Abstract
We have analyzed the role of the thyroid hormone receptors (TRs) in epidermal homeostasis. Reduced keratinocyte proliferation is found in interfollicular epidermis of mice lacking the thyroid hormone binding isoforms TRα1 and TRβ (KO mice). Similar results were obtained in hypothyroid animals, showing the important role of the liganded TRs in epidermal proliferation. In addition, KO and hypothyroid animals display decreased hyperplasia in response to 12-O-tetradecanolyphorbol-13-acetate. Both receptor isoforms play overlapping functional roles in the skin because mice lacking individually TRα1 or TRβ also present a proliferative defect but not as marked as that found in double KO mice. Defective proliferation in KO mice is associated with reduction of cyclin D1 expression and up-regulation of the cyclin-dependent kinase inhibitors p19 and p27. Paradoxically, ERK and AKT activity and expression of downstream targets, such as AP-1 components, are increased in KO mice. Increased p65/NF-κB and STAT3 phosphorylation and, as a consequence, augmented expression of chemokines and proinflammatory cytokines is also found in these animals. These results show that thyroid hormones and their receptors are important mediators of skin proliferation and demonstrate that TRs act as endogenous inhibitors of skin inflammation, most likely due to interference with AP-1, NF-κB, and STAT3 activation.
Keywords: Hormone Receptors, Signal Transduction, Skin, Thyroid, Transcription Factors
Introduction
The actions of thyroid hormones are mediated by binding to nuclear receptors, TRα4 and TRβ, that act as ligand-dependent transcription factors by association, generally as heterodimers with retinoid X receptors, with thyroid hormone response elements located in regulatory regions of target genes (1). TRs can also modulate expression of genes that do not contain a hormone response element by modulating the activity of other transcription factors and signaling pathways (2), including activator protein-1 (AP-1)-, cyclic AMP-response element-, and nuclear factor-κB (NF-κB)-dependent pathways (3–7).
Studies with knock-out (KO) mice for TRs obtained by homologous recombination have indicated that KO mice for α1 and β TR isoforms have different phenotypes (8–10) and that the double KO mice, surprisingly, survive (11), indicating that these receptors are not required for viability. Although these animals show extreme resistance to thyroid hormones, they exhibit a milder phenotype than hypothyroid mice, indicating divergent consequences for hormone versus receptor deficiency for some actions.
Although the skin is a target tissue for thyroid hormone action, no studies on the skin phenotype in TR KO mice have been reported. TRα and TRβ mRNAs are present in the skin (12–15), and these receptors can regulate either positively or negatively the expression of selected keratins in cultured cells (16–19). Clinical evidence (19–27) as well as studies in hypothyroid mice and rats (28–30) also suggest that thyroid hormones could be involved in epidermal proliferation and differentiation, hair growth, and wound healing besides affecting the function of dermal fibroblasts. A question emerging from these studies is how to distinguish between effects due to altered thyroid hormone levels and effects due to expression of specific TR isoforms.
The TR KO mice represent an excellent model for the analysis of the role of these receptors in the skin and its response to hyperproliferative stimuli. Topical application of 12-O-tetradecanolyphorbol-13-acetate (TPA) to mouse skin is a well known model for induction of skin hyperproliferation and also promotes a strong inflammatory reaction that activates multiple immunostimulatory pathways. The skin response to TPA is associated with activation of several intracellular pathways (e.g. mitogen-activated protein kinases (MAPKs), AKT, NF-κB, STAT3, and AP-1) as well as an increase in the content of chemical mediators, such as cytokines, chemokines, vasoactive peptides, prostaglandins, leukotrienes, and nitric oxide among others (31–34).
In this work, we have investigated skin proliferation and inflammation, before and after TPA application, in mice lacking TRα1, TRβ, or both genes, comparing these responses with those of hypothyroid animals to distinguish the specific contributions of receptor expression and activation. We found that TRs and thyroid hormones are required for skin homeostasis after TPA treatment and that both receptor genes contribute to attain a normal proliferative response to the tumor promoter. Reduced proliferation in animals lacking TRs correlates with increased expression of cyclin-dependent kinase inhibitors in the interfollicular epidermis and with strongly reduced cyclin D1 expression in the keratinocytes of the basal layer. In addition, skins from TR KO mice show signs of inflammation with increased production of several proinflammatory cytokines, which is associated with enhanced phosphorylation of p65/NF-κB and STAT3 transcription factors. These results show a novel role for TRs in the skin response to proliferative signals and demonstrate that these receptors act as endogenous inhibitors of cutaneous inflammation.
EXPERIMENTAL PROCEDURES
Animals and Treatments
All animal work was done in compliance with European Community law (86/609/EEC) and Spanish law (R.D. 1201/2005), with approval of the Ethics Committee of the Consejo Superior de Investigaciones Científicas. Experiments were performed in adult female mice. TRα1−/−/TRβ−/− double KO mice in a hybrid genetic background of 129/OLa+129/Sv+ BALB/c+C57BL/6, single TRα1−/−/TRβ+/+ (KOα), and TRα1+/+/TRβ−/− (KOβ) mice, and wild-type TRα1+/+/TRβ+/+ animals with the same background were genotyped (11) and used for the studies. Wild-type mice were made hypothyroid (hT) by treatment with 0.02% methymazole and 0.1% sodium perchlorate in the drinking water (35). Treatment started when animals were 1 month old and was continued for 4 months. Dorsal skins were shaved and exhaustively depilated with hair-removing cream 24 h before the treatment with TPA (obtained from Sigma). TPA (12 μg) was applied topically twice (at days 1 and 3), and mice were sacrificed at day 4 or 7. In the control group, animals were treated with vehicle (acetone) only. At the end of the experiments, skin was excised and either frozen to obtain RNA and protein or fixed for immunohistochemical analysis. Between 3 and 6 animals/experimental group were examined, and most experiments were repeated at least three times.
Histological and Immunohistochemical Analysis
Skin samples were fixed with paraformaldehyde or ethanol and embedded in paraffin. Skin sections (4 μm) were stained with H&E or processed for immunohistochemistry. Immunohistochemistry was performed using standard protocols on deparaffinized sections as described previously (36). The slides were microwaved with citrate buffer after deparaffinization to enhance the staining. Sections were preincubated for 30 min with 5% horse serum in PBS and incubated with primary antibodies (dilution 1:100) at 4 °C overnight and with a secondary antibody for 2 h at room temperature. They were resolved with avidin-biotin peroxidase complex using Vectastain ABC kit (Vector Laboratories) and were revealed with diaminobenzidine (DAB kit, Vector Laboratories) and counterstained with hematoxylin. Slides were mounted and analyzed by light microscopy (Leica DM RXA2), and microphotographs were taken. Frozen sections of skin were used to detect by immunofluorescence K5, K10, and loricrin as well as dermal inflammatory cells. T lymphocytes were detected by expression of CD3ϵ, T and B lymphocytes by expression of CD45, and macrophages by expression of CD11b/Mac1.
BrdU Labeling
Epithelial cell proliferation was measured by intraperitoneal injection of BrdU (0.1 mg/g weight in 0.9% NaCl) 1 h prior to sacrifice. BrdU incorporation was detected by immunohistochemistry of paraffin-embedded sections using a mouse anti-BrdU monoclonal antibody (Roche Applied Science, catalog no. 1170376). The number of BrdU-positive and total basal keratinocytes cells was counted in at least five areas from five animals of each experimental group. The mean ± S.D. percentage of proliferating over total basal cells (>200) was then estimated as reported previously (36).
