Abstract
Platelets contain high levels of Src family kinases (SFKs), but their functional role downstream of G protein pathways has not been completely understood. We found that platelet shape change induced by selective G12/13 stimulation was potentiated by SFK inhibitors, which was abolished by intracellular calcium chelation. Platelet aggregation, secretion, and intracellular Ca2+ mobilization mediated by low concentrations of SFLLRN or YFLLRNP were potentiated by SFK inhibitors. However, 2-methylthio-ADP-induced intracellular Ca2+ mobilization and platelet aggregation were not affected by PP2, suggesting the contribution of SFKs downstream of G12/13, but not Gq/Gi, as a negative regulator to platelet activation. Moreover, PP2 potentiated YFLLRNP- and AYPGKF-induced PKC activation, indicating that SFKs downstream of G12/13 regulate platelet responses through the negative regulation of PKC activation as well as calcium response. SFK inhibitors failed to potentiate platelet responses in the presence of Gq-selective inhibitor YM254890 or in Gq-deficient platelets, indicating that SFKs negatively regulate platelet responses through modulation of Gq pathways. Importantly, AYPGKF-induced platelet aggregation and PKC activation were potentiated in Fyn-deficient but not in Lyn-deficient mice compared with wild-type littermates. We conclude that SFKs, especially Fyn, activated downstream of G12/13 negatively regulate platelet responses by inhibiting intracellular calcium mobilization and PKC activation through Gq pathways.
Keywords: Calcium, G Protein-coupled Receptor (GPCR), Platelet, Signal Transduction, Src
Introduction
Src family kinases (SFKs)2 are nonreceptor protein-tyrosine kinases that are involved in the control of a variety of cellular processes such as proliferation, differentiation, motility, and adhesion (1). There are nine members of the Src family of tyrosine kinases, which include Src, Lck, Hck, Blk, Fyn, Lyn, Yes, Fgr, and Yrk (1, 2). Earlier studies reported that both human and rodent platelets contain high levels of Src as well as Fyn, Lyn, Hck, Yes, Lck, and Fgr (3–5). Among these, c-Src kinase is present in the most abundant amounts and represents about 0.2–0.4% of the total platelet protein (6). Although platelets contain high levels of SFKs, suggesting an important role for these enzymes in platelet function, the role of SFKs in platelet function has been complex and not fully understood. In platelets, SFKs, particularly Lyn and Fyn, play a crucial role downstream of collagen receptors (7). In addition, platelets from Fyn−/− mice exhibit delayed spreading on immobilized fibrinogen (8), whereas platelets from Lyn−/− mice spread poorly on von Willebrand factor (9). Moreover, c-Src has been shown to play an important role in integrin αIIbβ3-dependent outside-in signaling (10, 11), but Lyn has been reported to regulate integrin αIIbβ3-mediated signaling in platelets negatively (12). It is also known that SFKs play a role in thromboxane generation (13), shape change (14), as well as regulation of phosphorylation of Akt (15) and ERK (16, 17).
An important mechanism for regulation of c-Src tyrosine kinase activity is through control of its phosphorylation status. There are two major phosphorylation sites present on human c-Src, Tyr416 and Tyr527. A short C-terminal tail of SFKs contains an autoinhibitory phosphorylation site, Tyr527 (18, 19), whereas Tyr416 is a target for intermolecular autophosphorylation (19). When Tyr416 is phosphorylated, it is displaced from the substrate binding pocket, allowing the kinase access to substrates. Thus, when Tyr416 is phosphorylated, it is a positive regulator of Src activity.
Shape change is considered to be the first measurable physiological response produced by platelets following exposure to an agonist. ADP-induced platelet shape change is involved in Gq-coupled calcium mobilization and the activation of RhoA (20). A number of receptors can couple to G12/13 including thrombin and thromboxane A2, and platelet shape change induced by these agonists is mediated by both calcium-dependent and -independent mechanisms that occur through Gq and G12/13 pathways, respectively (20). G12/13 has been shown to regulate Rho-dependent response (21), and G12/13-mediated shape change is abolished in the presence of the Rho kinase p160ROCK inhibitor Y27632 (22), suggesting an important role of Rho/Rho kinase pathway downstream of G12/13 in the calcium-independent pathway leading to platelet shape change. Activation of phospholipase C (PLC) β2 is essential for agonist-induced physiological responses in platelets (23). Upon activation of PLC, phosphatidylinositol 4,5-bisphosphate in the plasma membrane inner leaflet is hydrolyzed to diacylglycerol and inositol 1,4,5-trisphosphate (24, 25). Although diacylglycerol serves as a stimulatory cofactor for the activation of protein kinase C (PKC), inositol 1,4,5-trisphosphate induces a rapid rise in intracellular calcium (23, 26). Calcium regulates various platelet functions, including secretion, integrin activation, annexin V binding, thromboxane A2 generation, and thrombin generation (27–30). Various PKC- and calcium-dependent events ensue, two of which are the secretion of contents stored in the dense granules of the platelet and the phosphorylation of pleckstrin, a 47-kDa substrate of PKC.
In the current study, we have investigated the regulation of platelet function by SFKs downstream of G12/13 pathways. Our studies indicate that inhibition of SFKs leads to potentiation of platelet aggregation and secretion mediated by PAR agonists, whereas ADP-induced platelet aggregation is not affected by SFK inhibitors. Moreover, inhibiting SFKs downstream of G12/13 led to the potentiation of intracellular calcium mobilization and PKC activation. Interestingly, we have found that platelet aggregation and PKC activation mediated by AYPGKF are potentiated in Fyn-deficient mouse platelets compared with the wild-type mouse platelets. These studies demonstrate that Fyn downstream of G12/13 negatively regulate platelet responses by inhibiting intracellular Ca2+ and PKC through modulation of Gq pathways. These studies clearly demonstrate the intricate signaling pathways that negatively regulate Gq activation in platelets.
