Abstract
Somatodendritic (STD) dopamine (DA) release is a key mechanism for the autoregulatory control of DA release in the brain. However, its molecular mechanism remains undetermined. We tested the hypothesis that differential expression of synaptotagmin (Syt) isoforms explains some of the differential properties of terminal and STD DA release. Down-regulation of the dendritically expressed Syt4 and Syt7 severely reduced STD DA release, whereas terminal release required Syt1. Moreover, we found that although mobilization of intracellular Ca2+ stores is inefficient, Ca2+ influx through N- and P/Q-type voltage-gated channels is critical to trigger STD DA release. Our findings provide an explanation for the differential Ca2+ requirement of terminal and STD DA release. In addition, we propose that not all sources of intracellular Ca2+ are equally efficient to trigger this release mechanism. Our findings have implications for a better understanding of a fundamental cell biological process mediating transcellular signaling in a system critical for diseases such as Parkinson disease.
Keywords: Calcium, Calcium Channels, Exocytosis, Mouse, Neurotransmitters, Dopamine, Somatodendritic, Synaptotagmin
Introduction
Dopamine (DA),4 like other monoamine neurotransmitters, is released from the cell body and dendrites in addition to axon terminals (1). This process, called somatodendritic (STD) release, is important in the ventral tegmental area (VTA) for induction of behavioral sensitization to amphetamine through activation of local D1 receptors (2, 3) and in the substantia nigra (SN) for control of motor performance (4, 5). In addition, STD DA release modulates DA neuron firing activity through D2 autoreceptor activation (6, 7) and increases firing activity of SN pars reticulata γ-aminobutyric acid-releasing neurons, a process that might activate feedback signals regulating DA neuron activity (8), thereby influencing axonal DA release.
Two mechanisms have been proposed to mediate STD DA release: reversal of the DA transporter (9) and a vesicular exocytotic-like mechanism. In agreement with the second mechanism, STD DA release is activity-dependent (6, 10), sensitive to depletion of vesicular stores with reserpine (6, 11, 12), and Ca2+-dependent (6, 10, 12, 13). Moreover, disruption of SNARE proteins with botulinum toxins blocks STD DA release (10, 13). Vesicular exocytosis requires the concerted action of SNARE proteins and a synaptotagmin (Syt). During release, SNAREs have a direct role in vesicle-membrane fusion, and Syt acts as a Ca2+ sensor. Of the 15 Syt isoforms identified so far, Syt1, 2, 3, 5, 6, 7, 9, and 10 have been reported to drive Ca2+-dependent vesicular fusion (14), and only Syt1, 2, and 9 are confirmed as Ca2+ sensors for synaptic neurotransmitter release from axon terminals (15).
One of the hallmarks of STD DA release is its relative persistence at reduced levels of extracellular Ca2+ concentrations: although release from axon terminals is drastically reduced at extracellular Ca2+ levels lower than 1 mm, STD DA release persists at Ca2+ levels between 0.5 and 1 mm (Refs. 10, 12, and 13; but see also Ref. 16). This differential Ca2+ sensitivity between STD and terminal DA release suggests differences in either SNARE composition or Ca2+ sensors between these compartments.
Here, we focused on the role of Syt isoforms in mediating STD DA release. We determined the repertoire of Syt isoforms expressed in DA neurons, examined their distribution, and analyzed their implication in STD DA release triggered by basal firing activity. We also investigated the participation of different Ca2+ sources in triggering basal STD DA release. Our results propose a novel function for Syt7 as a critical mediator of STD neurotransmitter release and show that Syt7, together with Syt4, is part of the exocytotic machinery that controls STD DA release.
EXPERIMENTAL PROCEDURES
Animals and Tissue Processing
All of the experiments were performed using the TH-EGFP/21-31 mouse line; it carries enhanced GFP under the control of the tyrosine hydroxylase (TH) promoter (17) in a C57BL/6 genetic background. SN and VTA containing slices were prepared from mice at postnatal day 0 (P0), P14, and P45. P0 pups were cryoanesthesized prior to decapitation, and brains were collected in ice-cold dissociation solution (90 mm Na2SO4, 30 mm K2SO4, 5.8 mm MgCl2, 0.25 mm CaCl2, 10 mm HEPES, 20 mm glucose, 0.001% phenol red, pH 7.4). P14 and P45 mice were anesthetized with Halothane (Sigma-Aldrich) and decapitated, brains were collected in oxygenated saline solution (130 mm NaCl, 20 mm NaHCO3, 1.25 mm KH2PO4, 1.3 mm MgSO4, 3 mm KCl, 10 mm glucose, 1.2 mm CaCl2). All of the animal handling procedures were approved by the Université de Montréal animal ethics committee.
Mesencephalic Cell Suspension, Cell Sorting, and DA Neuron Cultures
Detailed procedures were described previously (18–20). Briefly, acutely dissociated mesencephalic cell suspensions were obtained by trituration of the slices mentioned above using glass pipettes of decreasing diameter after enzymatic treatment, and dead cells and debris were eliminated by differential gradient centrifugation. When the material was intended for FACS purification (supplemental Fig. S1, A and B), cells were resuspended in PBS with 1% FBS and injected into a BD FACSAria (BD BioSciences; San Jose, CA) flow cytometer/cell sorter. Double immunocytochemistry on cells plated on polyethyleneimine-coated coverslips after FACS purification showed that 96% of purified cells (n = 5) expressed both GFP and TH. No expression of GAD-67 (γ-aminobutyric acid neuron marker) or GFAP (astrocytic marker) was detected under RT-PCR on RNA obtained from FACS-purified cells (supplemental Fig. S1C).
When cells were intended for cultures, P0 mesencephalic cell suspensions were plated onto astrocytic monolayers (standard cultures), whereas cells were plated at a density of 350,000 cells/ml for cultures used for evaluation of DA release, the cells were plated at 50,000 cells/ml (low density cultures) for cultures used to evaluate expression and localization of Syts. In these cultures, DA neurons are identified by the expression of GFP; however, ectopic expression of GFP can be found in a small percentage of non-DA neurons, when evaluated by single-cell RT-PCR. In 17-day-old cultures, 90% (18 of 20) of GFP expressing neurons were found to be TH-positive when evaluated by immunocytochemistry. In 21-day-old cultures, over 95% of GFP positive cells were also TH-positive (results not shown).
Brain Slices
250-μm-thick horizontal brain slices were prepared from decapitated mice deeply anesthetized with halothane. The brain was quickly removed and placed in ice-cold artificial cerebrospinal fluid (125 mm NaCl, 25 mm NaHCO3, 2.5 mm KCl, 1.25 mm NaH2PO4, 2 mm CaCl2, 2 mm MgCl2, 23 mm glucose) saturated with 95% O2, 5% CO2. The slices were cut using a vibrating microtome (Leica Microsystems Canada Inc.) in ice-cold oxygenating artificial cerebrospinal fluid and left to recuperate in artificial cerebrospinal fluid at room temperature until use.
RNA Extraction and RT-PCR
Total RNA was extracted from FACS-purified cells as well as from tissues used as controls and standard primary culture coverslips by using TRIzol (Invitrogen). To purify RNA from FACS-purified cells, 5 μg of glycogen (Invitrogen) was added. Total RNA was dissolved in diethylpyrocarbonate-treated water and stored at −80 °C until use. cDNA synthesis was carried out for 1 h using 0.5 mm dNTP mix (Qiagen), 2.5 μm random hexamers (Applied Biosystems, Foster City, CA), 10 mm DTT, 40 units of RNaseOUT, 200 units of Moloney murine leukemia virus RT (Invitrogen), 50 mm Tris-HCl, 75 mm KCl, and 3 mm MgCl2, pH 8.3. RT enzyme was denatured, and the cDNAs were stored at −80 °C until use. PCRs were performed using 1.5 mm MgCl2, 0.5 mm dNTP mix, 10 pmol of each primer, 2.5 units of Taq DNA polymerase (Qiagen), 20 mm Tris-HCl, 50 mm KCl, pH 8.3, and 35 cycles with 55 °C of annealing temperature. All of the PCRs were resolved in 1.5% agarose gels.