Morphometric Analysis
Quantification of the epidermal thickness was performed in dorsal skin sections stained with H&E. At least 10 individual fields of 1 mm per slide were counted using the software MetaMorph (Premier Offline 7.0, Molecular Devices, Downingtown, PA). Experiments were performed in at least five mice of each group.
Immunoblotting
For preparation of whole-cell extracts, skins were ground with a mortar on liquid nitrogen and homogenized in lysis buffer composed of 200 mm Hepes, pH 7.9, 25% glycerol, 400 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1.25 mm DTT, 100 mm sodium pyrophosphate, 200 mm sodium orthovanadate, 500 mm NaF, 0.1 mm PMSF, 10 mg/ml aprotinin, 10 mg/ml leupeptin, and 1% Nonidet P-40. Nuclear extracts were isolated with the Nuclear Extraction Reagents kit, according to the manufacturer's protocol. The amount of proteins was measured by the Bradford method. Fifty μg of proteins were boiled in Laemmli buffer, separated on NuPAGE 4–12% BisTris gel (Invitrogen), and then transferred to nitrocellulose membranes (Millipore). Membranes were blocked with 4% BSA in TBS plus 0.5% Tween 20 and incubated with the indicated antibodies at 4 °C overnight. Dephosphorylation reactions were inhibited by the presence of 50 mm NaF in blocking reagent and antibody diluents. After washing, membranes were incubated with a peroxidase-conjugated secondary antibody for 2 h at room temperature and analyzed using the enhanced chemiluminescence method (Immun-Star HRP, Bio-Rad), according to the manufacturer's recommendations.
Antibodies
The antibodies used included antibodies to P-ERK (sc-7383), ERK2 (sc-154), AKT1,2 (sc-1619), c-Jun (sc-1694), c-Fos (sc-52), p27 (sc-528), p50 (sc-114), IkBα (sc-371), lamin B (sc-6217), and actin (sc-1616) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against P-p65 (3033), IκB kinase α (2682), STAT3 (4904), P-STAT3Tyr705 (9145s), and P-STAT3Ser727 (9134) were obtained from Cell Signaling Technology Inc. (Beverly, MA), and antibodies against p19 (Ab-7960) and p21 (Ab-80) were purchased from Abcam Inc. (Cambridge, UK). Antibodies used for K5 and loricrin detection by immunofluorescence were from Covance (Princeton, NJ) and were used at 1:500 or 1:1000 dilutions. Keratin K10 was detected using a monoclonal antibody (Sigma) at a 1:1000 dilution. Cleaved caspase 3 (Asp175) was detected with an antibody from Cell Signaling at a 1:250 dilution. Immunodetection of CD3ϵ, CD45, and CD11b/Mac1 was performed using fluorescein isothiocyanate-labeled specific rat anti-mouse monoclonal antibodies (BD Pharmigen) at 1:50 dilution. Secondary peroxidase-conjugated anti-rabbit antibody was from Amersham Biosciences, and secondary peroxidase-conjugated anti-mouse antibody and biotin-conjugated anti-rabbit or anti-mouse antibodies were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Cytokine and Chemokine Quantification
R&D Systems Mouse Cytokine Array, Panel A (catalogue no. ARY006) was used to simultaneously detect the levels of 40 different cytokines and chemokines in 1 mg of whole-cell protein extracts obtained from skins from 3 animals/group and following the manufacturer's specifications. Samples were analyzed in duplicate, and signals were detected by chemiluminescence and were quantitated by scanning the x-ray films after different exposures (between 1 and 10 min) with Quantity One-4.6.6 (Basic mode) one-dimensional analysis software Bio-Rad 200.
Electrophoretic Mobility Shift Assays (EMSAs)
EMSAs were performed by incubating total or nuclear cell extracts from mouse skin with a labeled double-stranded oligonucleotide corresponding to consensus AP-1 (5′-CGCTTGATGACTCAGCCGGAA-3′) or NF-κB (5′-GATCCAACGGCAGGGGAATTCCCCTCTCCTTA-3′) sites. Complexes were separated on 5.5% native polyacrylamide gels in 0.25× Tris borate-EDTA buffer, dried, and exposed to Hyperfilm-MP (Amersham Biosciences) at −70 °C.
Primary Cultures
Primary keratinocytes were derived from newborn double KO mice and the corresponding wild-type controls. Tails from each pup were used for genotyping. Skin was removed, and epidermis was obtained by trypsinization. Cells were plated in Eagle's minimal essential medium (BioWhittaker, Inc.) supplemented with 4% Chelex-treated fetal bovine serum and 0.2 mm Cl2Ca. After 15 h, cells were transferred to medium containing 0.05 mm Cl2Ca. To analyze proliferation, cells were incubated for 1 h with 10 mm BrdU.
Quantitative Real-time PCR Assays
Total RNA was extracted from keratinocytes using Tri Reagent (Sigma). mRNA levels were analyzed in triplicate samples by quantitative RT-quantitative PCR. RT was performed with 2 μg of RNA following the specifications of the SuperScriptTM first strand synthesis system (Invitrogen). The primers used were 5′-GAACTGG CAGAAGAGGCACT-3′ (forward) and 5′-AGGGTCTGGGCCATAGAACT-3′ (reverse) for TNFα, 5′-GGAATCACCATGCCCTCTA-3′ (forward) and 5′-TGGCTGTCTTTGTGAGATGC-3′ (reverse) for S100A8, and 5′-TCATCGACACCTTCCATCAA-3′ (forward) and 5′-GTCCTGGTTTGTCCAGGT-3′ (reverse) for S100A9. PCRs were performed using a MX3005P instrument (Stratagene) and detected with SYBR Green. Data analysis was done using the comparative CT method, and data were corrected with the GAPDH mRNA levels obtained with primers 5′-ACACTGCATGCCATCACTGCC-3′ (forward) and 5′-GCCTGCTTCACCACCTTCTTG-3′ (reverse).
Statistical Analysis
Differences with respect to wild-type animals were assessed by using Student's t test, with statistical significance when p was <0.05.
