figs. 2C, 3D, and 4D and supplemental Table S1), other unique interactions are also observed. For example, the backbone nitrogen, rather than the side chain hydroxyl, of Ser107 participates in a hydrogen bond with an α-phosphoryl-derived oxygen atom of FVMAPP (Fig. 4, D and E). Furthermore, several interactions that are crucial to the proposed catalytic mechanism are also seen. This includes a hydrogen bond (3.4 Å distance) between the side chain of Asp283 and C3 hydroxyl of FMVAPP, and each of the nitrogen atoms of the Arg144 guanidinium group binds to a different oxygen of the FMVAPP C1 carboxylate (2.9 and 3.1 Å). Finally, the fluoromethyl group of FMVAPP participates in an F···(H–N) hydrogen bond (3.1 Å) with the backbone amine of Ala284.
FIGURE 4.
2.20 Å cocrystal structure of MDD bound to the inhibitor FMVAPP. A, Fo − Fc map (green mesh at 3.0σ contour) of the refined structure in the absence of modeled ligand. Active site side chains within interaction distance are depicted in ball and stick format (cyan). MDD backbone is depicted in ribbon format (purple). B, 2Fo − Fc map (blue mesh at 2.0σ contour) of the refined structure with one molecule FMVAPP modeled per enzyme. Color scheme is the same as A, with FMVAPP colored yellow. C, overlay of FMVAPP molecules from chains A (yellow) and B (cyan). D, active site side chains within 2.6–3.4 Å of FMVAPP. Further information on these distances can be found in supplemental Table S2. E, overlay of the MDD active site from cocrystal structures of DPGP (green) and FMVAPP (yellow). Active site side chains from the FMVAPP cocrystal structure are depicted in ball and stick format (cyan).
Identification of Ser192 as New Residue That Contributes to MDD Catalysis
Earlier structure/function studies on MDD proteins have identified residues Asp283 (7) and Arg144 (11) as playing essential roles in catalysis. Furthermore, mutagenesis studies on Ser139 and Ser141 strongly suggest the influence of these residues in binding the diphosphorylated substrate MVAPP (19). Whereas these roles are readily interpreted in light of the DPGP- and FMVAPP-bound structures described above, the contributions of other residues are less certain. One such residue is Ser192, which is also invariant among a large and diverse cohort of MDD proteins (supplemental Fig. S1).
Ser192 is positioned on the opposite side of the active site cleft from Ser139 and Ser141, whose side chains participate directly in substrate binding. Nevertheless, Ser192 is also a far distance from the MVAPP atoms whose bonding patterns are altered during the MDD reaction, which suggests a role for this residue in substrate binding. Consistent with this idea, this side chain participates in hydrogen bonds with atoms from the inhibitor α-phosphoryl group in both the DPGP- and FMVAPP-bound structures. To test the role of Ser192 more directly, a S192A mutant of S. epidermidis MDD was constructed and characterized. Mutagenic substitutions of this side chain resulted in a near wild type Km value for substrate binding (Table 1), a feature that agrees well with the 2.10-Å cocrystal structure of FMVAPP bound to this mutant enzyme (Fig. 5A and Table 2). Despite these similarities, however, the mutant exhibited a greater than thousand-fold loss of catalytic activity (kcat) (Table 1). Although a detailed explanation for this effect may require a ternary ATP-FMVAPP-MDD structure, it is noteworthy that distances between the established catalytic residues (Asp283 and Arg144) and their cognate groups on FMVAPP are greater in the mutant cocrystal structure when compared with wild type MDD (Fig. 5B and supplemental Table S2). Furthermore, the dihedral angle between the Arg144 guanidinium group and the FMVAPP C1 carboxylate is noticeably steeper in the mutant structure relative to that of wild type (−29.3 ± 1.6° versus −18.4 ± 2.8°, respectively).
FIGURE 5.