Multiplex Single-cell RT-PCR
GFP-expressing neurons were randomly selected to avoid a selection bias toward cells that express high levels of GFP. Neurons were individually collected under RNase-free conditions using autoclaved borosilicate patch pipettes; each cell was collected by applying light negative pressure to the pipette. The content of each pipette was transferred immediately into individual prechilled 200-μl tubes containing 6 μl of a freshly prepared solution of 20 units of RNaseOUT and 8.3 mm DTT; the samples were immediately frozen on dry ice until use. Single cells were processed as described previously (18). Briefly, frozen samples were thawed on ice and subjected to the RT reaction as describe above but using 1.25 μm random hexamers, 100 units of Moloney murine leukemia virus RT, and 20 additional units of RNaseOUT. A first round of PCR was done as mentioned above using half of the RT reaction in 25 μl of final volume and 28 cycles. A second round of PCR was performed using 10% of the first PCR in 15 μl of final volume and 35 cycles. All of the PCR products were resolved in 1.5% agarose gels. The identity of each PCR products was confirmed by sequencing. Primers were designed based upon sequences deposited in the GenBankTM data base. They do not interact with each of the other primers in the multiplex PCR. Right primers are followed by left primers: GFP, 5′-aagttcatctgcaccaccg-3′ and 5′-tgctcaggtagtggttgtcg-3′; β-actin, 5′-ctcttttccagccttccttctt-3′ and 5′-agtaatctccttctgcatcctgtc-3; TH, 5′-gttctcaacctgctcttctcctt-3′ and 5′-ggtagcaatttcctcctttgtgt-3′; TH-nested, 5′-gtacaaaaccctcctcactgtctc-3′ and 5′-cttgtattggaaggcaatctctg-3′; GAD-67, 5′-atatcattggtttagctggtgaatg-3′ and 5′-gtgactgtgttctgaggtgaagag-3′; GFAP, 5′-agaagctccaagatgaaaccaa-3′ and 5′-ctttaccacgatgttcctcttga-3′; SNAP, 5′-gtaatgaactggaggagatgcaga-3′ and 5′-atttaagcttgttacagggacacaca-3′; Syt1, 5′-gaaagacttagggaagaccatgaa-3′ and 5′-tggacttttgtctcaaacttcttct-3′; Syt2, 5′-agaaacatcttcaagaggaaccag-3′ and 5′-aggttctctggctctttctcct-3′; Syt4, 5′-atatctacccagaaaacctaagtagcc-3′ and 5′-aaaacctgtcaaaactcagaactgtaa-3′; Syt7, 5′-cttagcgtcactatcgtcctctg-3′ and 5′-gtagccaacactgaactggattc-3′; Syt9, 5′-aaccagagttatacaaacagaggtca-3′ and 5′-tcaaagtcatacacagagaagtgaag-3′; and Syt11, 5′-tcgatgagaccttcaccttctac-3′ and 5′-tgacataaggattacctgagagacc-3′. For the single-cell RT-PCR experiments, only TH detection required the use of a nested reaction in the second round of PCR. Syt7 primers were designed to detect its three reported variants in mouse.
Immunocytochemistry
Cultures of cells plated on polyethyleneimine-coated coverslips and brain slices were fixed with 4% paraformaldehyde in PBS and processed using protocols published elsewhere (10, 18). A mouse monoclonal anti-TH antibody (Sigma-Aldrich) was used in combination with the following rabbit polyclonal antibodies: anti-GFP (Abcam), anti-Syt1 (Synaptic Systems, Goettingen, Germany), anti-Syt4 (developed by Mitsunori Fukuda in Japan), anti-Syt7 (Synaptic Systems), or anti-SNAP25 (Synaptic Systems). Because GFP fluorescence is negligible after paraformaldehyde fixation, TH was detected using a secondary antibody coupled to Alexa-488, whereas synaptotagmins and SNAP25 were detected using a secondary antibody coupled to Alexa-647. In experiments localizing the position within the mesencephalon of collected single cells, biocytin was added to the patch pipette and was then detected using Alexa-labeled streptavidin. Cellular localization of Syt was performed using low density cultures. The images were acquired using a point-scanning confocal microscope, equipped with 488 argon and 633-nm helium-neon lasers (Prairie Technologies LLC, Middleton, WI). The images were analyzed with Metamorph 4.5 (Universal Imaging Corp, Downingtown, PA).
DA Release Detection by Radioassay
STD DA release evoked by spontaneously firing DA neurons was detected by a radioassay using standard cultures on coverslips plated at a concentration of 350,000 cells/ml. This technique was selected because other approaches such as cyclic voltammetry cannot detect DA release evoked by spontaneous firing. After two rinses with Krebs-Ringer buffer (KRB) (140 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, 10 mm glucose, 6 mm sucrose, pH 7.35, and 305 mOsm), coverslips were incubated at 37 °C in KRB containing monoamine oxidase inhibitors (5 μm clorgyline and 100 μm pargyline, KRB-CP) and the D2 autoreceptor antagonist sulpiride (4 μm) for 5 min. Intracellular DA stores were next emptied by depolarization with 40 mm KCl. Labeling of intracellular DA was performed by incorporation of 0.2 μm (20 μCi/ml) of l-[2,3,5,6-3H]tyrosine (GE Healthcare) for 30 min in KRB-CP at 37 °C. Unincorporated radioactivity was removed by three rinses with KRB-CP, and the cells were left to rest for 3 min at room temperature. The coverslips were then placed in wells containing 400 μl of KRB-CP, and every 3 min a sample of 100 μl was collected (and immediately replaced with fresh KRB-CP) and mixed with scintillation mixture to determine radioactivity. The coverslips were fixed and the DA neurons were counted after immunocytochemistry. The values reported throughout the paper were obtained as follows: background cpm values from glial monolayers were subtracted from values of neuronal cultures and then divided by the number of DA neurons, counted blindly following TH immunocytochemistry performed after each experiment. When striatal (γ-aminobutyric acid) neurons were used, 8.6 ± 4.3 cpm/cell were detected at 2 mm Ca2+, whereas in DA neuron cultures, 148 ± 11.3 cpm/cell were detected.
The identity of the released radioactive substance as DA was confirmed by the observation that when radioactive labeling was performed without blockade of D2 autoreceptor (but with clorgyline and pargyline to prevent transmitter catabolism), therefore allowing feedback inhibition of TH activity (21), the release of radioactively labeled DA was decreased over 80 times (supplemental Fig. S1D). This observation is incompatible with the possibility that some of the radioactivity we measured in our experiments comes from 3H2O as a byproduct of tyrosine metabolism, especially from the tritium molecule in position C5. Thus the vast majority of radioactivity recovered was thus [3H]DA. Some experiments were also performed using 14C-labeled tyrosine (Moraveck Biochemicals, Brea, CA) obtaining results similar to those with l-[2,3,5,6-3H]-Tyrosine (data not shown). When experiments involved constant treatment, the drugs were added 20 min before collection and were present in the bath until the end of the experiment. When experiments required acute treatment to measure time course responses, the drugs were dissolved into the 100 μl of replacement volumes and administered after the control period. The composition of KRB was modified according to experiments to maintain osmolarity and concentration of divalent cations; NaCl concentration was lowered by 40 mm for the KRB containing 40 mm KCl, and MgCl2 was raised to 3.5 mm for experiments in 0.5 mm Ca2+. In all of the experiments, DA radioactivity labeling was performed in 2 mm Ca2+, whereas resting period and sampling was performed at 0.5 or 2 mm Ca2+, as required.