RESULTS
TRs Are Required for Skin Proliferation
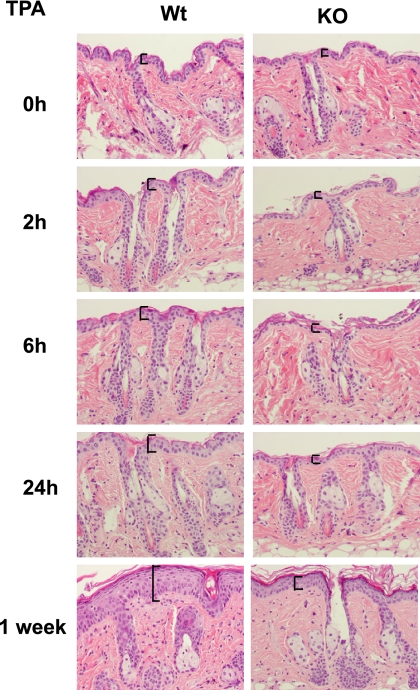
The skin of TRα1−/−/TRβ−/− double KO mice appeared to have a normal morphology (Fig. 1 and supplemental Fig. S1), and no overt differentiation alterations were observed because expression and distribution of keratin 10 and loricrin was normal in TR-deficient mice (supplemental Fig. S2). However, BrdU incorporation analysis in paraffin sections incubated with an anti-BrdU antibody demonstrated a reduced proliferation in KO mouse epidermis (supplemental Fig. S1; see also Fig. 2C). Reduced proliferation was not accompanied by changes in apoptosis, as examined by caspase 3 cleavage, which was undetectable both in WT and TR-deficient mice (supplemental Fig. S3). To further characterize the role of TRs in epidermal function and proliferation, we examined the response of mice to topical application of TPA, a tumor promoter known to induce epidermal hyperplasia. After a topical dose of TPA, the number of cell layers in WT skin increased significantly within the first 24 h, whereas TPA-treated KO skin did not display a detectable hyperplasia. A strongly reduced hyperplasia in mice lacking TRs was also observed after 1 week of treatment (Fig. 1).
FIGURE 1.
Reduced hyperplasia after TPA treatment in mice lacking TRs. Dorsal skins of female wild-type mice (Wt) and double knock-out mice lacking TRα1 and TRβ (KO), were treated topically with vehicle (acetone) or with TPA and sacrificed at the indicated time periods. H&E staining shows that epidermal hyperplasia was greatly reduced in KO skin. Epidermal thickness is indicated with brackets.
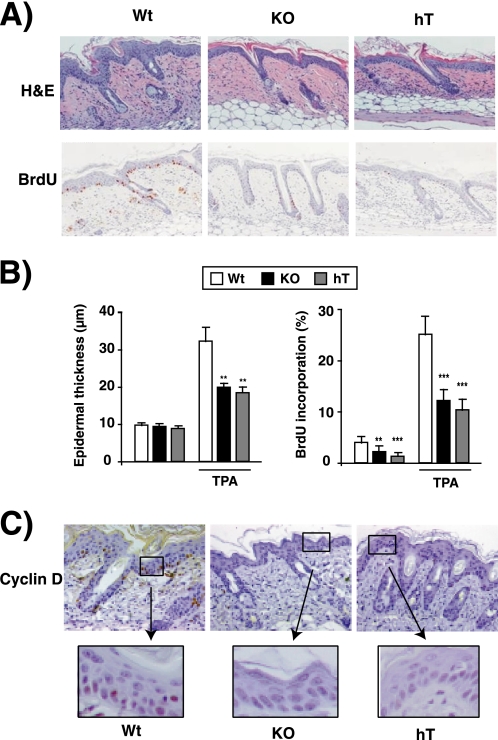
FIGURE 2.
Reduced epidermal proliferation in hypothyroid and TR knock-out mice. A, the upper panels show representative H&E staining of dorsal skin from WT, KO, and hT mice after treatment with vehicle or TPA for 5 days. The animals were injected with BrdU 1 h prior to sacrifice, and the lower panels show BrdU immunostaining. B, morphometric analysis were performed to determine epidermal thickness in WT, KO, and hT mice before and after TPA treatment (left panel). Proliferation rate of the interfollicular epidermal keratinocytes was quantified in the indicated groups and is represented as the percentage of positive BrdU cells versus total cells in the right panel. Data are shown as mean values ± S.E. (error bars), and asterisks denote statistically significant differences relative to WT mice (*, p < 0.05; ***, p < 0.001). C, immunolocalization of cyclin D1 in dorsal skin paraffin sections from WT, KO, and hT mice. Larger magnifications of the indicated areas are also shown.
To study if thyroid hormone loss has an effect similar to that of the absence of TRs on skin proliferation, skin morphology before and after TPA treatment was also analyzed in animals treated with anti-thyroidal drugs for 4 months. No evident differences between the skin of WT and hT mice treated with vehicle alone were found, although BrdU incorporation was also reduced in these animals (supplemental Fig. S1). As shown in Fig. 2A, after two topical doses of TPA, a strong reduction in skin hyperplasia was observed in hT mice. To analyze if the reduced hyperplasia in these animals is a consequence of reduced keratinocyte proliferative activity, BrdU incorporation was examined in TPA-treated animals. As can be seen in Fig. 2A, BrdU incorporation was strongly decreased in hT and KO mice with respect to WT animals. Fig. 2B shows the quantification of skin thickness and interfollicular BrdU incorporation in vehicle and TPA-treated groups. Although vehicle-treated hT and KO animals have a detectable reduction in BrdU incorporation, differences in skin thickness with respect to WT mice were not found. However, after TPA treatment, epidermal thickness was reduced to a similar extent in KO and hT mice with respect to WT. In WT mice, ∼3% of the interfollicular basal cells were labeled, and this index was significantly reduced in hT and KO groups. Furthermore, after two topical doses of TPA, the BrdU labeling index in basal cells reached up to ∼25% in WT mice, whereas it was strongly reduced in animals lacking thyroid hormones or their receptors. Altogether, these findings clearly indicate that the hyperplastic response to TPA was reduced dramatically in hT and KO mice, underscoring the important role of the liganded TRs in the proliferation of the mouse epidermis.
To investigate the possible molecular mechanisms responsible for the phenotypic alterations observed in the skin of TR KO mice, we examined by immunohistochemistry the expression of cyclin D1, a protein that plays a key role in skin proliferation (37). Cyclin D1 expression was very low in vehicle-treated skins (not shown), but it was increased after TPA treatment in basal cells of WT animals (Fig. 2C), in agreement with the proliferation index found under these conditions. In addition, the reduced proliferation observed in hT and KO mice was accompanied by a dramatic reduction in cyclin D1 expression in basal keratinocytes.
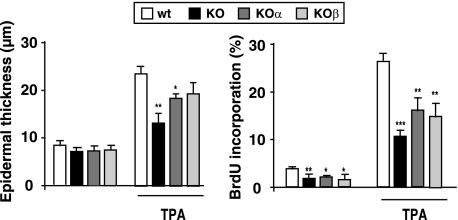
Both TRα1 and TRβ Contribute to Skin Proliferative Responses
To analyze the role of specific TR isoforms in skin proliferation, we compared the effect of TPA treatment in double KO mice with that obtained in single KOα and KOβ animals. The results illustrated in Fig. 3 demonstrate that both receptor isoforms contribute to skin homeostasis because in the single KO mice hyperplasia and interfollicular BrdU incorporation after treatment with the tumor promoter were between those found in WT and double KO mice.
FIGURE 3.
TRα1 and TRβ contribute to skin proliferative responses. Epidermal thickness (left) and BrdU incorporation (right) were determined in WT mice, double knock-out mice (KO), and single knock-out mice lacking either TRα1 (KOα) or TRβ (KOα) 4 days after the start of treatment with vehicle or TPA. Data are shown as mean values ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus WT animals.