EXPERIMENTAL PROCEDURES
Materials
2-MeSADP, epinephrine, MRS-2179, apyrase (type V), CDP-Star® chemiluminescent substrates, sodium citrate, and BSA (fraction V) were purchased from Sigma. YFLLRNP, SFLLRN, and AYPGKF were custom synthesized by Invitrogen. Ser(P) PKC substrate and β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA). Phospho-Fyn (Tyr530) and phospho-Lyn (Tyr507) antibodies were purchased from Abcam (Cambridge, MA). Alkaline phosphatase-labeled secondary antibody was from Kirkegaard & Perry Laboratories (Gaithersburg, MD). PP1, PP2, and Y27632 were from Enzo Life Sciences, Inc. (Plymouth Meeting, PA). 5,5′-Dimethyl-BAPTA and Fura-2/AM were obtained from Molecular Probes. Bisindolylmaleimide I (GF 109203X) and PP3 were from Calbiochem. YM254890 was a generous gift from Yamanouchi Pharmaceutical (Ibaraki, Japan). All other reagents were reagent grade, and deionized water was used throughout.
Animals
Gαq-deficient mice were obtained from T. Kent Gartner (31), with permission from Stefan Offermanns (University of Heidelberg, Heidelberg, Germany). Fyn- and Lyn-deficient mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Preparation of Human Platelets
Human blood was obtained from a pool of healthy volunteers in a one-sixth volume of acid-citrate-dextrose (2.5 g of sodium citrate, 1.5 g of citric acid, and 2.0 g of glucose in 100 ml of H2O). Platelet-rich plasma was prepared by centrifugation of citrated blood at 230 × g for 20 min at room temperature. Acetylsalicylic acid was added to platelet-rich plasma to a final concentration of 1 mm, and the preparation was incubated for 45 min at 37 °C followed by centrifugation at 980 × g for 10 min at room temperature. The platelet pellet was resuspended in Tyrode's buffer (138 mm NaCl, 2.7 mm KCl, 2 mm MgCl2, 0.42 mm NaH2PO4, 5 mm glucose, 10 mm HEPES, pH 7.4, and 0.2% BSA) containing 0.05 units/ml apyrase. The platelet count was adjusted to 2 × 108 cells/ml.
Preparation of Mouse Platelets
Blood was collected from anesthetized mice into syringes containing 1/10 blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100 × g for 10 min at room temperature. Platelet-rich plasma was recovered, and platelets were pelleted at 400 × g for 10 min at room temperature. The platelet pellet was resuspended in Tyrode's buffer, pH 7.4, containing 0.05 unit/ml apyrase to a density of 2 × 108 cells/ml.
Platelet Aggregation, Secretion, and Intracellular Ca2+ Mobilization
Platelet aggregation was measured using a lumiaggregometer (Chrono-Log, Havertown, PA) at 37 °C under stirring conditions. A 0.5-ml sample of aspirin-treated washed platelets was stimulated with different agonists, and change in light transmission was measured. Platelets were preincubated with different inhibitors where noted before agonist stimulation. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/s.
Platelet secretion was determined by measuring the release of ATP by adding luciferin-luciferase reagent. Platelet ATP release and aggregation were performed in a lumiaggregometer at 37 °C simultaneously.
Platelet Ca2+ mobilization was also measured. Platelet-rich plasma was incubated with 5 μm Fura-2/AM and 1 mm aspirin. Fluorescence was measured, and the Ca2+ concentration was calculated as described previously (32).
Western Blotting
Platelets were stimulated with agonists for the appropriate time, and the reaction was stopped by the addition of 3 × SDS sample buffer. In some experiments, PP2 (10 μm) was added and incubated for 5 min at 37 °C without stirring before agonist stimulation. Samples were separated on 10% SDS-PAGE and transferred onto polyvinylidene difluoride membrane. Nonspecific binding sites were blocked by incubation in Tris-buffered saline/Tween (TBST; 20 mm Tris, 140 mm NaCl, 0.1% (v/v) Tween 20) containing 0.5% (w/v) milk protein and 3% (w/v) BSA for 30 min at room temperature, and membranes were incubated overnight at 4 °C with primary antibody (1:1000 in TBST/2% BSA) with gentle agitation. After three washes for 5 min each with TBST, the membranes were probed with the alkaline phosphatase-labeled goat anti-rabbit IgG (1:5000 in TBST/2% BSA) for 1 h at room temperature. After additional washing steps, membranes were then incubated with a CDP-Star® chemiluminescent substrates for 10 min at room temperature, and chemiluminescence was measured using Fujifilm LAS-3000 Luminescent Image Analyzer (Fuji, Tokyo, Japan).