Electrophysiology
Patch clamp recordings under whole cell configuration were performed at room temperature on GFP-expressing neurons in culture following protocols published before (19, 22). In experiments evaluating spontaneous synaptic currents, the intrapipette solution contained 145 mm potassium methylsulfate, 10 mm NaCl, 0.1 mm EGTA, 2 mm MgATP, 0.6 mm GTP (Tris salt), 10 mm HEPES, 10 mm phosphocreatine, pH 7.35, and 300 milliosmol/liter. The neurons were voltage-clamped at −70 mV. After recordings, the synaptic currents were detected and analyzed using MiniAnalysis software (version 6) with a detection threshold of 100 pA to exclude a majority of miniature synaptic currents. In experiments measuring Ca2+ currents, voltage-clamp recordings were performed using a cesium intrapipette solution (68 mm CsF, 56 mm CsCl, 2.2 mm MgCl2, 4.5 mm EGTA, 14 mm creatine phosphate (Tris salt), 4 mm ATP (magnesium salt), 0.3 mm GTP (Tris salt), 9 mm HEPES, pH 7.35 (adjusted with CsOH 50%), and 300 milliosmol/liter). Ca2+ currents were isolated using a saline solution containing 160 mm TEA, 5 mm BaCl2, 10 mm HEPES, 5 × 10−4 tetrodotoxin, pH 7.35 (adjusted with TEAOH 20%), and 300 milliosmol/liter. When Ca2+ currents were stable for at least 5 min, nifedipine was applied for 5 min.
Small Interfering RNA
All of the siRNA, except GFP siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) and Cy3-labeled scrambled siRNA (Ambion, Austin, TX), were synthesized in vitro using the Silencer® siRNA construction kit from Ambion. Targeted sequences were chosen from published reports: Syt1 (23), Syt4 (24), and Syt7 (25). The siRNA against Syt7 acts against its three reported isoforms in mouse; it binds within exon 2, 145 bases before the alternative splicing site located after exon 4. The cells were transfected at day 17 in culture, and the effects on DA release were measured 4–5 days after. Transfection was performed using 40 pmol of each siRNA and Lipofectamine 2000 (Invitrogen). siRNA against GFP was used as a heterologous control. Nontransfected controls followed the transfection protocol, but no siRNA was added. The transfection efficiency was evaluated 24–36 h after transfection by detecting the Cy3-labeled scrambled siRNA; this evaluation was performed by counting the proportion of GFP expressing cells that also contained the Cy3 signal.
Calcium Imaging
The cells were incubated with 5 μm Fura2-AM and 0.02% pluronic acid (Invitrogen) for 45 min at room temperature in KRB. The coverslips were washed and left to stabilize for 15 min to allow for de-esterification of Fura2. Image ratio pairs (340/380 nm) were taken every 10 s under perfusion with KRB using a Hamamatsu Orca-II digital cooled CCD camera with a Lambda DG-4 excitation system (Sutter Instruments, Novato, CA) controlled with the AFA (Advanced Fluorescence Acquisition) module of the Image Pro Plus suite (Media Cybernetics) and analyzed with the ratio macro of the AFA module. At the end of each experiment, neurons that did not respond to a 40 mm KCl depolarization were considered as nonviable and discarded. The minimal Fura-2 ratio was calculated using 0 mm extracellular Ca2+ and 4 mm EGTA, and the maximal ratio was determined using 2 mm Ca2+ and 5 μm of ionomycin (Sigma).
Statistics
The data shown throughout are the means ± S.D. Statistically significant differences among conditions were analyzed either by a one-way analysis of variance with Dunnett's multiple comparison test or with a t test, as appropriate.
RESULTS
DA Neurons Express Synaptotagmin 1, 4, and 7
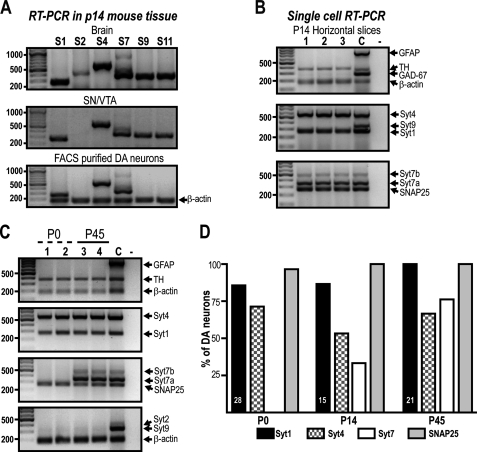
Because several Syt isoforms have been reported to be expressed in the SN and VTA (26–28), we first evaluated which of them are indeed expressed by DA neurons. We performed RT-PCR on RNA from FACS-purified DA neurons obtained from TH-GFP transgenic mice (supplemental Fig. S1, A and B). We found that at P14, purified DA neurons express Syt1, 4, and 7 (Fig. 1A), whereas Syt9 and 11 were detected in RNA from mesencephalon but not in RNA obtained from FACS-purified DA neurons (Fig. 1A) and thus are likely to be expressed by non-DA mesencephalic neurons. Syt2 was undetectable in RNA from mesencephalon but was found in whole brain RNA, used as control (Fig. 1A). We next confirmed our findings by performing multiplex single-cell RT-PCR using cytoplasm of individual GFP-expressing neurons obtained in horizontal slices of P14 mice; the samples were aspirated during patch clamp recordings in whole cell configuration from both SN and VTA. Once again, Syt2, 9, and 11 could not be detected. However, Syt1, 4, and 7 were found in DA neurons (Fig. 1B). In addition, these neurons contained mRNA for SNAP25 (Fig. 1B), a SNARE protein necessary for exocytosis that plays a fundamental role in basal STD DA release (10).
FIGURE 1.
Expression profile of Syt isoforms in DA neurons in vivo. A, FACS-purified DA neurons from the SN and VTA of TH-GFP mice were subjected to RT-PCR; their Syt mRNA expression pattern was compared with that of whole brain and mesencephalon (SN/VTA) of the same age (P14). B, the expression of Syt isoforms was evaluated in situ by single-cell RT-PCR performed on cytoplasm of DA neurons collected from SN and VTA after whole cell patch clamp recordings performed on horizontal slices. Three representative neurons are shown. C, the expression of Syt isoforms was evaluated during postnatal development by single-cell RT-PCR using freshly dissociated DA neurons. Two single DA neurons from P0 (cells 1-2) and two from P45 (cells 3-4) are shown. D, graph presenting the proportion of DA neurons expressing each of the Syt isoform detected at each age. The numbers inside the graph indicate the number of neurons collected per group from at least three different experiments. The identity of DA neurons was validated by the expression of TH. Syt7 can be detected as any of three variants in mouse; DA cells preferentially express the smallest one. Lane C, RNA from whole mesencephalon plus cortex used as positive control; lane −, collecting solution used as internal control.
Given that some of the Syt isoforms reported to be expressed in the mesencephalon could not be detected at P14, we decided to examine DA neurons at different developmental time periods. The Syt expression profile was thus assessed by single-cell RT-PCR using neurons acutely dissociated from newborns (P0) and young adults (P45). We again detected no expression of Syt2, 9, or 11 (Fig. 1C and data not shown) at these ages. However, we found a marked age-dependent increase in the proportion of DA neurons expressing Syt7 (Fig. 1, C and D). In comparison, there was no notable change in the proportion of DA neurons expressing Syt1, Syt4, and SNAP25.