Expression of Signaling Molecules in Skin of TR KO Mice
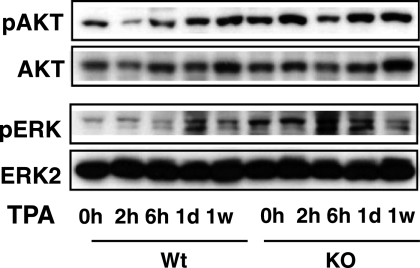
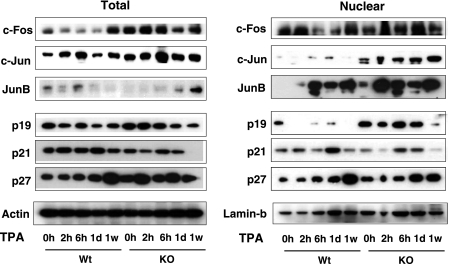
Because both AKT and the mitogen-activated protein kinase (MAPK) ERK, which are activated following topical treatment with tumor promoters, are important signaling pathways that stimulate cyclin D1 expression in skin (38, 39), we analyzed by Western blot the kinetics of phosphorylation of these kinases in pooled samples of skins from WT and KO mice in response to TPA. Surprisingly, phospho-ERK and phospho-AKT levels were not reduced, but were instead moderately higher, in protein lysates from KO mice (Fig. 4). The signaling cascades activated by these kinases control the expression of cell cycle molecules. Therefore, we also analyzed total and nuclear levels of AP-1 components and several cyclin kinase inhibitors (CKIs) that are crucial regulatory proteins for cell proliferation (Fig. 5). AP-1 is a dimeric transcription factor complex consisting of homo- and heterodimers of the Jun and Fos protein families (40). Expression of c-Jun is regarded as a positive regulator of keratinocyte proliferation in skin, whereas JunB can antagonize proliferation of keratinocytes (41–43). Despite the reduced proliferative response, c-Jun and c-Fos levels were significantly elevated in the skin of the KO mice. Interestingly, this increase was detected mainly in the nuclear compartment in the case of c-Jun, whereas total c-Fos levels were preferentially elevated, showing that lack of TRs can affect differentially expression and subcellular localization of these proteins. On the other hand, basal JunB levels were higher in the nuclei from KO mice, and an earlier and stronger induction by TPA, which could at least partially counteract the functional effects of increased c-Jun levels, was also found. When AP-1 complexes were analyzed in EMSAs, it could be observed that in WT mice, the abundance of proteins that bind a consensus AP-1 site is transiently increased by TPA, reverting at 1 week of treatment, whereas in mice lacking TRs, they were high in the absence of the tumor promoter and remained high even after 1 week, in agreement with the elevated levels of Jun proteins found in these animals (supplemental Fig. S4).
FIGURE 4.
TR elimination enhances in vivo ERK and AKT phosphorylation in the skin. Shown is immunoblotting using whole-cell extracts from dorsal skin of WT and TRα1 and TRβ knock-out mice (KO) that were treated with TPA for 0 h, 2 h, 6 h, 1 day, and 1 week to check the expression of total and phosphorylated AKT (pAKT) and ERK (pERK). Each band represents a pool from three individual animals.
FIGURE 5.
TR inactivation leads to increased skin expression of AP-1 proteins and cyclin kinase inhibitors. Total and nuclear skin cell lysates from WT and KO mice treated topically with TPA for the indicated time periods, ranging between 0 h and 1 week, were prepared for Western blotting with the indicated antibodies. Actin and lamin B were used as a loading control for total and nuclear extracts, respectively.
When skin expression of CKIs was examined, it was observed that whereas the level of p21 was not significantly different between WT and KO mice, the total levels of p27 were higher in the skin of the mice lacking TRs, and a very strong increase of p19 was found in the nuclei of these animals, suggesting that enhanced expression of cell cycle inhibitors could also contribute to the reduced proliferative response observed in the absence of TRs (Fig. 5 and supplemental Fig. S5).
Increased Production of Proinflammatory Cytokines and Chemokines in the Skin of TR KO Mice
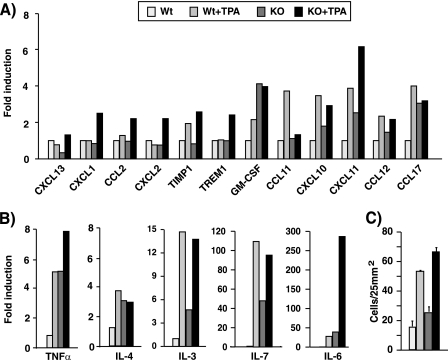
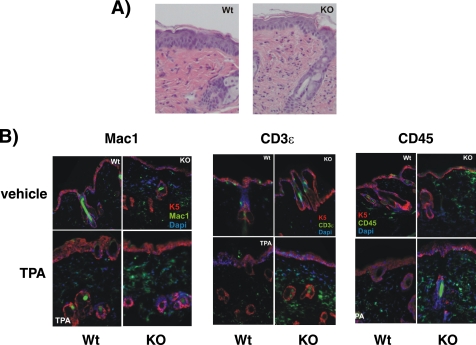
Topical application of TPA on the mouse skin not only induces hyperplasia, but it is also a well known model for induction of cutaneous inflammation (32, 33). We thus sought to analyze the skin inflammatory response in TR KO mice. With this purpose, we measured the levels of chemokines and proinflammatory cytokines, which play pivotal roles in the recruitment of inflammatory cells, in the skin of vehicle- and TPA-treated WT and KO mice using cytokine arrays. Many of the assayed proteins presented changes between both groups (Fig. 6). Some of them (CXCL1, CXCL2, CCL2, or TREM1) increased after TPA treatment in KO but not in WT animals, and others, such as GM-CSF, CXCL10, CXCL11, CCL12, and CCL17, that increased in response to TPA in WT mice were already elevated in vehicle-treated KO mice. Only one protein, CCL11, responded to TPA in the WT but not in KO mice (Fig. 6A). The more relevant changes were found with the interleukins IL-3, IL-4, IL-6, and IL-7 and tumor necrosis factor α (TNFα). The levels of these cytokines, which were strongly stimulated by the tumor promoter in the WT animals, were also significantly higher under basal conditions in vehicle-treated KO skin when compared with the normal skin (Fig. 6B). These results suggest that the mice lacking TRs present biochemical signs of skin inflammation, and in agreement with this idea, the number of dermal cells was increased in the KO mice (Figs. 6C and 7A). Increased infiltration of inflammatory cells in TR-deficient animals was demonstrated by immunofluorescence analysis of skins performed with antibodies recognizing Mac1, CD3ϵ, and CD45 (Fig. 7B). Therefore, these receptors appear to play a physiological role as endogenous inhibitors of cutaneous inflammation.
FIGURE 6.
TRs regulate proinflammatory cytokines in vivo. A and B, relative levels of cytokines and chemokines that showed a different expression between WT and KO mice using a mouse cytokine array. Whole cell extracts from skins of animals treated with vehicle or with TPA for 1 week were used in the assay. Values representing expression levels of vehicle-treated WT mice were set to 1, and -fold induction is represented relative to this basal level. Note that axis labeling is different for different proteins. C, dermal cellularity in skin of the same groups of animals. Data are mean ± S.D. (error bars).
FIGURE 7.
Increased immune cell infiltration in the skin of TR-deficient mice. A, representative H&E staining of TPA-treated skins from WT and TR KO mice, showing increased dermal cellularity. B, double immunofluorescence images of keratin 5 (K5; red immunofluorescence) with the immune cell markers Mac-1, CD3ϵ, and CD45 (green immunofluorescence) in skins from wild-type and TR KO mice treated with vehicle (acetone) or TPA. Nuclei were stained with DAPI (blue). Mac-1 labels macrophages, CD3ϵ labels T lymphocytes, and CD45 is a common lymphocyte antigen.