2.10 Å cocrystal structure of MDD mutant S192A bound to the inhibitor FMVAPP. A, active site side chains within 2.6–3.6Å of FMVAPP are depicted in ball and stick format (cyan). Further information on these distances can be found in supplemental Table S2. MDD backbone is depicted in ribbon format (purple). B, overlay of FMVAPP molecules from wild type MDD (green) and mutant S192A MDD (yellow). Color scheme is the same as A.
Conservation and Divergence in the Vicinity of the MDD Active Site Cleft
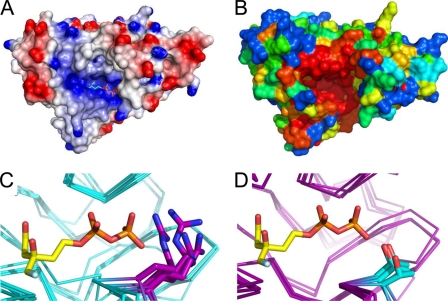
The dual substrate nature of MDD (i.e. MVAPP and ATP) places strict requirements on the solvent accessibility of the enzyme active site. This is magnified by the polyanionic nature of the substrates, which together carry a formal charge of −8 at physiological pH values. Consistent with these limitations, the residues lining the MDD active site entrance are overwhelmingly electropositive, and the charge density in this area is a striking feature of the MDD surface (Fig. 6A). The importance of this positively charged patch to substrate binding is likewise underscored by evolutionary analysis of surface-exposed MDD residues. Whereas much of the MDD surface exhibits variable levels of sequence conservation, the entrance to the active site cleft is practically invariant since the divergence of bacteria and humans (Fig. 6B).
FIGURE 6.
Conserved nature of the active site cleft. A, surface representation of electrostatic potential, generated by DelPhi, of chain A from MDD bound to FMVAPP (cyan). Color scheme represents regions of negative (red) and positive (blue) charge density contoured at ±5 e/kT. B, conservation plot of surface-exposed MDD amino acid side chains from S. epidermidis, as generated by CONSURF. Color scheme depicts variable (blue to green), average (yellow), and strong (orange to red) conservation. C, conserved nature of Arg193 (purple stick) within the active site of prokaryotic MDD proteins (backbone atoms colored cyan). Superimposed MDD structures are from S. epidermidis, S. aureus, S. pyogenes, and L. pneumophila. D, conserved nature of Thr (cyan stick) in eukaryotic MDD proteins (backbone atoms in purple) at the structurally equivalent position to Arg193. Superimposed MDD structures are from H. sapiens, M. musculus, T. brucei, and S. cerevisiae. FMVAPP is shown in C and D according to its position in the wild type MDD + FMVAPP structure (Fig. 4).
The many conserved features of the MDD mechanism are highlighted by high levels of identity among residues found in the active sites of various MDD proteins. However, the ability to identify and develop highly selective competitive inhibitors requires some level of divergence between target enzymes (i.e. those found in bacterial cells) and those found in the human host. In this regard, examination of microbial MDD sequences and structures along with those from their protozoan, mammalian, and human counterparts reveals one obvious and significant difference within the active site (Figs. 6C and 2C and supplemental Fig. S1). Specifically, although higher order organisms have a Thr at the equivalent position to residue 193, bacterial enzymes instead harbor an Arg at this location. It is worth noting that the side chain of Arg193 is well ordered in all cocrystal structures determined here (e.g. average atomic B factor of 27.9 Å2 relative to the mean value of 33.0 Å2 for protein atoms in the MDD + FMVAPP structure), as the atoms from its guanidinium group facilitate binding to the β-phosphoryl moiety of both DPGP and FMVAPP. As a consequence, exploitation of this particular amino acid substitution may allow for future development of compounds that specifically target only bacterial MDD proteins.