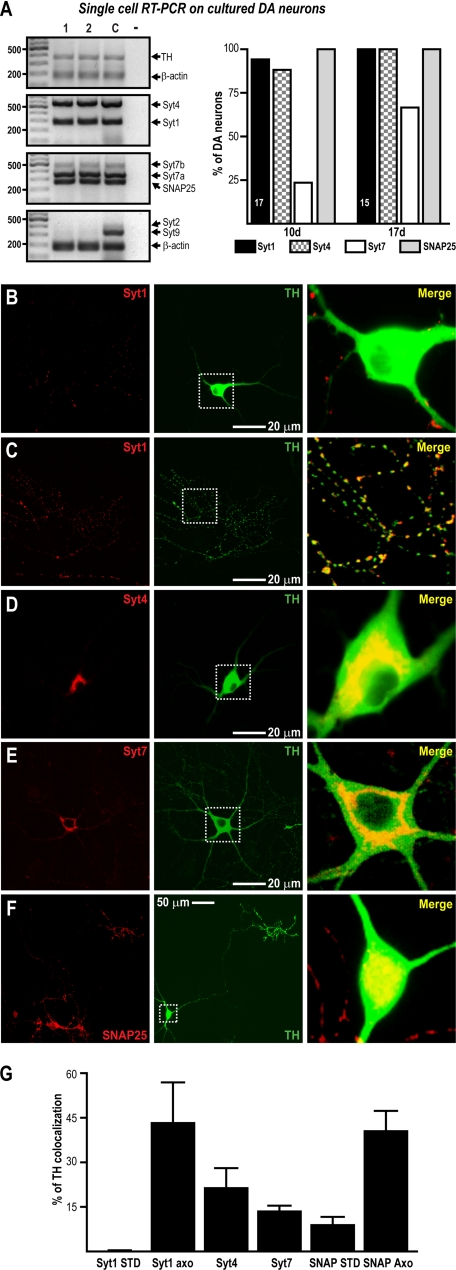
Because further experiments aiming to determine the mechanism of basal STD DA release required the use of primary DA neuron cultures, we next evaluated whether the Syt expression profile of DA neurons was the same in culture as in vivo. Using single-cell RT-PCR, we confirmed that DA neurons cultured for 10 or 17 days expressed only Syt1, 4, and 7 (Fig. 2A). Moreover, we found that the proportion of cells expressing Syt7 increased over time as it does in vivo (Fig. 2A). Furthermore, when evaluated by immunochemistry in low density cultures, all of the DA neurons expressed Syt1 (13 of 13 neurons) and Syt4 (18 of 18 neurons), whereas Syt7 was found in 66% of DA neurons (12 of 18 neurons).
FIGURE 2.
Expression profile of Syt isoforms in cultured DA neuron. The expression of Syt isoforms in cultured DA neurons was similar to that in vivo. A, the expression pattern was examined in DA neurons cultured for 10 or 17 days. Two representative cells are shown. The graph shows the proportion of DA neurons expressing each isoform at the two time points; the numbers in the bars indicate the number of cells collected from three different experiments. The presence of β-actin was assayed to confirm the presence of mRNA. Lane C, RNA from whole mesencephalon plus cortex used as positive control; lane −, collecting solution used as internal control. B–F, the subcellular localization of Syt1 (B and C), Syt4 (D), Syt7 (E), and SNAP25 (F) within the STD compartment of DA neurons was evaluated by immunocytochemistry in 17-day-old low density cultures. The immunolabeling of the proteins of interest (red signal) is shown in the left panels, whereas TH immunolabeling (green signal) is shown in the central panels. A higher magnification of the areas shown by the white boxes is presented in the right panels. Note the lack of colocalization of Syt1 and TH within the STD compartment of DA neurons (B) and the extensive presence of Syt1 within axonal-like varicosities (C). The axonal varicosities shown in C originate from the cell body of a DA neuron not visible in the micrograph. Note also the extensive presence of Syt4, Syt7, and SMAP25 within the STD compartment of DA neurons (D–F). G, summary graph presenting the results of quantitative image analysis to evaluate the colocalization of each protein of interest with TH. The level of colocalization of Syt1 and SNAP25 was quantified in both the STD and axonal-like (Axo) compartments.
Syt4 and 7 Are Localized in the Somatodendritic Compartment of DA Neurons
If Syt1, 4, or 7 is involved in triggering STD DA release, these proteins should accordingly be localized in the STD compartment of DA neurons. We thus determined their subcellular localization in low density cultures using immunocytochemistry (Fig. 2, B–F). Syt1 was not found within the cell body or dendrites of DA neurons (Fig. 2B). It was found to be restricted to fine axonal-like varicosities (Fig. 2C). However, Syt4 and Syt7 were always found in the soma and major dendrites of DA neurons (Fig. 2, D and E). In addition, Syt7 was found in a subset of axon terminals (supplemental Fig. S2A). Interestingly, SNAP25 was immunodetected in the STD compartment of DA neurons (Fig. 2F) in addition to its expected localization to axonal-like processes. Quantitative analysis of the colocalization of Syt1, Syt4, Syt7, or SNAP25 with TH (Fig. 2G) corroborated the presence of Syt4, Syt7, and SNAP25 in the STD compartment. In addition, as expected, no significant presence of Syt1 was found within the STD compartment, but both Syt1 and SNAP25 were colocalized with TH in axonal-like varicosities.
Syt4 and Syt7 Are Necessary for Basal Somatodendritic DA Release
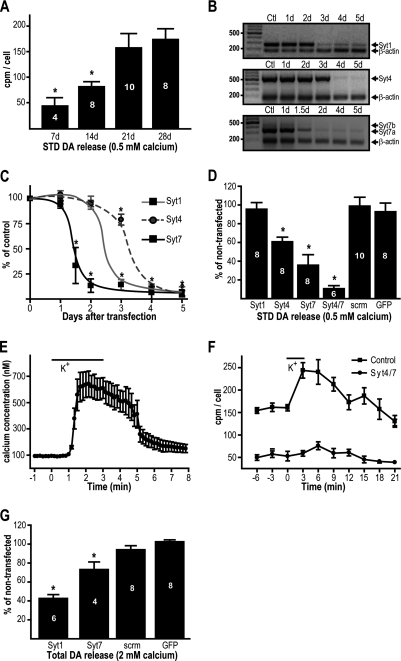
To evaluate the participation of Syt isoforms in basal STD DA release, we used a recently developed mesencephalic primary DA neuron culture system (10) in which activity-dependent (10, 29, 30) STD DA release is selectively isolated from terminal DA release by reducing extracellular Ca2+ levels to 0.5 mm (10, 13) and can be measured by radioassay or HPLC, both giving similar results (10). As previously demonstrated, we found that lowering extracellular Ca2+ from 2 mm to 0.5 mm reduced but did not prevent the extracellular accumulation of DA (supplemental Fig. S2B). In addition, accumulation of extracellular DA at 0.5 mm of extracellular Ca2+ was reduced by preventing firing with TTX (supplemental Fig. S2C) and increased in response to depolarization with 40 mm KCl (supplemental Fig. S2D). Finally, treatment with 1 μm of GBR1209 to block DA transporter resulted in increased DA levels at 0.5 mm Ca2+, showing that release did not occur by reverse transport (supplemental Fig. S2E). Also confirming complete blockade of axonal neurotransmitter release in the presence of 0.5 mm of extracellular Ca2+, glutamate-mediated synaptic currents were completely blocked under these conditions, leaving only action potential-independent miniature synaptic currents (supplemental Fig. S2F). Interestingly, when evaluating cultures of 7–28 days of maturation, we found a time-dependent increase in STD DA release (Fig. 3A), a time course reminiscent of that of Syt7 expression.
FIGURE 3.