Increased Phosphorylation of p65/NF-κB and STAT3 in Skin of Mice Lacking TRs
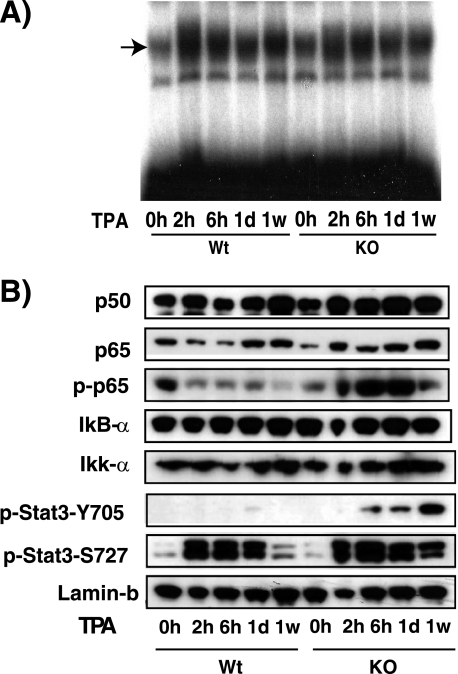
Given that quite a few of the analyzed cytokines are controlled by the transcription factor NF-κB or STAT3 or both (44), our results suggest that TR deletion could increase the expression and/or activity of these factors. NF-κB transcription factors are bound in the cytoplasm to inhibitory proteins called IκBs, and their activation is commonly mediated by the IκB kinase complex. IκB kinase-mediated IκB phosphorylation leads to their ubiquitin-dependent degradation and the subsequent nuclear entry of the released NF-κB dimers (45). Once in the nucleus, transcriptional activity of NF-κB dimers can be further stimulated by protein phosphorylation (46, 47). To analyze NF-κB status, we first prepared nuclear skin extracts from control and KO mice and assayed their NF-κB-binding complexes by EMSA. In mouse skin, NF-κB complexes consist mainly of p50 homodimers and contain also p50/p65 heterodimers as identified by supershift assays (48) (supplemental Fig. S6). Basal NF-κB binding was detected in the skin of both WT and KO animals, and this binding increased similarly after topical application of TPA in both groups (Fig. 8A). These results suggest that expression of NF-κB proteins was not altered by TR deletion, and in agreement with this idea, the nuclear levels of p50, p65, IκB-α, and IκB kinase α were not changed in the KO mice (Fig. 8B). In contrast, the levels of phosphorylated p65 were significantly higher in these mice, indicating that TRs do not decrease nuclear NF-κB content but rather negatively interfere with p65 activation in the skin.
FIGURE 8.
Increased phosphorylation of p65/NF-κB and STAT3 in skin of mice lacking TRs. A, nuclear extracts from WT and KO mice treated with TPA between 0 h and 1 week were used in an EMSA assay with a labeled oligonucleotide conforming to a consensus NF-κB binding site. B, the same extracts were used to detect the indicated proteins by Western blotting. Lamin B was used as a loading control. p-p65, pStat-Y705, and pStat-S727, phosphorylated p65, STAT Tyr705, and STAT Ser727, respectively.
Activation of STAT3, which belongs to the signal transducer and activator of transcription (STAT) family of signal-responsive transcription factors, is mediated by phosphorylation of a critical tyrosine residue (Tyr705) that induces STAT3 dimerization (49). As illustrated in Fig. 8B, phosphorylation of STAT3 was strongly augmented upon TPA treatment in the skin of KO mice. In addition, STAT3 transcriptional activity and DNA binding can be further enhanced through serine (Ser727) phosphorylation (50), and this phosphorylation was more sustained in skin from the mice lacking TRs. According to these results, the TR anti-inflammatory action in skin could be, at least in part, due to the interference of this receptor with NF-κB and STAT3 activation.
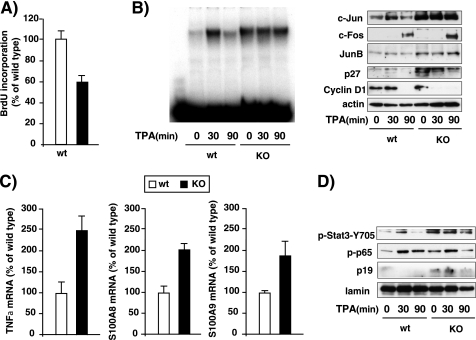
Decreased Proliferation and Increased Inflammatory Mediators in Primary Keratinocytes from TR KO Mice
In order to analyze if the reduced skin proliferation observed in the KO mice is the result of a direct effect of the TRs on the keratinocytes, we analyzed BrdU incorporation in primary cultures of keratinocytes isolated from skin of newborn WT and KO mice. As illustrated in Fig. 9A, the keratinocytes that lack TRs show an intrinsic proliferative defect because BrdU incorporation was reduced by 40% with respect to that found in the cultured keratinocytes from WT mice. In agreement with the results obtained in vivo in the skin, despite presenting reduced proliferation, cultured keratinocytes from TR-deficient mice had higher levels of AP-1 complexes than those of WT mice, measured in gel retardation assays (Fig. 9B). After incubation with TPA, AP-1 complexes increased transiently in WT keratinocytes but not in KO cells. c-Jun levels followed a similar pattern because the basal levels of this protein were also strongly increased in KO keratinocytes, and they did not increase further upon TPA treatment. The levels of JunB and p27 were also constitutively elevated in the KO keratinocytes. c-Fos levels were low in unstimulated cells, but a stronger induction by TPA was also found in cultured keratinocytes from TR-deficient mice. On the other hand, and in contrast with the results obtained in vivo, cyclin D1 was not induced by the tumor promoter in the primary cultures; rather, cyclin D1 levels were reduced, this reduction being observed earlier in the KO keratinocytes (Fig. 9B).
FIGURE 9.
Reduced proliferation and increased immune mediators in cultured keratinocytes from mice lacking TRs. Primary cultures of keratinocytes were prepared from skin of newborn WT and KO mice. A, proliferation, assessed by measuring BrdU incorporation, in WT and KO cultures. B, WT and KO keratinocyte cultures were treated with TPA for 0, 30, and 90 min and whole cell protein extracts were used in EMSA with a nucleotide conforming a consensus AP-1 motif (left) or for detection of c-Jun, c-Fos, JunB, p27, and cyclin D1 in Western blots, using actin as a loading control (right). C, determination by quantitative RT-PCR of the mRNA levels of TNFα, S100A8, and S100A9 in WT and KO keratinocytes. Data are mean ± S.D. (error bars) and are expressed as a percentage of the value obtained in WT cultures. D, nuclear extracts from untreated and TPA-treated WT and KO keratinocyte cultures were used for determination of p19 (p-p19) and phosphorylated STAT-3 (p-Stat3-Y705) and p65 (p-p65) by Western blot. Lamin was used as a loading control.