DISCUSSION
MDD is a member of the large GHMP kinase family of proteins, which includes not only phosphoryl transfer enzymes but also proteins involved in development. A recent article (40) on a bacterial threonine kinase, which fits into this protein family, pointed out that family members appear to use different kinetic and catalytic mechanisms despite their common tertiary structures. These enzymes typically catalyze sequential reactions in which ATP reacts with a phosphoryl acceptor to form ADP and a phosphorylated product. Some GHMP kinases bind ATP first and acceptor substrate second (e.g. liver galactokinase (41) and Salmonella enterica threonine kinase (40)). By contrast, others bind acceptor before ATP (e.g. liver mevalonate kinase (42) and liver MDD (43). Yet others still appear to utilize a random order of substrate binding in proceeding to a ternary reaction complex (E. coli galactokinase (44) and Streptococcus pneumoniae phosphomevalonate kinase (45). Based upon the crystal structures presented here, it seems unlikely that MDD could function through any mechanism other than binding the MVAPP acceptor prior to ATP. This is because both the MVAPP-binding site and the catalytic residues of the enzyme lie at the bottom of the MDD active site cleft. This active site cleft resembles a funnel, rather than a shallow groove on the protein surface, so there is no conceivable route to the catalytic site that would not be greatly or completely occluded by binding to the comparatively larger nucleotide substrate. Still, a conclusive statement regarding this aspect of MDD function will require determination of a ternary complex structure. However, these observations on MDD binary complexes are in accord with the kinetic results of Jabalquinto and Cardemil (43), who productively used the dead-end competitive inhibitor method (well established by Fromm (46)) to distinguish between random, ordered, and ping-pong kinetic mechanisms for MDD. They employed mevalonate, mevalonate phosphate, ATPγS, and AMP-PCP as inhibitors and tested these against the substrates mevalonate diphosphate and ATP. The inhibition patterns that were documented unambiguously established that MDD follows a sequential kinetic mechanism in which mevalonate phosphate binds before ATP. Thus, their results represent an important test and provide independent confirmation of the structural data.
Reports suggest that different catalytic mechanisms may be used by the various GHMP kinases. For homoserine kinase, there is no structural evidence for an active site base that would enhance attack of acceptor substrate on the γ-phosphoryl of ATP, so, by default, a direct nucleophilic attack mediated by transition state stabilization has been proposed (47). For yeast galactokinase, pH dependence results were interpreted to discount any need for a catalytic base in this enzyme (48). Subsequently, these authors collaborated on a structural investigation of Pyrococcus furiosus galactokinase; the conclusions suggested that an active site aspartate may indeed function as a catalytic base (49). For two GHMP kinases in the mevalonate pathway, we addressed this issue by interpreting structural results in the context of functional data. In the case of rat mevalonate kinase, the binary enzyme-ATP structure indicates the proximity between Asp204 to the γ-phosphoryl of ATP (37). Mutagenesis results indicate that exchange of the aspartate carboxyl for either asparagine or alanine side chains that do not function as a base reduces the catalytic rate by greater than 104-fold. Such an effect is appropriate in magnitude for removal of a catalytic base (50). Similarly, substitution of Asp302 in yeast MDD with either asparagine or alanine results in ∼103- and ∼105-fold decreases in catalysis, respectively. Consistent with these previous observations, the results reported here indicate that the corresponding Asp283 is situated only 3.4 Å from the C3 hydroxyl oxygen of FMVAPP. Thus, a functional assignment of Asp283 as a catalytic base can now be justified on the basis of both structural proximity and mutagenesis results. When considered together, the body of literature on both mevalonate kinase and MDD strongly suggests that GHMP kinases include a subclass of enzymes that do indeed utilize an active site base to deprotonate the phosphoryl acceptor.