Syt4 and Syt7 are important for STD DA release, whereas Syt1 is essential for terminal DA release. A, STD DA release increases with time in culture. Extracellular [3H]DA levels were measured in DA neuron cultures in the presence of 0.5 mm Ca2+. B and C, down-regulation of Syt by siRNA. Cultured DA neurons were transfected at day 17 in culture using isoform-specific siRNA. The efficiency of transfection was 95%, and its effects on RNA (B) and protein (C) levels were assessed by semiquantitative RT-PCR and immunochemistry, respectively, at different days after transfection, as indicated. B, a representative gel of each Syt mRNA levels after siRNA transfection is shown. The presence of β-actin mRNA was evaluated to confirm the presence of mRNA and as a loading control. Note that Syt7 can be expressed as three different isoforms in mouse; DA neurons preferentially express Syt7a, the smallest one. C, summary graph presenting the Syt/TH ratio measured at different days after transfection, as indicated. The cells were fixed and subjected to immunochemistry, 10 images were captured from random fields at 40× (n = 6) and quantified with Metamorph software. D, effect of Syt mRNA down-regulation on STD [3H]DA release. Transfection was performed at day 17 in culture; their effects were evaluated 4 days later. STD [3H]DA release was evaluated in the presence of 0.5 mm extracellular Ca2+. E, summary graph showing that elevation of extracellular K+ in the presence of 0.5 mm extracellular Ca2+causes a substantial increase in [Ca2+]i levels in cultured DA neurons. F, after down-regulation of Syt4/7, high potassium depolarization fails to significantly elevate extracellular DA levels in the presence of 0.5 mm extracellular Ca2+. G, the effect of Syt mRNA down-regulation on total STD [3H]DA release (STD plus terminal) was also evaluated (in the presence of 2 mm Ca2+). Syt1 down-regulation caused a large decrease in DA levels, whereas Syt7 down-regulation caused a more modest decrease. scrm, cells transfected with scrambled siRNA; GFP, cells transfected with siRNA against GFP (heterologous control); Ctl, no siRNA used during transfection. The number of experiments for each condition is indicated inside the bars. *, p < 0.05 versus 21d (A) or versus Ctl (C–G). The error bars represent S.D.
To determine the participation of Syt isoforms in basal STD DA release, we down-regulated their expression using siRNA. We first evaluated the efficiency of each siRNA (Fig. 3B). Maximal inhibition of Syt1 mRNA was after 3 days of transfection; for Syt4, it was after 4 days, and for Syt7, it was after 48 h. As heterologous control, we used a siRNA against GFP, which showed maximal inhibition at 4 days post-transfection (results not shown). In all cases, mRNA levels were still down-regulated for at least 5 days post-transfection (Fig. 3B). In addition, the protein levels were evaluated by immunocytochemistry to confirm their inhibition (Fig. 3C).
By using the DA radioassay at 0.5 mm of extracellular Ca2+, we next evaluated the effect of Syt down-regulation on STD DA release (Fig. 3D). We found that Syt1 down-regulation did not affect extracellular DA levels, whereas down-regulation of siRNA against Syt4 or Syt7 had a marked effect, with Syt7 down-regulation being the most efficient. Moreover, down-regulation of Syt4 and 7 together almost completely blocked STD DA release. Transfection of siRNA against GFP or scrambled siRNA had no effect on extracellular DA levels (Fig. 3D). We also evaluated the effect of Syt4/Syt7 knockdown on STD DA release evoked by potassium depolarization, a stimulus that causes a marked increase in intracellular Ca2+, even in 0.5 mm extracellular Ca2+ (Fig. 3E). We found STD DA release evoked in this way was also completely blocked by Syt4/7 knockdown (Fig. 3F), thus showing that these isoforms are critical for both basal and stimulated STD DA release.
The lack of effect of Syt1 down-regulation on STD DA release is compatible with its localization in axon terminals and with the fact that axonal DA release is blocked under 0.5 mm Ca2+ under our conditions. It is also compatible with the previous observation that Syt1 does not trigger neurotransmitter release at 0.5 mm of external Ca2+ (31). However, to control for the functional effectiveness of Syt1 down-regulation, we evaluated its effect on DA release in 2 mm extracellular Ca2+, which allows both axonal and STD DA release. We found that under these conditions, Syt1 siRNA reduced extracellular DA levels by ∼60%, thus confirming the important role of Syt1 in axonal DA release (Fig. 3G). In contrast, compatible with an effect limited to STD DA release, Syt7 down-regulation caused an only 25% decrease on DA release measured in 2 mm Ca2+ (Fig. 3G). As expected, no effect on extracellular DA levels was observed when cells were transfected with siRNA against GFP or scrambled siRNA (Fig. 3G).
Somatodendritic DA Release Is Selectively Dependent on Calcium Influx through N and P/Q Voltage-gated Calcium Channels
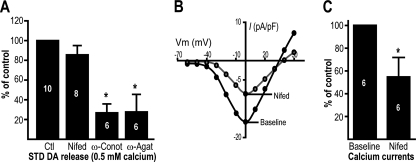
Because Ca2+ sensor such as synaptotagmins typically require coupling to a local source of intracellular Ca2+ influx, we next examined the role of voltage-gated Ca2+ channels in STD DA release. Although the broad spectrum voltage-gated Ca2+ channel (VGCC) blocker cadmium has been reported to be effective at reducing STD DA release (32, 33), the specific Ca2+ channel subtypes driving STD DA release remain unclear. In addition, recent work has suggested a partial implication of intracellular Ca2+ ([Ca2+]i) stores in STD DA release evoked by action potential trains (33). Therefore, we tested the participation of both extracellular and intracellular Ca2+ in triggering STD DA release. Because it has been reported that N-, P/Q-, and L-type VGCC account for 85% of peak Ca2+ current in the cell body of DA neurons (34, 35), we evaluated the effect of the L-type Ca2+ channel blocker nifedipine (20 μm), the N-type blocker ω-conotoxin GVIA (100 nm) and the P/Q-type blocker ω-agatoxin IVA (100 nm). We found that blocking of N- or P/Q-type channels efficiently reduced STD DA release (Fig. 4A). In contrast, the L-type blocker nifedipine failed to reduce extracellular DA levels (Fig. 4A). The lack of effect of nifedipine was not caused by inefficient block of Ca2+ channels because, compatible with previous reports (35), we found that this antagonist blocks half of the total Ca2+ current recorded in cultured DA neurons (Fig. 4, B and C).
FIGURE 4.
Somatodendritic DA release depends on N- and P/Q-type voltage-gated Ca2+ channels. A, 21-day-old DA neuron cultures were treated with 20 μm nifedipine (Nifed), 100 nm ω-conotoxin GVIA (ω-Conot), or 100 nm ω-agatoxin IVA (ω-Agat), and STD [3H]DA release was measured in the presence of 0.5 mm extracellular Ca2+. The graph represents the [3H]DA levels normalized against the control (Ctl, vehicle-treated) group. B, nifedipine (20 μm) efficiently blocks Ca2+ currents in DA neurons. Ca2+ currents, carried by barium, were measured in DA neurons cultured for 21 days. B and C, a representative Ca2+ current density (current size (pA)/membrane capacity (pF) ratio)-voltage plot is shown in B, whereas the measurement of peak Ca2+ current amplitude is summarized in C. The numbers inside the bars indicate the number of experiments performed in each group. *, p < 0.05 versus control. The error bars represent S.D.
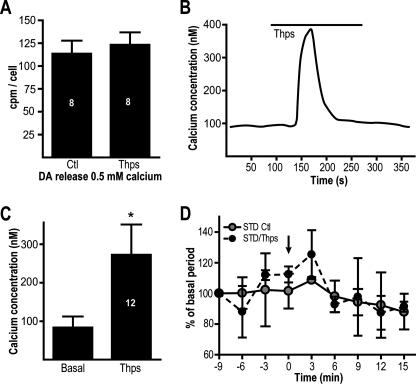
To examine the involvement of [Ca2+]i stores, we used thapsigargin, an inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase. We reasoned that if basal STD DA release relies on ER Ca2+ stores, their depletion with thapsigargin (1 μm) should impair STD DA release. We found that preapplication of thapsigargin failed to cause a significant change in extracellular DA levels (Fig. 5A). Furthermore, although an acute application of thapsigargin caused the expected transient increase in [Ca2+]i in cultured DA neurons (Fig. 5, B and C), this treatment did not cause a significant increase in DA levels 3 min after thapsigargin application (Fig. 5D).