RESULTS
Effect of SFK Inhibition on Ca2+-independent, G12/13-induced Platelet Shape Change Mediated by YFLLRNP
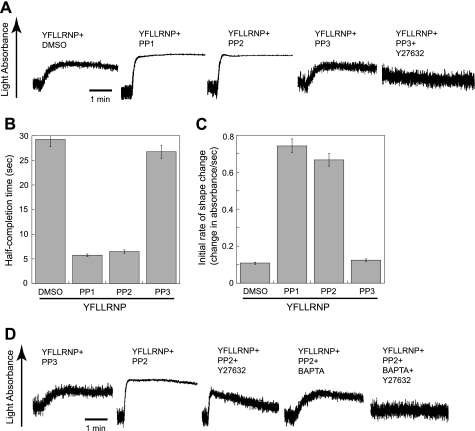
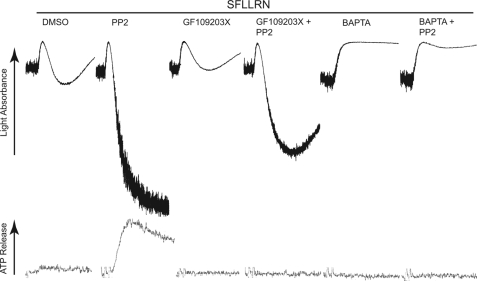
It has been shown that PAR agonists can couple to G12/13 pathways that are involved in Rho kinase p160ROCK activation and the subsequent shape change in platelets (33, 34). Low concentrations of YFLLRNP, a heptapeptide binding to PAR1, causes slow shape change without calcium mobilization in platelets (22, 35), and the chelation of intracellular Ca2+ by BAPTA/AM does not inhibit this shape change (36). As seen in Fig. 1A, YFLLRNP-induced shape change was slow as it took about 1 min to complete the shape change as light absorbance increases slowly. YFLLRNP-induced shape change was completely inhibited by the p160ROCK inhibitor Y27632 (20, 22, 37), and the Gq-selective inhibitor YM254890 (38) did not inhibit this shape change (data not shown), confirming that YFLLRNP induces Rho kinase-dependent shape change without calcium mobilization in this condition. However, YFLLRNP-induced shape change was complete by 15 s as evidenced by dramatic increase in the rate and amplitude of light absorbance with diminished oscillations in the presence of SFK inhibitors PP1 and PP2 (39), but not control compound PP3. We and others have used PP1 and PP2 as a selective SFK inhibitor in platelets at the concentrations used in our study (10, 15, 40). The time for half-completion of shape change was dramatically decreased (∼25% of DMSO or PP3) in the presence of PP1 and PP2 (Fig. 1B), and a significant increase in the initial rate of shape change was also observed in the presence of PP1 and PP2 (Fig. 1C). The changes in shape change tracings in the presence of PP1 and PP2 indicate the contribution of both Rho kinase and calcium components and suggest that inhibition of SFKs downstream of G12/13 can induce mobilization of intracellular calcium in platelets.
FIGURE 1.
Effect of Src kinase inhibition on platelet shape change induced by YFLLRNP. A, aspirin-treated, washed human platelets were preincubated at 37 °C with DMSO (vehicle), 10 μm Y27632, 10 μm PP1, 10 μm PP2, or 10 μm PP3 for 5 min prior to platelet stimulation with 60 μm YFLLRNP. B and C, the time to half-complete shape change (point at which half-maximal light absorbance was reached) (B) and the initial rate of light absorbance during platelet shape change (change in absorbance/sec) (C) are presented. D, aspirinated human platelets were pretreated at 37 °C with either 10 μm PP3 or 10 μm PP2. Platelet samples that were preincubated with 10 μm Y27632 or 10 μm 5,5′-dimethyl-BAPTA were stimulated with 60 μm YFLLRNP as noted. All tracings are representative of at least three experiments from different donors.
To confirm the contribution of cytosolic Ca2+ in shape changes in the presence of SFK inhibitors, we used the Ca2+ chelator 5,5′-dimethyl-BAPTA (41). Fig. 1D shows that YFLLRNP-induced shape change in the presence of PP2 was not inhibited by Y27632. Shape change tracing shows the steadily increased oscillation following an initial increase in light absorbance which is a characteristic feature of the shape change induced by calcium-dependent pathways (20, 22, 42). In 5,5′-dimethyl-BAPTA-treated platelets, YFLLRNP-induced shape change in the presence of PP2 was reversed to Ca2+-independent, RhoA/p160ROCK-dependent shape change, characterized by the slower rate of shape change with constant prestimulatory oscillations. The combination of Y27632 and 5,5′-dimethyl-BAPTA completely prevented shape change, indicating that YFLLRNP-induced shape change in the presence of PP2 is caused by both calcium-dependent and RhoA-dependent pathways and that the inhibition of SFKs might be involved in an increase in cytosolic Ca2+.
Effect of SFK Inhibition on Agonist-induced Platelet Aggregation and Secretion
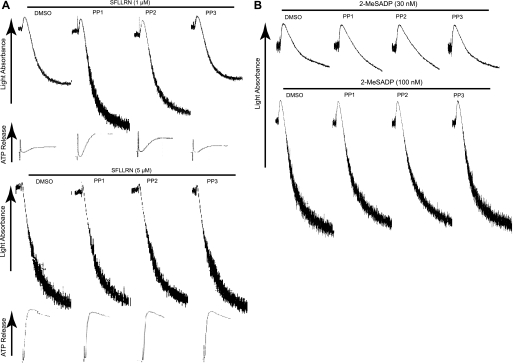
We further investigated the effect of inhibition of SFKs in platelet aggregation and secretion mediated by different concentrations of PAR1-activating peptide SFLLRN and 2-MeSADP. As shown in Fig. 2A, we have observed that platelet aggregation and secretion mediated by low concentrations of SFLLRN (1 μm) were potentiated in the presence of PP1 and PP2, whereas these inhibitors showed no effect at higher concentrations of SFLLRN (5 μm). We also observed that the concentration response curves for PAR4-activating peptide AYPGKF- and thrombin-induced platelet aggregation and secretion were shifted to the left in the presence of SFK inhibitors (data not shown). However, both low (30 nm) and high (100 nm) concentrations of 2-MeSADP-induced platelet aggregation, through activation of Gq and Gi, were not affected by PP1 and PP2 (Fig. 2B). Taken together, these findings indicate that SFK inhibition leads to the potentiation of platelet responses induced by only those agonists that activate G12/13 pathways. These results also indicate the important inhibitory role of SFKs activated downstream of G12/13, but not Gq/Gi, pathways in agonist-induced platelet aggregation and secretion.
FIGURE 2.
Effect of Src kinase inhibition on platelet aggregation and secretion induced by SFLLRN and 2-MeSADP. Aspirinated, washed human platelets were preincubated at 37 °C with DMSO (vehicle), 10 μm PP1, 10 μm PP2, or 10 μm PP3 for 5 min prior to platelet stimulation with different concentrations of either (A) SFLLRN or (B) 2-MeSADP as noted, and platelet aggregation and ATP secretion were measured as described under “Experimental Procedures.” Results shown are representative of at least three experiments from different donors.