Perhaps there may be a reasonable alternative explanation for the role of active site aspartates in mevalonate kinase, MDD, and other GHMP kinase enzymes whose future characterization may add to this mechanistic class. This hypothesis would involve a role for aspartate in precisely positioning the substrate so that the acceptor oxygen (i.e. C5 oxygen in mevalonate; C3 oxygen in mevalonate diphosphate) is optimally aligned for efficient in-line phosphoryl transfer of the ATP γ-phosphoryl group. Such an explanation (17) has been proposed to account for the large diminution in catalysis (4,000-fold) that results for mevalonate kinase upon alanine substitution of Ser146 which, by hydrogen bonding, optimizes orientation of the phosphoryl chain of ATP (37). It is therefore likely that more structural data on GHMP kinase ternary complexes will allow a consensus to develop regarding whether general base catalysis is operative or whether substrate alignment accounts for observations such as those documented for binary complexes of mevalonate kinase and MDD. As such data develop for MDD, a more complete understanding of binding differences of DPGP or FMVAPP to MDD should become possible, although the difference in how the carboxyl groups of these analogs interact with Arg144 already seems clear. That being said, previous functional characterization of mutations at serines comparable to Ser139 and Ser141 was not unambiguous, as these data indicated effects on both phosphoryl acceptor and donor binding sites (19). In this regard, Ser107 appears to interact with phosphoryl acceptors in the binary complexes, albeit in slightly different ways, even though precedent strongly suggests that this residue is situated in a consensus binding motif for the ATP phosphoryl donor. Here again, information obtained from a ternary complex structure is likely to refine our understanding of these MDD-binding site determinants.
Because several related taxa of important human pathogens rely on the mevalonate pathway to provide the isoprenoid building blocks necessary for ATP biosynthesis and for synthesis and maintenance of the bacterial cell wall, the potential for antibiotic discovery within this pathway remains both justified and intriguing. In this regard, the absolute requirement of MDD for a functional mevalonate pathway renders this enzyme an attractive target for such work. Because crystallographic data can play a significant part in guiding inhibitor design, the crystals and structures presented here constitute a promising starting point for several reasons. First, the S. epidermidis MDD crystals provide the opportunity to study an enzyme from the biologically relevant situation (i.e. a Gram-positive pathogen) at relatively high resolution. Second, unlike the crystals described for closely related proteins (e.g. S. aureus MDD), the S. epidermidis MDD crystals grow in a condition that is free of both high concentrations of sulfate and phosphate anions. This serendipitous discovery prevents undesirable, adventitious binding of such anions in a region of the active site that contributes important determinants for recognizing the diphosphorylated substrate MVAPP. Along similar lines, the modest ionic strength of the precipitant solution (0.25 m sodium formate) appears to be amenable to cocrystallization or ligand soaking of large numbers of potential small molecule inhibitors. In this respect, determination of cocrystal structures with both DPGP and FMVAPP, two molecules whose relative affinities for the enzyme differ considerably, provides a valuable proof of principle moving forward. Finally, this approach has already uncovered a significant difference between bacterial and eukaryotic MDD active sites that may be important to designing selective inhibitors. While this work is still ongoing, it represents an exciting basis from which future structure-activity relationship studies on small molecule inhibitors can be launched.
Supplementary Material
Acknowledgments
We acknowledge the generous technical assistance of Drs. Rod Salazar and Andy Howard during x-ray diffraction data collection and Dr. Samuel Bouyain for comments on the manuscript. Drs. Imogen Wilding and William Hunter generously provided plasmids used for production and characterization of S. aureus MDD and T. brucei MDD, respectively. Use of the Advanced Photon Source was supported by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract W-31-109-Eng-38. Data were collected at Southeast Regional Collaborative Access Team (SER-CAT) beamlines at the Advanced Photon Source, Argonne National Laboratory. A list of supporting member institutions may be found on the SER-CAT website.
This work was supported in part by National Institutes of Health Grants AI071028 and AI090149.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Tables S1 and S2.
The atomic coordinates and structure factors (codes 3QT5, 3QT6, 3QT7, and 3QT8) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
Numbering of all residues in this work reflects their position in the S. epidermidis MDD sequence.