FIGURE 5.
[Ca2+]i elevation induced by thapsigargin does not trigger STD DA release. A, [Ca2+]i stores were depleted by preapplication of 1 μm thapsigargin (Thps), and the release of [3H]DA was measured in the presence of 0.5 mm Ca2+. B, acute application of thapsigargin (1 μm) triggered a transient [Ca2+]i elevation in DA neurons. C, summary graph presenting the average peak increase in [Ca2+]i in response to thapsigargin. The graph represents results from 12 independent experiments. D, [Ca2+]i elevation caused by an acute application of thapsigargin was unable to trigger the release of [3H]DA measured in the presence of 0.5 mm of extracellular Ca2+. The arrow indicates the time of application of thapsigargin (0 min). The number of experiments per group is indicated in the bars. *, p < 0.05 versus control (A) or basal (C). The error bars represent S.D. Ctl, control.
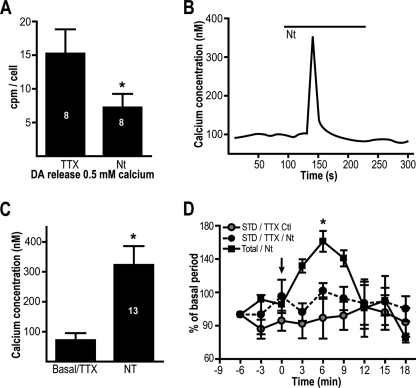
The finding that Ca2+ influx through L-type channels is unable to trigger STD DA release argues that this form of DA release cannot be nonselectively triggered by any [Ca2+]i elevation. To further test this hypothesis, we used the neuropeptide neurotensin, a modulator well known to elevate [Ca2+]i in DA neurons through activation of nonselective cationic channels of the transient receptor potential family and through [Ca2+]i mobilization (22). In the presence of TTX (0.5 μm) to prevent action potential firing and secondary Ca2+ influx through VGCC, preapplication of neurotensin (10 nm) failed to induce any increase in STD DA release. To the contrary, it actually induced a significant decrease in extracellular DA levels (Fig. 6A). In addition, although acute application of neurotensin caused a robust elevation of [Ca2+]i (Fig. 6, B and C), it failed to enhance STD DA release (Fig. 6D). However, in normal extracellular Ca2+, total DA release was elevated by neurotensin, compatible with the well known ability of this peptide to enhance DA release from axon terminals. These results suggest that any elevation of [Ca2+]i cannot indiscriminately trigger STD DA release: an elevation at release sites of a sufficient amplitude and duration is likely to be required.
FIGURE 6.
[Ca2+]i elevation induced by the peptide neurotensin does not trigger STD DA release. A, 21-day-old DA neuron cultures were pretreated with 10 nm neurotensin (Nt) to activate Ca2+ influx and [Ca2+]i mobilization. The release of [3H]DA was measured in the presence of 0.5 mm extracellular Ca2+ and 0.5 μm TTX to prevent firing. Neurotensin (10 nm) caused a decrease in STD DA release. The control group was treated only with TTX. B, acute treatment of DA neurons with 10 nm neurotensin induced a fast transient increase in [Ca2+]i in the presence of 0.5 μm TTX. C, summary data presenting the effect of neurotensin on [Ca2+]i in DA neurons (n = 13). D, effect of acute application of neurotensin on STD DA release. The release of [3H]DA upon treatment with 10 nm neurotensin was evaluated in the presence of 0.5 mm extracellular Ca2+ and in the presence of 0.5 μm TTX (black circles, STD DA release/TTX/neurotensin) or in the presence of 2 mm extracellular Ca2+ (black squares, total/neurotensin) to measure total DA release. A control group without neurotensin (with TTX) is also illustrated. The arrow indicates the time of application of neurotensin (0 min). The numbers inside the bars indicate the number of repetitions in each group. *, p < 0.05 versus control group (A) or basal (C and D). The error bars represent S.D.
DISCUSSION
Modulation of DA neuron activity is central to the encoding of motivated behavior and reward prediction. DA is also critical for the response to antipsychotic drugs, to psychostimulants used in the treatment of attention deficit disorder, and to drugs of addiction. STD DA release is a key mechanism regulating DA neuron activity because it activates D2 autoreceptors, leading to slow inhibitory synaptic responses in DA neurons (6, 29, 36, 37). However, identification of the underlying mechanism has proven to be controversial. Although reversal of the DA transporter has been suggested to be required for STD DA release evoked by glutamate (9) or veratridine (38), we and others (6, 29) have found that DA transporter is not required for STD DA release evoked by spontaneous firing or short trains. Compatible with the implication of a form of exocytosis, it was found that STD DA release requires SNAP25 and synaptobrevin (10, 13). Here we further explored the mechanism of STD DA release by evaluating the implication of synaptotagmin isoforms and Ca2+ sources in an effort to explain the differential Ca2+ sensitivity of STD and terminal DA release. Our results further strengthen the hypothesis of the implication of an exocytotic mechanism by demonstrating the selective involvement of Syt4 and Syt7 in STD DA release. Furthermore, we find that although Ca2+ is important for triggering STD DA release, not all sources of Ca2+ have the ability to trigger basal STD DA release caused by spontaneous firing. N- and P/Q-type Ca2+ channels play a preferential role, perhaps because of their known coupling to SNARE proteins.
Differential Roles of Synaptotagmin 1, 4, and 7 in Terminal and STD DA Release
The Syt isoforms that we detected in DA neurons are known to regulate exocytosis. The role of Syt1 in fast Ca2+-triggered axonal neurotransmitter release is very well documented (39). Our findings argue that Syt1 is the primary Ca2+ sensor in dopaminergic axon terminals; its lack of presence in the STD compartment makes it unlikely to participate in STD DA release. Syt7 regulates Ca2+-triggered exocytosis of lysosomes in fibroblast (40, 41), of dense core vesicles (25), and of glucagon in pancreatic cells (42). Syt4 regulates glutamate release from hippocampal astrocytes (24), as well as from dense core vesicles in LβT2 cells (43) and from auditory ribbon synapses (44). It also appears to modulate vesicular transport in the trans-Golgi network (45) and to regulate the release of retrograde signals at the neuromuscular junction in Drosophila (46). Here, our findings argue that Syt7 is a critical regulator of STD DA release. Moreover, the fact that Syt7 has a 10-fold higher affinity than Syt1 for binding phospholipids and syntaxin (47) and can trigger neurotransmitter release at intracellular Ca2+ concentrations as little as 300 nm versus the 3 μm that Syt1 requires (48, 49) provides a possible explanation for the remarkable difference in Ca2+ requirement between terminal and STD DA release sites (10, 13). Our finding of robust Syt4/7-dependent STD DA release evoked by K+ depolarization elevating [Ca2+]i to values between 500 and 700 nm fit with this general hypothesis. Our finding of a partial requirement for Syt4 in STD DA release is less easily reconcilable with the hypothesis of a lower Ca2+ sensitivity of STD DA release. Syt4 is thought to be a nonfunctional Ca2+-dependent Syt and proposed to act as a negative modulator of exocytosis by competing with Syt1 for interaction with SNARE proteins (50); the knock-out of Syt4 increases synaptic vesicle exocytosis, whereas Syt4 overexpression decreases it (Ref. 51; but see Ref. 52). However, because Syt1 is not present in the STD compartment of DA neurons, a Syt1-Syt4 interaction should not occur. In addition, based on a recent proposal (53), it is possible that Syt4 acts to position vesicles close to release sites in a Ca2+-insensitive manner. Considering the affinities of Syts for Ca2+, one can argue that lowering extracellular Ca2+ favors artificially the activity of Syt7 over Syt1 in STD DA release. However, the absence of Syt1 within the STD compartment makes this scenario improbable.