In addition, there was an increase in the mRNA levels of TNFα in the KO cultures, in line with the higher levels of this cytokine observed in vivo in the skin of these animals (Fig. 9B). Moreover, TNFα activates expression of the proinflammatory S100A8 and S100A9 proteins in primary keratinocytes (51), and the transcripts for both proteins were also higher in the KO keratinocytes (Fig. 9C). S100A8 and S100A9 proteins are potent stimulators of neutrophils and are a hallmark of numerous pathological conditions associated with inflammation (52, 53). The observed increase of inflammatory mediators in the cultured keratinocytes from TR-deficient mice was also accompanied by increased STAT3 and p65 phosphorylation (Fig. 9D), reinforcing the idea that regulation of the activity of these transcription factors cold be involved in the effect of TRs on skin inflammation. Finally, and in agreement with the results obtained in the skin in vivo, the nuclear levels of p19 were significantly up-regulated in primary keratinocytes from TR KO mice (Fig. 9D).
DISCUSSION
In this work, we present evidence that the thyroid hormone receptors play an important role in skin proliferation. Although both skin structure and epidermal thickness appear to be normal in mice lacking TRα1 and TRβ genes, coding for the receptor isoforms able to bind thyroid hormone have been genetically inactivated, a significant reduction in BrdU incorporation in the interfollicular epidermis is observed in these animals under normal conditions. Furthermore, this difference is augmented after treatment with the hyperproliferative agent TPA. TR KO mice show a strongly reduced epidermal proliferation in response to the tumor promoter, indicating that they may be less sensitive to a two-stage skin carcinogenesis protocol. Indeed, we have previously reported that they develop fewer tumors than WT mice when subjected to that protocol, although the tumors formed become more aggressive after chronic TPA treatment (13). Because the proliferative defects observed in the skin of mice lacking TRs could be secondary to systemic effects or to changes in dermal fibroblasts rather than being mediated by direct effects on the keratinocytes, we also carried out studies with primary cultures of keratinocytes obtained from control and TR KO mice. Under these conditions, which mimic skin hyperproliferative states, proliferation was also reduced, indicating that keratinocytes are a direct target of these receptors.
Analysis of TR double KO mice has revealed that they do not exhibit a phenotype identical to that of hypothyroid mice, a difference generally attributed to the fact that these receptors in the absence of ligand can act as constitutive repressors because they bind corepressors that can specifically silence gene expression (54). However, our results show that skin proliferation was similarly reduced in KO and hypothyroid mice, indicating that receptor loss is equivalent to ligand loss in this tissue and that therefore thyroid hormone binding to TRs rather than the effect of the corepressor-bound receptors mediates their actions on skin proliferation. These results are in agreement with the previous observation that topical application of the thyroid hormone triiodothyronine stimulates epidermal proliferation in rodents (30). On the other hand, distinct phenotypes are found in mice in which TRα1 or TRβ (9, 10) are individually deleted, and when both genes are inactivated, an array of phenotypes, not found in the single receptor-deficient mice, are observed (11), indicating that TRα1 and TRβ can substitute for each other to mediate some actions of the thyroid hormones, but they can also mediate isoform-specific functions (54). Our results show that TR isoforms can play overlapping functional roles in the skin because single KO mice also present a proliferative defect but not as marked as that found in double KO mice.
The reduced epidermal proliferation observed in mice lacking TRs is accompanied by a strong reduction in cyclin D1 expression. Because this protein plays a key role in epidermal proliferation (37, 55), this decrease could be an important component of the observed phenotype. Additionally, the increased expression of CKIs in the epidermis of mutant mice can also contribute to the proliferative defect found. Because ERK and AKT are essential for skin proliferation and cyclin D1 expression (38, 39, 56), it would be expected a reduced activity of these kinases in TR KO mice, but paradoxically stimulation rather than inhibition was found in these animals, indicating that other still unidentified pathways dependent on the receptors are responsible for the reduction of cyclin D1. Because expression of the cyclin D1 gene is under the control of a panoply of transcription factors that are modulated by these kinases, the reduced cyclin D1 expression observed could indicate that the TRs and its ligand are required for the activity of those transcription factors or that they are essential direct modulators of cyclin D1 expression in keratinocytes. In contrast, we and others have previously shown that TRs can inhibit expression of the cyclin D1 gene in other types of cells in culture through interference with the activity of transcription factors that bind to its promoter (4, 57, 58). In addition, one may consider that the moderate increase in ERK and AKT activity found in KO mice was insufficient to allow for stimulation of cyclin D1 expression. In support of this possibility in KO mice, we have not observed the alterations in ectodermal organ development mediated by expression of active AKT kinase in transgenic mouse epidermis (59). However, we have observed a preferential increase in total versus nuclear p27 content in these mice, in agreement with the cytoplasmic localization of p27 mediated by AKT phosphorylation observed in cancer cells (60). It is clear that the study of the mechanisms by which TR or thyroid hormone depletion reduces cyclin D1 expression in keratinocytes in vivo warrants further investigation.
In contrast with the dissociation between ERK and AKT activation and cyclin D1 expression, other targets of the MAPK cascade are enhanced in the skin of the TR KO mice. Thus, ERK activates AP-1 transcription factor complex expression, and accordingly, we found increased c-Fos and Jun proteins in the absence of TRs both in vivo and in cultured keratinocytes. These receptors are known to oppose AP-1 activity (2), and this observation agrees with this antagonism. Because both c-Jun and JunB that play opposite roles in proliferation (41–43, 61) were increased in the KO mouse, overexpression of JunB could also counteract the proliferative effects of c-Jun. Furthermore, ERK and Akt have been identified as important regulators of NF-κB activation in the skin (48, 62, 63), and consistent with this, we found that p65 phosphorylation was increased in the TR KO mice.
Activation of NF-κB plays a key role in inflammatory skin processes (64, 65), and TPA is a potent activator of this factor as well as the transcription factor STAT3 (50, 66–68). In addition to p65/NF-κB phosphorylation, we found that mice lacking TRs presented enhanced levels of STAT3 phosphorylation. Prominent among STAT3 and NF-κB targets are genes encoding cytokines and chemokines that play pivotal roles in the recruitment of inflammatory cells (44). Furthermore, STAT3 induces the members of AP-1 family, and Jun proteins also enhance the expression of cytokines and chemotactic proteins (43). Hence, the up-regulation of NF-κB, STAT3, and AP-1 activity in the skin of TR KO mice should induce expression of inflammatory mediators, and accordingly, a significant increase in the production of an important number of cytokines and chemokines, together with increased infiltration of inflammatory cells, was found in these animals. Remarkably, some of these proteins were overexpressed in the untreated animals, indicating that in the absence of TRs, the skin already presents an inflammatory reaction under basal conditions. The ability of several members of the nuclear receptor superfamily to inhibit transcriptional activation by NF-κB and AP-1 by a mechanism that is referred to as transrepression, thereby attenuating inflammatory responses, is well known (69). Our present results showing increased activity of these transcription factors as well as augmented production of proinflammatory molecules in TR KO mice demonstrate that TRs share these properties and that these receptors act as an endogenous brake of skin inflammation in vivo. At least some of the effects of TRs are directly exerted in the keratinocytes because TNF-α expression is enhanced in primary cultures from mice lacking the receptors and activation of STAT3 and p65 was found in these cells. Furthermore, the chemotatic proteins S100A8 and S100A9 are also higher in the KO keratinocytes. These proteins are markedly overexpressed under cellular stress conditions and may play a role in the pathogenesis of epidermal disease and inflammation (52, 53). Thus, the increased release of S100A8 and S100A9 from keratinocytes might be one important molecular event that could contribute to increased immune cell invasion and skin inflammation in TR KO mice.