Effect of SFK Inhibition on Platelet Aggregation and Secretion Mediated by Co-stimulation of G12/13 and Gi/Gz Pathways
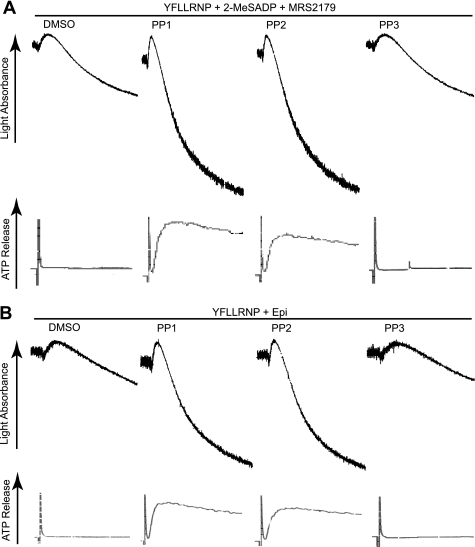
It is shown that YFLLRNP-mediated G12/13 signaling causes fibrinogen receptor activation in human platelets when combined with selective stimulation of Gi or Gz pathways (43, 44). To confirm the contribution of SFKs downstream of G12/13 to platelet activation, we measured the effect of SFK inhibitors on platelet aggregation and secretion mediated by the simultaneous stimulation of G12/13 and Gi/Gz pathways. To stimulate Gi pathways selectively, we used 2-MeSADP in the presence of MRS2179, a P2Y1-selective antagonist, to block ADP signaling through the Gq-coupled P2Y1 receptor. We also used epinephrine as an agonist to stimulate Gz pathways through the α2A adrenergic receptor. We found that platelet aggregation and secretion induced by combined stimulation of G12/13 and Gi (Fig. 3A) or G12/13 and Gz (Fig. 3B) were potentiated in the presence of PP1 and PP2, whereas the control compound PP3 had no effect. Previous work has shown that SFK inhibitors can partially inhibit platelet aggregation induced by combined stimulation of Gi and Gz signaling (45). Thus, these results strongly suggest the idea that SFKs downstream of G12/13, not Gi/Gz, contribute as negative regulators of platelet activation.
FIGURE 3.
Effect of Src kinase inhibition on platelet aggregation and secretion mediated by the simultaneous stimulation of G12/13 and Gi/Gz pathways. Washed human platelets were preincubated at 37 °C with DMSO (vehicle), 10 μm PP1, 10 μm PP2, or 10 μm PP3 for 5 min prior to platelet stimulation with either (A) 60 μm YFLLRNP + 100 nm 2-MeSADP + MRS2179 (100 μm) or (B) YFLLRNP (60 μm) + epinephrine (10 μm), and platelet aggregation and ATP secretion were measured. The data shown are representative of at least three experiments.
Effect of Inhibition of SFKs on Intracellular Calcium Mobilization and PKC Activation
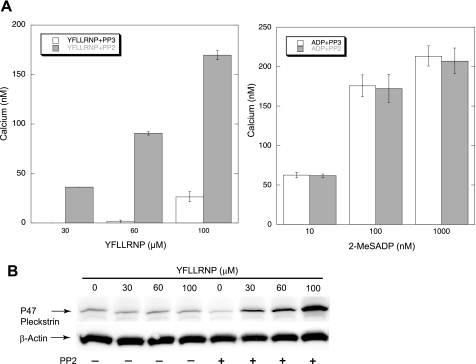
To determine the mechanism by which the SFK inhibition downstream of G12/13 pathways leads to the potentiation of platelet function, we first measured the effect of SFK inhibition on Gq pathways by monitoring Ca2+ and PKC activation. We first stimulated Fura-2-loaded platelets with different concentrations of PAR1-activating peptide YFLLRNP and 2-MeSADP in the presence of PP2 or PP3, and then measured the intracellular calcium mobilization. It is known that YFLLRNP, a heptapeptide binding to PAR1, initially couples to G12/13, resulting in shape change. While increasing the concentrations of YFLLRNP, it also couples to Gq, in addition to G12/13. YFLLRNP-induced calcium mobilization was potentiated in the presence of PP2, but the level of intracellular calcium in response to 2-MeSADP was not affected by PP2 (Fig. 4A), consistent with previous data showing that inhibition of Src by PP1 has no effect on the calcium response to ADP (46). Because 2-MeSADP does not couple to G12/13 signaling pathways (47), these findings indicate that SFKs downstream of G12/13, not Gq/Gi, have a role in the negative regulation of calcium response in platelets. We then measured the phosphorylation of the PKC substrate pleckstrin in response to different concentrations of YFLLRNP in the presence and absence of PP2. As shown in Fig. 4B, YFLLRNP-induced pleckstrin phosphorylation was increased in the presence of PP2, indicating enhanced PKC activation. Taken together, SFK inhibition downstream of G12/13 potentiates platelet function maybe through enhanced intracellular calcium mobilization and PKC activation.
FIGURE 4.
Effect of PP2 on agonist-induced intracellular calcium mobilization and PKC activation. A, Fura-2 (2 μm)-loaded, aspirinated, washed human platelets were stimulated with different concentrations of YFLLRNP or 2-MeSADP in the presence of either 10 μm PP3 or 10 μm PP2 as noted. Ca2+ was measured by recording fluorescence at 510 nm following excitation at 340 and 380 nm. The results show composite data from three experiments, and values are mean ± S.E. (error bars) (n = 3). B, washed platelets were stimulated at 37 °C for 30 s with different concentrations of YFLLRNP in the absence and presence of PP2 (10 μm). Platelet proteins were separated by SDS-PAGE followed by Western blot analysis using Ser(P) PKC substrate or anti-β-actin (lane loading control) antibody. The blot shown is a representative of three independent experiments.