- IPP
- isopentenyl diphosphate
- MDD
- mevalonate diphosphate decarboxylase
- DPGP
- diphosphoglycolyl proline
- MVAPP
- mevalonate 5-diphosphate
- FMVAPP
- 6-fluoromevalonate 5-diphosphate
- GHMP
- galactokinase, homoserine kinase, mevalonate kinase, phosphomevalonate kinase
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- AMP-PCP
- adenosine 5′-(β,γ-methylenetriphosphate)
- PDB
- Protein Data Bank.
REFERENCES
- 1. Zetola N., Francis J. S., Nuermberger E. L., Bishai W. R. (2005) Lancet Infect. Dis. 5, 275–286 [DOI] [PubMed] [Google Scholar]
- 2. Tenover F. C., Biddle J. W., Lancaster M. V. (2001) Emerging Infect. Dis. 7, 327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lowy F. D. (1998) N. Engl. J. Med. 339, 520–532 [DOI] [PubMed] [Google Scholar]
- 4. Eisenreich W., Bacher A., Arigoni D., Rohdich F. (2004) Cell. Mol. Life Sci. 61, 1401–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilding E. I., Brown J. R., Bryant A. P., Chalker A. F., Holmes D. J., Ingraham K. A., Iordanescu S., So C. Y., Rosenberg M., Gwynn M. N. (2000) J. Bacteriol. 182, 4319–4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bloch K., Chaykin S., Phillips A. H., De Waard A. (1959) J. Biol. Chem. 234, 2595–2604 [PubMed] [Google Scholar]
- 7. Krepkiy D., Miziorko H. M. (2004) Protein Sci. 13, 1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alvear M., Jabalquinto A. M., Eyzaguirre J., Cardemil E. (1982) Biochemistry 21, 4646–4650 [DOI] [PubMed] [Google Scholar]
- 9. Michihara A., Sawamura M., Nara Y., Ikeda K., Yamori Y. (1997) J. Biochem. 122, 647–654 [DOI] [PubMed] [Google Scholar]
- 10. Chiew Y. E., O'Sullivan W. J., Lee C. S. (1987) Biochim. Biophys. Acta 916, 271–278 [DOI] [PubMed] [Google Scholar]
- 11. Voynova N. E., Fu Z., Battaile K. P., Herdendorf T. J., Kim J. J., Miziorko H. M. (2008) Arch. Biochem. Biophys. 480, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonanno J. B., Edo C., Eswar N., Pieper U., Romanowski M. J., Ilyin V., Gerchman S. E., Kycia H., Studier F. W., Sali A., Burley S. K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12896–12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byres E., Alphey M. S., Smith T. K., Hunter W. N. (2007) J. Mol. Biol. 371, 540–553 [DOI] [PubMed] [Google Scholar]
- 14. Geisbrecht B. V., Bouyain S., Pop M. (2006) Protein Expr. Purif. 46, 23–32 [DOI] [PubMed] [Google Scholar]
- 15. Brøns-Poulsen J., Nøhr J., Larsen L. K. (2002) Methods Mol. Biol. 182, 71–76 [DOI] [PubMed] [Google Scholar]
- 16. Reardon J. E., Abeles R. H. (1987) Biochemistry 26, 4717–4722 [DOI] [PubMed] [Google Scholar]
- 17. Cho Y. K., Ríos S. E., Kim J. J., Miziorko H. M. (2001) J. Biol. Chem. 276, 12573–12578 [DOI] [PubMed] [Google Scholar]
- 18. Vlattas I., Dellureficio J., Ku E., Bohacek R., Zhang X. (1996) Bioorg. Med. Chem. Lett. 6, 2091–2096 [Google Scholar]
- 19. Krepkiy D. V., Miziorko H. M. (2005) Biochemistry 44, 2671–2677 [DOI] [PubMed] [Google Scholar]
- 20. Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 21. McCoy A. J., Grosse-Kunstleve R. W., Storoni L. C., Read R. J. (2005) Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 [DOI] [PubMed] [Google Scholar]