In summary, the use of mice in which the TR isoforms that bind thyroid hormones have been inactivated allows the definition of these nuclear receptors and its ligand as important components of the skin homeostasis in vivo. Interestingly, although cutaneous proliferation and inflammation are normally associated, in the absence of TRs, there is a clear dissociation between both processes because reduced proliferation together with an increased inflammatory response is found in the skin of animals in which TRs have been inactivated. Future studies will be needed to validate which of the changes of the analyzed molecules play a causal role in the epidermal phenotype observed upon loss of TR signaling and which of them are the result of feedback mechanisms caused by those primary alterations. A more profound knowledge of the molecular mechanisms responsible for these actions will help to a better understanding of skin biology and thyroid diseases.
Supplementary Material
Acknowledgments
We thank M. Sánchez Prieto for technical help. We also thank Björn Vennström (Karolinska Institute, Sweden), who originally generated the KO mice, for kindly providing these mice.
This work was supported by Ministerio de Ciencia e Innovación Grants BFU2007-62402 and SAF2008-00121, Fondo de Investigaciones Sanitarias Grants RD06/0020/0036 and RD06/0020/0029, and European CRESCENDO Grant FP-018652.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- TR
- thyroid hormone receptor
- hT
- hypothyroid
- TPA
- 12-O-tetradecanolyphorbol-13-acetate
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- CKI
- cyclin kinase inhibitor.
REFERENCES
- 1. Flamant F., Baxter J. D., Forrest D., Refetoff S., Samuels H., Scanlan T. S., Vennström B., Samarut J. (2006) Pharmacol. Rev. 58, 705–711 [DOI] [PubMed] [Google Scholar]
- 2. Aranda A., Martínez-Iglesias O., Ruiz-Llorente L., García-Carpizo V., Zambrano A. (2009) Trends Endocrinol. Metab. 20, 318–324 [DOI] [PubMed] [Google Scholar]
- 3. Chiloeches A., Sánchez-Pacheco A., Gil-Araujo B., Aranda A., Lasa M. (2008) Mol. Endocrinol. 22, 2466–2480 [DOI] [PubMed] [Google Scholar]
- 4. García-Silva S., Aranda A. (2004) Mol. Cell Biol. 24, 7514–7523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lasa M., Gil-Araujo B., Palafox M., Aranda A. (2010) Mol. Endocrinol. 24, 412–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Méndez-Pertuz M., Sánchez-Pacheco A., Aranda A. (2003) EMBO J. 22, 3102–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez P., Palomino T., Schönthal A., Aranda A. (1994) Biochem. Biophys. Res. Commun. 205, 135–140 [DOI] [PubMed] [Google Scholar]
- 8. Forrest D., Erway L. C., Ng L., Altschuler R., Curran T. (1996) Nat. Genet. 13, 354–357 [DOI] [PubMed] [Google Scholar]
- 9. Fraichard A., Chassande O., Plateroti M., Roux J. P., Trouillas J., Dehay C., Legrand C., Gauthier K., Kedinger M., Malaval L., Rousset B., Samarut J. (1997) EMBO J. 16, 4412–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wikström L., Johansson C., Saltó C., Barlow C., Campos Barros A., Baas F., Forrest D., Thorén P., Vennström B. (1998) EMBO J. 17, 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Göthe S., Wang Z., Ng L., Kindblom J. M., Barros A. C., Ohlsson C., Vennström B., Forrest D. (1999) Genes Dev. 13, 1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Billoni N., Buan B., Gautier B., Gaillard O., Mahé Y. F., Bernard B. A. (2000) Br. J. Dermatol. 142, 645–652 [DOI] [PubMed] [Google Scholar]
- 13. Martínez-Iglesias O., Garcia-Silva S., Tenbaum S. P., Regadera J., Larcher F., Paramio J. M., Vennström B., Aranda A. (2009) Cancer Res. 69, 501–509 [DOI] [PubMed] [Google Scholar]
- 14. Slominski A., Wortsman J., Kohn L., Ain K. B., Venkataraman G. M., Pisarchik A., Chung J. H., Giuliani C., Thornton M., Slugocki G., Tobin D. J. (2002) J. Invest. Dermatol. 119, 1449–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahsan M. K., Urano Y., Kato S., Oura H., Arase S. (1998) J. Med. Invest. 44, 179–184 [PubMed] [Google Scholar]
- 16. Jho S. H., Radoja N., Im M. J., Tomic-Canic M. (2001) J. Biol. Chem. 276, 45914–45920 [DOI] [PubMed] [Google Scholar]
- 17. Radoja N., Diaz D. V., Minars T. J., Freedberg I. M., Blumenberg M., Tomic-Canic M. (1997) J. Invest. Dermatol. 109, 566–572 [DOI] [PubMed] [Google Scholar]
- 18. Radoja N., Stojadinovic O., Waseem A., Tomic-Canic M., Milisavljevic V., Teebor S., Blumenberg M. (2004) Mol. Cell Biol. 24, 3168–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramot Y., Paus R., Tiede S., Zlotogorski A. (2009) BioEssays 31, 389–399 [DOI] [PubMed] [Google Scholar]
- 20. Bodó E., Kromminga A., Bíró T., Borbíró I., Gáspár E., Zmijewski M. A., van Beek N., Langbein L., Slominski A. T., Paus R. (2009) J. Invest. Dermatol. 129, 1126–1139 [DOI] [PubMed] [Google Scholar]
- 21. Freinkel R. K., Freinkel N. (1972) Arch. Dermatol. 106, 349–352 [PubMed] [Google Scholar]
- 22. Li J. J., Mitchell L. H., Dow R. L. (2010) Bioorg. Med. Chem. Lett. 20, 306–308 [DOI] [PubMed] [Google Scholar]
- 23. Messenger A. G. (2000) Br. J. Dermatol. 142, 633–634 [DOI] [PubMed] [Google Scholar]
- 24. Paus R. (2010) J. Invest. Dermatol. 130, 7–10 [DOI] [PubMed] [Google Scholar]
- 25. Slominski A., Wortsman J. (2000) Endocr. Rev. 21, 457–487 [DOI] [PubMed] [Google Scholar]
- 26. Thiboutot D. M. (1995) J. Clin. Endocrinol. Metab. 80, 3082–3087 [DOI] [PubMed] [Google Scholar]
- 27. van Beek N., Bodó E., Kromminga A., Gáspár E., Meyer K., Zmijewski M. A., Slominski A., Wenzel B. E., Paus R. (2008) J. Clin. Endocrinol. Metab. 93, 4381–4388 [DOI] [PubMed] [Google Scholar]
- 28. Safer J. D., Crawford T. M., Holick M. F. (2004) Endocrinology 145, 2357–2361 [DOI] [PubMed] [Google Scholar]