To confirm the role of Ca2+ and PKC activation, we measured the potentiating effect of SFK inhibitors in response to a low dose of SFLLRN in the presence and absence of PKC inhibitor GF109203X (48) and a high affinity Ca2+ chelator 5,5′-dimethyl-BAPTA (41). As shown in Fig. 5, SFK inhibitor PP2 was still able to potentiate platelet aggregation without secretion in the presence of GF109203X, suggesting that PKC has a minor role in this potentiating effect. However, PP2 failed to potentiate platelet aggregation in the presence of 5,5′-dimethyl-BAPTA, indicating that potentiation of cytosolic calcium is essential for the enhanced platelet responses by SFK inhibition.
FIGURE 5.
Effect of 5,5′-dimethyl-BAPTA and GF109203X on the potentiation of platelet aggregation and secretion in the presence of PP2. Washed platelets were pretreated with either DMSO or 10 μm PP2. Platelets that were preincubated with 10 μm GF109203X or 10 μm 5,5′-dimethyl-BAPTA were stimulated with 1 μm SFLLRN. All aggregation tracings are representative of at least three experiments.
Role of Gq Signaling in the Potentiation of G12/13-induced Shape Change in the Presence of SFK Inhibitors
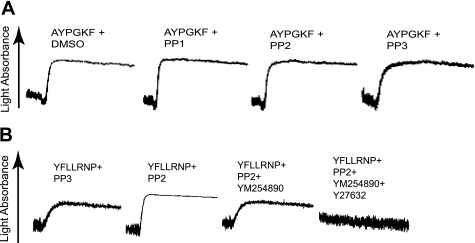
To confirm the effect of SFK inhibition on Gq signaling, we used Gαq-deficient mouse platelets (31) with AYPGKF to activate G12/13 pathways selectively. PP1 and PP2 failed to potentiate platelet shape change induced by selective stimulation of G12/13 by AYPGKF in Gq-deficient platelets (Fig. 6A), indicating that SFKs negatively regulate platelet responses through modulation of Gq signaling pathways. Moreover, platelet aggregation induced by combined G12/13 and Gi signaling by AYPGKF and 2-MeSADP in Gq-deficient platelets failed to potentiate in the presence of PP2 (data not shown). To confirm the effect of SFK inhibition on Gq signaling in human as well as murine platelets, we selectively activated G12/13 signaling with YFLLRNP and measured the effect of SFK inhibition on shape change in the presence of the Gq-selective inhibitor YM254890 (38). Fig. 6B shows that YFLLRNP-induced shape change in the presence of PP2 was reversed to Ca2+-independent, Rho-dependent shape change in the presence of YM254890, which was completely inhibited by the addition of Y27632. Taken together, these results confirm the negative regulation of Gq pathways by SFKs downstream of G12/13 pathways.
FIGURE 6.
Role of Gq signaling in G12/13-induced platelet shape change in the presence of Src kinase inhibitors. A, Gq-deficient mouse platelets were preincubated at 37 °C with DMSO, 10 μm PP1, 10 μm PP2, or 10 μm PP3 for 5 min prior to platelet stimulation with AYPGKF (500 μm). B, washed human platelets were preincubated with either 10 μm PP3 or 10 μm PP2. Platelet samples that were preincubated with 100 nm YM254890 or 10 μm Y27632 were stimulated with 60 μm YFLLRNP as noted. All tracings are representative of at least three experiments from different donors.
Potentiation of Platelet Responses in Fyn-deficient Platelets
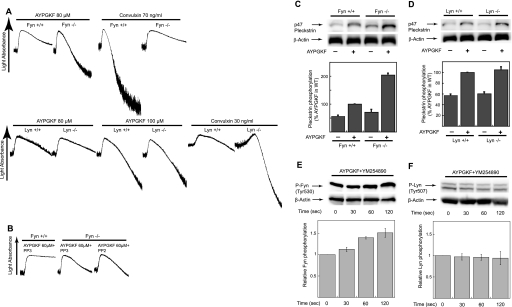
To distinguish the role of individual SFKs in this negative regulation of platelet responses, we stimulated mouse platelets deficient in Fyn and Lyn with different concentrations of AYPGKF. We found that platelet aggregation induced by low concentrations of AYPGKF was potentiated in Fyn-deficient platelets compared with the platelets from age/sex-matched wild-type littermates, whereas platelet aggregation induced by different concentrations of AYPGKF showed no difference between Lyn−/− and wild-type mice (Fig. 7A). Convulxin was used as a positive control in this experiment and showed the inhibition of platelet aggregation in Fyn-deficient platelets whereas Lyn-deficient platelets showed a potentiation of convulxin-induced aggregation consistent with previous findings (7). Similar to results shown in Fig. 3B, low and high concentrations of 2-MeSADP-induced platelet aggregation were not potentiated in both Fyn- and Lyn-deficient platelets compared with the wild-type platelets (data not shown). In addition, potentiation of AYPGKF-induced platelet aggregation in Fyn-deficient platelets was not significantly affected by PP2 (Fig. 7B). Fig. 7C shows that phosphorylation of the PKC substrate pleckstrin in response to a low concentration of AYPGKF was significantly increased in Fyn-deficient platelets compared with the wild-type platelets, whereas Lyn-deficient platelets showed no difference in pleckstrin phosphorylation compared with the wild-type platelets (Fig. 7D), indicating increased PKC activity in Fyn−/− mice. In addition, selective activation of G12/13 signaling with AYPGKF in the presence of YM254890 resulted in the phosphorylation of Fyn (Fig. 7E), but not Lyn (Fig. 7F), confirming the specificity of Fyn in potentiation. These results strongly support our data showing the potentiating effect of SFK inhibitors on human platelet responses and indicate that Fyn downstream of G12/13 pathways plays a major role in the negative regulation of platelet responses, whereas Lyn is not involved in this process.
FIGURE 7.