Although Syt1, 4, and 7 are also expressed by non-DA neurons (Fig. 1A and data not shown), it is unlikely that the results obtained in our siRNA experiments result indirectly from modified synaptic inputs to DA neurons because (i) neither Syt4 nor Syt7 participate in fast exocytosis at synapses and (ii) at 0.5 mm Ca2+, the presence or absence of Syt1 does not change STD DA release.
Participation of Voltage-gated Ca2+ Channels in STD DA Release
The participation of VGCCs in STD DA release has remained unclear, and contradictory results have been obtained using different models. STD DA release in response to stimulation trains in adult mouse mesencephalic slices has suggested the involvement of N-type Ca2+ channels (6). Using acutely dissociated rat DA neurons from P9–P14 animals, others have reported that KCl-evoked STD DA release is reduced by ∼50% by the broad spectrum VGCC blockers cobalt and cadmium (30). Using adult guinea pig mesencephalic brain slices, cadmium was found to completely block STD DA release evoked by stimulus trains (33); however, STD DA release evoked by single pulses was found to be unaffected by VGCC antagonists (54). Finally, using in vivo microdialysis in adult rats, it was found that the basal nonstimulated STD DA release is reduced only by T- and R-type VGCC blockers (55, 56). However, P/Q-type channels were reported to be critical for STD DA release evoked by KCl depolarization in the same preparation (38). Here we show that under conditions of nonstimulated spontaneous firing activity, N- and P/Q-type channels support a substantial component of STD DA release in cultured mouse DA neurons. Our findings are in agreement with other studies conducted in mouse tissue (6, 16). Species differences could perhaps explain some of the inconsistencies observed across different models. In addition, it is possible that different stimulation protocols such as single pulses, spontaneous firing, action potential trains, or KCl depolarization differentially recruit various Ca2+ channel subtypes. However, the involvement of N- or P/Q-type channels observed here fits well with earlier findings that these channel subtypes selectively associate with SNARE proteins to support neurotransmitter release at synapses in various brain regions (57–59). Despite their known abundance in the cell body of DA neurons, the inability of L-type channels to associate with SNARE proteins could explain their inability to support STD DA release.
Sources of Calcium for STD DA Release
Our findings that only [Ca2+]i elevations occurring via Ca2+ influx through N- and P/Q-type channels trigger STD DA release raise the hypothesis that Ca2+ elevation occurring in close proximity to SNARE proteins and Syt7 are required to trigger STD DA release. Although our findings suggest that global elevations in [Ca2+]i through the opening of transient receptor potential-like channels or through mobilization of intracellular stores are unable to trigger exocytosis in this cellular compartment, it is possible that the amplitude and duration of Ca2+ elevation in the vicinity of the STD DA release sites is more important than the source of Ca2+ influx: perhaps large, local elevations near release sites are efficient, whereas elevations with a slower rise time are relatively inefficient. Nonetheless, we hypothesize that mobilization of [Ca2+]i stores might act as a positive regulator of STD DA release as recently demonstrated (33). Such global [Ca2+]i elevations could, for example, lead to enhanced priming of vesicles, as previously demonstrated in hypothalamic neurosecretory cells (60).
Using mouse brain slices and electrical stimulation trains (five stimuli at 40 Hz), it has been shown recently that both axonal and STD DA release appear to display rather similar dependences on extracellular Ca2+ (16). Nonetheless, a statistically higher proportion of STD DA release remained in 0.5 mm Ca2+ in comparison with release from axon terminals. The fact that in this study, the Ca2+ dependence between the two compartments was not as striking as in the previous studies of Rice and co-workers (12, 13) is surprising, and additional experiments will be required to examine this further and explore the contributions of Syt isoforms in this preparation. Our present findings, together with the previous demonstration of a requirement of SNAP25 and synaptobrevin for STD DA release, clearly show that STD DA release is mediated through a form of regulated exocytosis similar to that occurring in axon terminals. However, the requirement for Syt4 and 7, but not Syt1, distinguishes the release mechanism occurring in these two compartments. It also provides a possible molecular explanation for the differential Ca2+ sensitivity of these two forms of DA release. Because STD DA release is important for regulation of the excitability and function of SN/VTA DA neurons (6, 16), as well as for the development of amphetamine (2, 3) and alcohol (61) addiction, the determination of its mechanisms may lead to novel insights in the basic function and pathophysiological perturbation of this neurotransmitter system.
Supplementary Material
Acknowledgment
We thank Dr. Mitsunori Fukuda for the kind gift of the Syt4 antibody.
This work was funded by grants from the Canadian Institutes of Health Research, National Alliance for Research on Schizophrenia and Depression, and Neuroscience Canada (to L.-E. T.) and by a Fonds de la Recherche en Santé du Québec infrastructure grant to the Groupe de Recherche sur le Système Nerveux Central.

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Figs. S1 and S2.
- DA
- dopamine
- SN
- substantia nigra
- STD
- somatodendritic
- Syt
- synaptotagmin
- VGCC
- voltage-gated Ca2+ channel(s)
- VTA
- ventral tegmental area
- TH
- tyrosine hydroxylase
- Pn
- postnatal day n
- KRB
- Krebs-Ringer buffer
- SNAP
- soluble NSF attachment protein
- TTX
- tetrodotoxin.