- 29. Safer J. D., Crawford T. M., Holick M. F. (2005) Endocrinology 146, 4425–4430 [DOI] [PubMed] [Google Scholar]
- 30. Safer J. D., Fraser L. M., Ray S., Holick M. F. (2001) Thyroid 11, 717–724 [DOI] [PubMed] [Google Scholar]
- 31. Argyris T. S. (1985) Crit. Rev. Toxicol. 14, 211–258 [DOI] [PubMed] [Google Scholar]
- 32. Moore R. J., Owens D. M., Stamp G., Arnott C., Burke F., East N., Holdsworth H., Turner L., Rollins B., Pasparakis M., Kollias G., Balkwill F. (1999) Nat. Med. 5, 828–831 [DOI] [PubMed] [Google Scholar]
- 33. Mueller M. M. (2006) Eur. J. Cancer 42, 735–744 [DOI] [PubMed] [Google Scholar]
- 34. Raick A. N. (1973) Cancer Res. 33, 269–286 [PubMed] [Google Scholar]
- 35. Martínez-Iglesias O., García-Silva S., Regadera J., Aranda A. (2009) PLoS One 4, e6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Segrelles C., Lu J., Hammann B., Santos M., Moral M., Cascallana J. L., Lara M. F., Rho O., Carbajal S., Traag J., Beltrán L., Martínez-Cruz A. B., García-Escudero R., Lorz C., Ruiz S., Bravo A., Paramio J. M., DiGiovanni J. (2007) Cancer Res. 67, 10879–10888 [DOI] [PubMed] [Google Scholar]
- 37. Robles A. I., Rodriguez-Puebla M. L., Glick A. B., Trempus C., Hansen L., Sicinski P., Tennant R. W., Weinberg R. A., Yuspa S. H., Conti C. J. (1998) Genes Dev. 12, 2469–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khavari T. A., Rinn J. (2007) Cell Cycle 6, 2928–2931 [DOI] [PubMed] [Google Scholar]
- 39. Segrelles C., Moral M., Lara M. F., Ruiz S., Santos M., Leis H., García-Escudero R., Martínez-Cruz A. B., Martínez-Palacio J., Hernández P., Ballestín C., Paramio J. M. (2006) Oncogene 25, 1174–1185 [DOI] [PubMed] [Google Scholar]
- 40. Eferl R., Wagner E. F. (2003) Nat. Rev. Cancer 3, 859–868 [DOI] [PubMed] [Google Scholar]
- 41. Passegué E., Wagner E. F. (2000) EMBO J. 19, 2969–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Szabowski A., Maas-Szabowski N., Andrecht S., Kolbus A., Schorpp-Kistner M., Fusenig N. E., Angel P. (2000) Cell 103, 745–755 [DOI] [PubMed] [Google Scholar]
- 43. Zenz R., Wagner E. F. (2006) Int. J. Biochem. Cell Biol. 38, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 44. Grivennikov S. I., Karin M. (2010) Cytokine Growth Factor Rev. 21, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DiDonato J. A., Hayakawa M., Rothwarf D. M., Zandi E., Karin M. (1997) Nature 388, 548–554 [DOI] [PubMed] [Google Scholar]
- 46. Vallabhapurapu S., Karin M. (2009) Annu. Rev. Immunol. 27, 693–733 [DOI] [PubMed] [Google Scholar]
- 47. Viatour P., Merville M. P., Bours V., Chariot A. (2005) Trends Biochem. Sci. 30, 43–52 [DOI] [PubMed] [Google Scholar]
- 48. Santos M., Paramio J. M., Bravo A., Ramirez A., Jorcano J. L. (2002) J. Biol. Chem. 277, 19122–19130 [DOI] [PubMed] [Google Scholar]
- 49. Yoshimura A., Naka T., Kubo M. (2007) Nat. Rev. Immunol. 7, 454–465 [DOI] [PubMed] [Google Scholar]
- 50. Wen Z., Zhong Z., Darnell J. E., Jr. (1995) Cell 82, 241–250 [DOI] [PubMed] [Google Scholar]
- 51. Banno T., Gazel A., Blumenberg M. (2005) J. Biol. Chem. 280, 18973–18980 [DOI] [PubMed] [Google Scholar]
- 52. Eckert R. L., Broome A. M., Ruse M., Robinson N., Ryan D., Lee K. (2004) J. Invest. Dermatol. 123, 23–33 [DOI] [PubMed] [Google Scholar]
- 53. Gebhardt C., Németh J., Angel P., Hess J. (2006) Biochem. Pharmacol. 72, 1622–1631 [DOI] [PubMed] [Google Scholar]
- 54. Wondisford F. E. (2003) J. Investig. Med. 51, 215–220 [DOI] [PubMed] [Google Scholar]
- 55. Rodriguez-Puebla M. L., Robles A. I., Johnson D. G., LaCava M., Conti C. J. (1998) Cell Growth Differ. 9, 31–39 [PubMed] [Google Scholar]
- 56. Peng X. D., Xu P. Z., Chen M. L., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W. S., Crawford S. E., Coleman K. G., Hay N. (2003) Genes Dev. 17, 1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. García-Silva S., Martínez-Iglesias O., Ruiz-Llorente L., Aranda A. (2011) Oncogene 30, 854–864 [DOI] [PubMed] [Google Scholar]
- 58. Porlan E., Vidaurre O. G., Rodríguez-Peña A. (2008) Oncogene 27, 2795–2800 [DOI] [PubMed] [Google Scholar]
- 59. Segrelles C., Moral M., Lorz C., Santos M., Lu J., Cascallana J. L., Lara M. F., Carbajal S., Martínez-Cruz A. B., García-Escudero R., Beltran L., Segovia J. C., Bravo A., DiGiovanni J., Paramio J. M. (2008) Mol. Biol. Cell 19, 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Viglietto G., Motti M. L., Fusco A. (2002) Cell Cycle 1, 394–400 [DOI] [PubMed] [Google Scholar]
- 61. Zenz R., Scheuch H., Martin P., Frank C., Eferl R., Kenner L., Sibilia M., Wagner E. F. (2003) Dev. Cell 4, 879–889 [DOI] [PubMed] [Google Scholar]
- 62. Chung W. Y., Park J. H., Kim M. J., Kim H. O., Hwang J. K., Lee S. K., Park K. K. (2007) Carcinogenesis 28, 1224–1231 [DOI] [PubMed] [Google Scholar]
- 63. Medeiros R., Figueiredo C. P., Passos G. F., Calixto J. B. (2009) Biochem. Pharmacol. 78, 390–395 [DOI] [PubMed] [Google Scholar]
- 64. Bell S., Degitz K., Quirling M., Jilg N., Page S., Brand K. (2003) Cell. Signal. 15, 1–7 [DOI] [PubMed] [Google Scholar]
- 65. Kaufman C. K., Fuchs E. (2000) J. Cell Biol. 149, 999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aziz M. H., Manoharan H. T., Sand J. M., Verma A. K. (2007) Mol. Carcinog. 46, 646–653 [DOI] [PubMed] [Google Scholar]
- 67. Chan K. S., Carbajal S., Kiguchi K., Clifford J., Sano S., DiGiovanni J. (2004) Cancer Res. 64, 2382–2389 [DOI] [PubMed] [Google Scholar]
- 68. Chan K. S., Sano S., Kiguchi K., Anders J., Komazawa N., Takeda J., DiGiovanni J. (2004) J. Clin. Invest. 114, 720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pascual G., Glass C. K. (2006) Trends Endocrinol. Metab. 17, 321–327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.