Platelet aggregation and PKC activation in Fyn- and Lyn-deficient platelets. Platelets were isolated from Fyn−/−, Lyn−/−, or their wild-type littermates. A, platelets isolated from Fyn−/− and Lyn−/− mice were stimulated with 80 μm AYPGKF and different concentration of convulxin as indicated, and platelet aggregation was measured. B, platelets isolated from Fyn−/− mice were preincubated with either 10 μm PP3 or 10 μm PP2 and stimulated with 60 μm AYPGKF and platelet aggregation were measured. C and D, platelets isolated from Fyn−/− (C) and Lyn−/− (D) mice were stimulated at 37 °C for 30 s with AYPGKF (100 μm). Equal amounts of proteins were analyzed by Western blot analysis with Ser(P) PKC substrate or anti-β-actin (lane loading control) antibody. Densitometric measurement of Ser(P) PKC is shown, expressed as percentage of agonist control in wild-type platelets. E and F, washed human platelets preincubated with 100 nm YM254890 were stimulated for the time point indicated with 500 μm AYPGKF, and platelet proteins were analyzed by Western blot analysis with phospho-Fyn, phospho-Lyn, or anti-β-actin (lane loading control) antibodies. The Western blot analysis shown is a representative of three independent experiments. Error bars, S.E.
DISCUSSION
Although SFKs are known to be expressed abundantly in platelets, their role in platelet activation has not been fully elucidated. We have previously shown that AYPGKF and thrombin were able to induce an increase in Src phosphorylation in Gαq-deficient mouse platelets, indicating that selective G12/13 activation results in activation of SFKs (15). In this study, we have further elucidated the contribution of SFKs selectively activated downstream of G12/13 pathways in platelet functional responses.
To determine the role of SFKs downstream of G12/13, we first evaluated the effect of inhibition of SFKs on G12/13-induced shape change. It is known that platelet shape change involves both calcium-dependent and calcium-independent mechanisms as evidenced by the prevention of shape change in both the absence of calcium mobilization and RhoA/p160ROCK activity (20, 42). Platelet shape change induced by selective stimulation of G12/13 is mediated by RhoA/p160ROCK activity without the contribution of calcium mobilization (33, 34). Our results show that inhibition of SFK converts platelet shape change induced by selective G12/13 stimulation with YFLLRNP from a RhoA/p160ROCK-dependent event to a both RhoA/p160ROCK-dependent and calcium-dependent event, indicating the contribution of intracellular calcium mobilization in the presence of SFK inhibitors. Thus, it appears that SFKs downstream of G12/13 negatively regulates platelet responses with the contribution of calcium-dependent component.
If G12/13-mediated SFKs negatively regulate platelet function, we would expect that SFK inhibition leads to the potentiation of platelet responses induced by only those agonists that activate G12/13 pathways. It is known that PARs couple to Gq and G12/13 pathways (49), but 2-MeSADP cannot couple to G12/13 signaling pathways (47). Our results indicate that PP1 or PP2 caused a potentiation of platelet aggregation and secretion induced by low concentrations of PAR agonists, but not by 2-MeSADP, suggesting that SFKs downstream of G12/13, but not Gq/Gi, pathways are involved in the negative regulation of platelet responses. Moreover, platelet aggregation and secretion caused by co-stimulation of G12/13 and Gi/Gz (YFLLRNP + 2-MeSADP with P2Y1 antagonist/epinephrine) were potentiated in the presence of PP1 or PP2. Previous work has also shown that SFK inhibitors have no effect on calcium mobilization in response to ADP (46), confirming the role of SFKs downstream of G12/13, not Gq/Gi, as negative regulators of platelet activation.
It has been shown that G13-deficient platelets display the defective platelet shape change and aggregation in response to thrombin (50). It is well known that G12/13-mediated shape change is mediated by Rho kinase (22, 33) and RhoA downstream of G12/13 contributes to platelet aggregation and secretion (51, 52). Thus, we postulate that the positive contribution of RhoA downstream of G12/13 pathways might be more than the negative contribution of Fyn, which resulted in the observed defects in platelet shape change and aggregation in G13−/− mice.
We next sought to identify the molecular mechanism underlying the regulation of platelet function by SFKs downstream of G12/13. It is known that Gq-mediated PLCβ activation leads to the generation of inositol 1,4,5-trisphosphate and diacylglycerol, which further promote mobilization of intracellular calcium and activation of PKC, respectively (23). We observed the potentiation of calcium mobilization induced by PAR agonist in the presence of PP2 and not by 2-MeSADP. In addition, PP2 caused an increase in PAR-induced phosphorylation of PKC substrate pleckstrin, indicating the possible involvement of PKCs. These results indicate that SFKs downstream of G12/13 might negatively regulate PLCβ activation, resulting in the regulation of PKC activation as well as calcium mobilization. Moreover, PP2 failed to potentiate platelet aggregation in the presence of 5,5′-dimethyl-BAPTA, whereas PP2 was still able to partially potentiate platelet aggregation in the presence of GF109203X, suggesting the minor role of PKCs in this potentiating effect. These data confirm that the potentiation of cytosolic calcium in the presence of SFK inhibitors is essential for the enhanced platelet aggregation and secretion and indicate that platelets have the overriding need for calcium to secrete their granule contents or to aggregate.
In our study, PP1 or PP2 failed to potentiate platelet responses in the presence of Gq-selective inhibitor YM254890 in human platelets or in Gq-deficient mouse platelets. Thus, it is possible that the SFKs downstream of G12/13 negatively regulate PLC through the modulation of Gq pathways which results in the PLC-dependent production of inositol 1,4,5-trisphosphate-mediated calcium mobilization. When platelets are stimulated with low concentrations of thrombin, PARs initially couple to G12/13 pathways which results in platelet shape change without aggregation. Thus, at low doses, there might be weak Gq activation, although PARs predominantly couple to G12/13 pathways. It is also possible that Gq pathways are masked by the SFK-sensitive signal mediated by G12/13 pathways at these low doses. Thus, it appears that low concentrations of PAR-activating peptides, including YFLLRNP, stimulate Gq as well as G12/13 pathways when SFK, especially Fyn, is inhibited. Therefore under normal physiological conditions when platelets are exposed to low concentrations of thrombin, we can speculate that SFKs downstream of G12/13 negatively regulate Gq pathways and hence prevent platelets from aggregating and keep the platelets quiescent.