REFERENCES
- 1. Björklund A., Lindvall O. (1975) Brain Res. 83, 531–537 [DOI] [PubMed] [Google Scholar]
- 2. Vezina P. (1996) J. Neurosci. 16, 2411–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjijou Y., Stinus L., Le Moal M., Cador M. (1996) J. Pharmacol. Exp. Ther. 277, 1177–1187 [PubMed] [Google Scholar]
- 4. Andersson D. R., Nissbrandt H., Bergquist F. (2006) Eur. J. Neurosci. 24, 617–624 [DOI] [PubMed] [Google Scholar]
- 5. Kim Y., Park M. K., Chung S. (2009) Neurosci. Lett. 465, 31–35 [DOI] [PubMed] [Google Scholar]
- 6. Beckstead M. J., Grandy D. K., Wickman K., Williams J. T. (2004) Neuron 42, 939–946 [DOI] [PubMed] [Google Scholar]
- 7. Vandecasteele M., Glowinski J., Deniau J. M., Venance L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4904–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F. W., Jin Y., Matta S. G., Xu M., Zhou F. M. (2009) J. Neurosci. 29, 10424–10435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falkenburger B. H., Barstow K. L., Mintz I. M. (2001) Science 293, 2465–2470 [DOI] [PubMed] [Google Scholar]
- 10. Fortin G. D., Desrosiers C. C., Yamaguchi N., Trudeau L. E. (2006) J. Neurochem. 96, 1740–1749 [DOI] [PubMed] [Google Scholar]
- 11. Heeringa M. J., Abercrombie E. D. (1995) J. Neurochem. 65, 192–200 [DOI] [PubMed] [Google Scholar]
- 12. Rice M. E., Richards C. D., Nedergaard S., Hounsgaard J., Nicholson C., Greenfield S. A. (1994) Exp. Brain Res. 100, 395–406 [DOI] [PubMed] [Google Scholar]
- 13. Chen B. T., Rice M. E. (2001) J. Neurosci. 21, 7841–7847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrews N. W., Chakrabarti S. (2005) Trends Cell Biol. 15, 626–631 [DOI] [PubMed] [Google Scholar]
- 15. Xu J., Mashimo T., Südhof T. C. (2007) Neuron 54, 567–581 [DOI] [PubMed] [Google Scholar]
- 16. Ford C. P., Gantz S. C., Phillips P. E., Williams J. T. (2010) J. Neurosci. 30, 6975–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawamoto K., Nakao N., Kobayashi K., Matsushita N., Takahashi H., Kakishita K., Yamamoto A., Yoshizaki T., Terashima T., Murakami F., Itakura T., Okano H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6423–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendez J. A., Bourque M. J., Dal Bo G., Bourdeau M. L., Danik M., Williams S., Lacaille J. C., Trudeau L. E. (2008) J. Neurosci. 28, 6309–6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bourque M. J., Trudeau L. E. (2000) Eur. J. Neurosci. 12, 3172–3180 [DOI] [PubMed] [Google Scholar]
- 20. Fasano C., Thibault D., Trudeau L. E. (2008) Curr. Protoc. Neurosci. 44, 3.21.1–3.21.19 [DOI] [PubMed] [Google Scholar]
- 21. Lindgren N., Xu Z. Q., Herrera-Marschitz M., Haycock J., Hökfelt T., Fisone G. (2001) Eur. J. Neurosci. 13, 773–780 [DOI] [PubMed] [Google Scholar]
- 22. St-Gelais F., Legault M., Bourque M. J., Rompré P. P., Trudeau L. E. (2004) J. Neurosci. 24, 2566–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukuda M. (2004) Biochem. J. 380, 875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Q., Fukuda M., Van Bockstaele E., Pascual O., Haydon P. G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9441–9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fukuda M., Kanno E., Satoh M., Saegusa C., Yamamoto A. (2004) J. Biol. Chem. 279, 52677–52684 [DOI] [PubMed] [Google Scholar]
- 26. Glavan G., Zivin M. (2005) Neuroscience 135, 545–554 [DOI] [PubMed] [Google Scholar]
- 27. Huynh D. P., Scoles D. R., Nguyen D., Pulst S. M. (2003) Hum. Mol. Genet. 12, 2587–2597 [DOI] [PubMed] [Google Scholar]
- 28. Thuret S., Bhatt L., O'Leary D. D., Simon H. H. (2004) Mol. Cell Neurosci. 25, 394–405 [DOI] [PubMed] [Google Scholar]
- 29. Cragg S. J., Greenfield S. A. (1997) J. Neurosci. 17, 5738–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim Y., Park M. K., Chung S. (2008) Biochem. Biophys. Res. Commun. 373, 665–669 [DOI] [PubMed] [Google Scholar]
- 31. Rhee J. S., Li L. Y., Shin O. H., Rah J. C., Rizo J., Südhof T. C., Rosenmund C. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18664–18669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaffe E. H., Marty A., Schulte A., Chow R. H. (1998) J. Neurosci. 18, 3548–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel J. C., Witkovsky P., Avshalumov M. V., Rice M. E. (2009) J. Neurosci. 29, 6568–6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cardozo D. L., Bean B. P. (1995) J. Neurophysiol. 74, 1137–1148 [DOI] [PubMed] [Google Scholar]
- 35. Durante P., Cardenas C. G., Whittaker J. A., Kitai S. T., Scroggs R. S. (2004) J. Neurophysiol. 91, 1450–1454 [DOI] [PubMed] [Google Scholar]
- 36. Adell A., Artigas F. (2004) Neurosci. Biobehav. Rev. 28, 415–431 [DOI] [PubMed] [Google Scholar]
- 37. Bustos G., Abarca J., Campusano J., Bustos V., Noriega V., Aliaga E. (2004) Brain Res. Brain Res. Rev. 47, 126–144 [DOI] [PubMed] [Google Scholar]
- 38. Elverfors A., Jonason J., Jonason G., Nissbrandt H. (1997) Synapse 26, 359–369 [DOI] [PubMed] [Google Scholar]
- 39. Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Südhof T. C. (1994) Cell 79, 717–727 [DOI] [PubMed] [Google Scholar]
- 40. Martinez I., Chakrabarti S., Hellevik T., Morehead J., Fowler K., Andrews N. W. (2000) J. Cell Biol. 148, 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chakrabarti S., Kobayashi K. S., Flavell R. A., Marks C. B., Miyake K., Liston D. R., Fowler K. T., Gorelick F. S., Andrews N. W. (2003) J. Cell Biol. 162, 543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gustavsson N., Wei S. H., Hoang D. N., Lao Y., Zhang Q., Radda G. K., Rorsman P., Südhof T. C., Han W. (2009) J. Physiol. 587, 1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu Z. T., Chen M. R., Ping Z., Dong Y. M., Zhang R. Y., Xu T., Wu Z. X. (2008) Biochem. Biophys. Res. Commun. 371, 781–786 [DOI] [PubMed] [Google Scholar]
- 44. Johnson S. L., Franz C., Kuhn S., Furness D. N., Rüttiger L., Münkner S., Rivolta M. N., Seward E. P., Herschman H. R., Engel J., Knipper M., Marcotti W. (2010) Nat. Neurosci. 13, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arthur C. P., Dean C., Pagratis M., Chapman E. R., Stowell M. H. (2010) Neuroscience 167, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barber C. F., Jorquera R. A., Melom J. E., Littleton J. T. (2009) J. Cell Biol. 187, 295–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li C., Ullrich B., Zhang J. Z., Anderson R. G., Brose N., Südhof T. C. (1995) Nature 375, 594–599 [DOI] [PubMed] [Google Scholar]
- 48. Sugita S., Han W., Butz S., Liu X., Fernández-Chacón R., Lao Y., Südhof T. C. (2001) Neuron 30, 459–473 [DOI] [PubMed] [Google Scholar]
- 49. Fernández-Chacón R., Königstorfer A., Gerber S. H., García J., Matos M. F., Stevens C. F., Brose N., Rizo J., Rosenmund C., Südhof T. C. (2001) Nature 410, 41–49 [DOI] [PubMed] [Google Scholar]
- 50. Bhalla A., Chicka M. C., Chapman E. R. (2008) J. Biol. Chem. 283, 21799–21807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dean C., Liu H., Dunning F. M., Chang P. Y., Jackson M. B., Chapman E. R. (2009) Nat. Neurosci. 12, 767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ting J. T., Kelley B. G., Sullivan J. M. (2006) J. Neurosci. 26, 372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Young S. M., Jr., Neher E. (2009) Neuron 63, 482–496 [DOI] [PubMed] [Google Scholar]
- 54. Chen B. T., Moran K. A., Avshalumov M. V., Rice M. E. (2006) J. Neurochem. 96, 645–655 [DOI] [PubMed] [Google Scholar]
- 55. Bergquist F., Jonason J., Pileblad E., Nissbrandt H. (1998) J. Neurochem. 70, 1532–1540 [DOI] [PubMed] [Google Scholar]
- 56. Bergquist F., Nissbrandt H. (2003) Neuroscience 120, 757–764 [DOI] [PubMed] [Google Scholar]
- 57. Sheng Z. H., Rettig J., Takahashi M., Catterall W. A. (1994) Neuron 13, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 58. Sutton K. G., McRory J. E., Guthrie H., Murphy T. H., Snutch T. P. (1999) Nature 401, 800–804 [DOI] [PubMed] [Google Scholar]
- 59. Wadel K., Neher E., Sakaba T. (2007) Neuron 53, 563–575 [DOI] [PubMed] [Google Scholar]
- 60. Tobin V. A., Hurst G., Norrie L., Dal Rio F. P., Bull P. M., Ludwig M. (2004) Eur. J. Neurosci. 19, 2909–2912 [DOI] [PubMed] [Google Scholar]
- 61. Deng C., Li K. Y., Zhou C., Ye J. H. (2009) Neuropsychopharmacology 34, 1233–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.