- 22. Arnold K., Bordoli L., Kopp J., Schwede T. (2006) Bioinformatics 22, 195–201 [DOI] [PubMed] [Google Scholar]
- 23. Kiefer F., Arnold K., Künzli M., Bordoli L., Schwede T. (2009) Nucleic Acids Res. 37, D387–D392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., Terwilliger T. C. (2002) Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 [DOI] [PubMed] [Google Scholar]
- 25. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 26. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schüttelkopf A. W., van Aalten D. M. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 28. Thompson J. D., Higgins D. G., Gibson T. J. (1994) Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gouet P., Courcelle E., Stuart D. I., Métoz F. (1999) Bioinformatics 15, 305–308 [DOI] [PubMed] [Google Scholar]
- 30. Zemla A. (2003) Nucleic Acids Res. 31, 3370–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr., Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. (1977) Eur. J. Biochem. 80, 319–324 [DOI] [PubMed] [Google Scholar]
- 32. DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]
- 33. Rocchia W., Alexov E., Honig B. (2001) J. Phys. Chem. B 105, 6507–6514 [Google Scholar]
- 34. Rocchia W., Sridharan S., Nicholls A., Alexov E., Chiabrera A., Honig B. (2002) J. Comput. Chem. 23, 128–137 [DOI] [PubMed] [Google Scholar]
- 35. Glaser F., Pupko T., Paz I., Bell R. E., Bechor-Shental D., Martz E., Ben-Tal N. (2003) Bioinformatics 19, 163–164 [DOI] [PubMed] [Google Scholar]
- 36. Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fu Z., Wang M., Potter D., Miziorko H. M., Kim J. J. (2002) J. Biol. Chem. 277, 18134–18142 [DOI] [PubMed] [Google Scholar]
- 38. Krissinel E., Henrick K. (2007) J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 39. Nave J. F., d'Orchymont H., Ducep J. B., Piriou F., Jung M. J. (1985) Biochem. J. 227, 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan C., Fromm H. J., Bobik T. A. (2009) J. Biol. Chem. 284, 20240–20248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ballard F. J. (1966) Biochem. J. 101, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beytia E., Dorsey J. K., Marr J., Cleland W. W., Porter J. W. (1970) J. Biol. Chem. 245, 5450–5458 [PubMed] [Google Scholar]
- 43. Jabalquinto A. M., Cardemil E. (1989) Biochim. Biophys. Acta 996, 257–259 [DOI] [PubMed] [Google Scholar]
- 44. Gulbinsky J. S., Cleland W. W. (1968) Biochemistry 7, 566–575 [DOI] [PubMed] [Google Scholar]
- 45. Pilloff D., Dabovic K., Romanowski M. J., Bonanno J. B., Doherty M., Burley S. K., Leyh T. S. (2003) J. Biol. Chem. 278, 4510–4515 [DOI] [PubMed] [Google Scholar]
- 46. Fromm H. J. (1979) Methods Enzymol. 63, 467–486 [DOI] [PubMed] [Google Scholar]
- 47. Krishna S. S., Zhou T., Daugherty M., Osterman A., Zhang H. (2001) Biochemistry 40, 10810–10818 [DOI] [PubMed] [Google Scholar]
- 48. Timson D. J., Reece R. J. (2002) Biochimie 84, 265–272 [DOI] [PubMed] [Google Scholar]
- 49. Hartley A., Glynn S. E., Barynin V., Baker P. J., Sedelnikova S. E., Verhees C., de Geus D., van der Oost J., Timson D. J., Reece R. J., Rice D. W. (2004) J. Mol. Biol. 337, 387–398 [DOI] [PubMed] [Google Scholar]
- 50. Hellinga H. W., Evans P. R. (1987) Nature 327, 437–439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.