Because the pharmacological inhibitors do not distinguish various SFKs, we evaluated the contribution of individual SFKs in regulation of platelet function using knock-out mice. In this study, we used Fyn and Lyn knock-out mice. It is well known that the SFKs Fyn and Lyn play an important role in glycoprotein VI-mediated platelet activation (7), and we have also shown that Fyn, but not Lyn, regulates glycoprotein VI-mediated Akt phosphorylation (53). As evidenced by the results in Fig. 7A, our study clearly demonstrates that Fyn and Lyn differentially regulate platelet responses downstream of PARs and GPVI receptors. There are conflicting data in the literature regarding the role of Lyn in thrombin-induced GPCR signaling mostly likely due to the different platelet preparation conditions and agonists used. Quek et al. (7) have shown that aggregation in Lyn−/− platelets in response to α-thrombin is similar to control responses, whereas others have reported that Lyn is required for irreversible aggregation induced by γ-thrombin in platelet-rich plasma (54). Recently, Li et al. (40) have shown that Lyn−/− platelets have little effect on primary platelet aggregation induced by thrombin but have inhibitory effect on granule secretion and thus the secretion-dependent second wave of platelet aggregation. We have found that platelet aggregation and PKC activation induced by low concentrations of AYPGKF were potentiated in Fyn-, but not in Lyn-deficient platelets compared with the wild-type platelets, whereas 2-MeSADP-induced platelet aggregation was not affected in these platelets (data not shown). Moreover, potentiation of AYPGKF-induced platelet aggregation in Fyn-deficient platelets was not significantly affected by SFK inhibitors. In addition, selective activation of G12/13 resulted in the phosphorylation of Fyn, but not Lyn. Thus, we have identified that Fyn downstream of G12/13 plays a major role in the negative regulation of platelet function, whereas Lyn is not involved in this process. However, we cannot exclude that other SFKs such as Lck, Src, Yes, Fgr, or Hck might also be involved.
In conclusion, we demonstrate that SFKs downstream of G12/13, but not Gq/Gi, pathways negatively regulate platelet responses by inhibiting intracellular Ca2+ and PKC activation through the modulation of Gq signaling pathways. Specifically, we demonstrate that Fyn plays a major role in this negatively regulatory pathway.
This work was supported, in whole or in part, by National Institutes of Health Research Grants HL60683, HL93231, and HL81322 (to S. P. K.).
- SFK
- Src family kinase
- 5,5′-dimethyl-BAPTA
- 5,5′-dimethyl-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- DMSO
- dimethyl sulfoxide
- 2-MeSADP
- 2-methylthio-ADP
- PAR
- protease-activated receptor
- PLC
- phospholipase C.
REFERENCES
- 1. Thomas S. M., Brugge J. S. (1997) Annu. Rev. Cell Dev. Biol. 13, 513–609 [DOI] [PubMed] [Google Scholar]
- 2. Boggon T. J., Eck M. J. (2004) Oncogene 23, 7918–7927 [DOI] [PubMed] [Google Scholar]
- 3. Golden A., Nemeth S. P., Brugge J. S. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 7844–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stenberg P. E., Pestina T. I., Barrie R. J., Jackson C. W. (1997) Blood 89, 2384–2393 [PubMed] [Google Scholar]
- 6. Clark E. A., Shattil S. J., Brugge J. S. (1994) Trends Biochem. Sci. 19, 464–469 [DOI] [PubMed] [Google Scholar]
- 7. Quek L. S., Pasquet J. M., Hers I., Cornall R., Knight G., Barnes M., Hibbs M. L., Dunn A. R., Lowell C. A., Watson S. P. (2000) Blood 96, 4246–4253 [PubMed] [Google Scholar]
- 8. Reddy K. B., Smith D. M., Plow E. F. (2008) J. Cell Sci. 121, 1641–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin H., Liu J., Li Z., Berndt M. C., Lowell C. A., Du X. (2008) Blood 112, 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. (2002) J. Cell Biol. 157, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shattil S. J. (2005) Trends Cell Biol. 15, 399–403 [DOI] [PubMed] [Google Scholar]
- 12. Maxwell M. J., Yuan Y., Anderson K. E., Hibbs M. L., Salem H. H., Jackson S. P. (2004) J. Biol. Chem. 279, 32196–32204 [DOI] [PubMed] [Google Scholar]
- 13. Garcia A., Quinton T. M., Dorsam R. T., Kunapuli S. P. (2005) Blood 106, 3410–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bauer M., Maschberger P., Quek L., Briddon S. J., Dash D., Weiss M., Watson S. P., Siess W. (2001) Thromb. Haemost. 85, 331–340 [PubMed] [Google Scholar]
- 15. Kim S., Jin J., Kunapuli S. P. (2006) Blood 107, 947–954 [DOI] [PubMed] [Google Scholar]
- 16. Garcia A., Shankar H., Murugappan S., Kim S., Kunapuli S. P. (2007) Biochem. J. 404, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shankar H., Garcia A., Prabhakar J., Kim S., Kunapuli S. P. (2006) J. Thromb. Haemost. 4, 638–647 [DOI] [PubMed] [Google Scholar]
- 18. Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. (1986) Science 231, 1431–1434 [DOI] [PubMed] [Google Scholar]
- 19. Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. (1981) Proc. Natl. Acad. Sci. U.S.A. 78, 6013–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paul B. Z., Daniel J. L., Kunapuli S. P. (1999) J. Biol. Chem. 274, 28293–28300 [DOI] [PubMed] [Google Scholar]
- 21. Buhl A. M., Johnson N. L., Dhanasekaran N., Johnson G. L. (1995) J. Biol. Chem. 270, 24631–24634 [DOI] [PubMed] [Google Scholar]
- 22. Bauer M., Retzer M., Wilde J. I., Maschberger P., Essler M., Aepfelbacher M., Watson S. P., Siess W. (1999) Blood 94, 1665–1672 [PubMed] [Google Scholar]
- 23. Brass L. F., Manning D. R., Cichowski K., Abrams C. S. (1997) Thromb. Haemost. 78, 581–589 [PubMed] [Google Scholar]
- 24. Brass L. F., Hoxie J. A., Kieber-Emmons T., Manning D. R., Poncz M., Woolkalis M. (1993) Adv. Exp. Med. Biol. 344, 17–36 [DOI] [PubMed] [Google Scholar]
- 25. Brass L. F., Woolkalis M. J., Manning D. R. (1988) J. Biol. Chem. 263, 5348–5355 [PubMed] [Google Scholar]
- 26. Walker T. R., Watson S. P. (1993) Biochem. J. 289, 277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trotter P. J., Orchard M. A., Walker J. H. (1995) Biochem. J. 308, 591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feinstein M. B., Becker E. L., Fraser C. (1977) Prostaglandins 14, 1075–1093 [DOI] [PubMed] [Google Scholar]
- 29. Feinstein M. B., Fraser C. (1975) J. Gen. Physiol. 66, 561–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wörner P., Brossmer R. (1975) Thromb. Res. 6, 295–305 [DOI] [PubMed] [Google Scholar]
- 31. Cho M. J., Liu J., Pestina T. I., Steward S. A., Thomas D. W., Coffman T. M., Wang D., Jackson C. W., Gartner T. K. (2003) Blood 101, 2646–2651 [DOI] [PubMed] [Google Scholar]
- 32. Quinter P. G., Dangelmaier C. A., Quinton T. M., Kunapuli S. P., Daniel J. L. (2007) J. Thromb. Haemost. 5, 362–368 [DOI] [PubMed] [Google Scholar]
- 33. Klages B., Brandt U., Simon M. I., Schultz G., Offermanns S. (1999) J. Cell Biol. 144, 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Offermanns S., Toombs C. F., Hu Y. H., Simon M. I. (1997) Nature 389, 183–186 [DOI] [PubMed] [Google Scholar]
- 35. Rasmussen U. B., Gachet C., Schlesinger Y., Hanau D., Ohlmann P., Van Obberghen-Schilling E., Pouysségur J., Cazenave J. P., Pavirani A. (1993) J. Biol. Chem. 268, 14322–14328 [PubMed] [Google Scholar]
- 36. Negrescu E. V., de Quintana K. L., Siess W. (1995) J. Biol. Chem. 270, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 37. Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. (1997) Nature 389, 990–994 [DOI] [PubMed] [Google Scholar]
- 38. Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., Kobori M. (2004) J. Biol. Chem. 279, 47438–47445 [DOI] [PubMed] [Google Scholar]
- 39. Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. (1996) J. Biol. Chem. 271, 695–701 [DOI] [PubMed] [Google Scholar]
- 40. Li Z., Zhang G., Liu J., Stojanovic A., Ruan C., Lowell C. A., Du X. (2010) J. Biol. Chem. 285, 12559–12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jen C. J., Chen H. I., Lai K. C., Usami S. (1996) Blood 87, 3775–3782 [PubMed] [Google Scholar]
- 42. Paul B. Z., Kim S., Dangelmaier C., Nagaswami C., Jin J., Hartwig J. H., Weisel J. W., Daniel J. L., Kunapuli S. P. (2003) Platelets 14, 159–169 [DOI] [PubMed] [Google Scholar]
- 43. Dorsam R. T., Kim S., Jin J., Kunapuli S. P. (2002) J. Biol. Chem. 277, 47588–47595 [DOI] [PubMed] [Google Scholar]
- 44. Nieswandt B., Schulte V., Zywietz A., Gratacap M. P., Offermanns S. (2002) J. Biol. Chem. 277, 39493–39498 [DOI] [PubMed] [Google Scholar]
- 45. Dorsam R. T., Kim S., Murugappan S., Rachoor S., Shankar H., Jin J., Kunapuli S. P. (2005) Blood 105, 2749–2756 [DOI] [PubMed] [Google Scholar]
- 46. Hardy A. R., Jones M. L., Mundell S. J., Poole A. W. (2004) Blood 104, 1745–1752 [DOI] [PubMed] [Google Scholar]
- 47. Jin J., Kunapuli S. P. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 8070–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F., et al. (1991) J. Biol. Chem. 266, 15771–15781 [PubMed] [Google Scholar]
- 49. Offermanns S., Laugwitz K. L., Spicher K., Schultz G. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 504–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moers A., Nieswandt B., Massberg S., Wettschureck N., Grüner S., Konrad I., Schulte V., Aktas B., Gratacap M. P., Simon M. I., Gawaz M., Offermanns S. (2003) Nat. Med. 9, 1418–1422 [DOI] [PubMed] [Google Scholar]
- 51. Gong H., Shen B., Flevaris P., Chow C., Lam S. C., Voyno-Yasenetskaya T. A., Kozasa T., Du X. (2010) Science 327, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin J., Mao Y., Thomas D., Kim S., Daniel J. L., Kunapuli S. P. (2009) Biochem. Pharmacol 77, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim S., Mangin P., Dangelmaier C., Lillian R., Jackson S. P., Daniel J. L., Kunapuli S. P. (2009) J. Biol. Chem. 284, 33763–33772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cho M. J., Pestina T. I., Steward S. A., Lowell C. A., Jackson C. W., Gartner T. K. (2002) Blood 99, 2442–2447 [DOI] [PubMed] [Google Scholar]