Abstract
Several missense mutations in the von Willebrand Factor (VWF) gene of von Willebrand disease (VWD) patients have been shown to cause impaired constitutive secretion and intracellular retention of VWF. However, the effects of those mutations on the intracellular storage in Weibel-Palade bodies (WPBs) of endothelial cells and regulated secretion of VWF remain unknown. We demonstrate, by expression of quantitative VWF mutants in HEK293 cells, that four missense mutations in the D3 and CK-domain of VWF diminished the storage in pseudo-WPBs, and led to retention of VWF within the endoplasmic reticulum (ER). Immunofluorescence and electron microscopy data showed that the pseudo-WPBs formed by missense mutant C1060Y are indistinguishable from those formed by normal VWF. C1149R, C2739Y, and C2754W formed relatively few pseudo-WPBs, which were often short and sometimes round rather than cigar-shaped. The regulated secretion of VWF was impaired slightly for C1060Y but severely for C1149R, C2739Y, and C2754W. Upon co-transfection with wild-type VWF, both intracellular storage and regulated secretion of all mutants were (partly) corrected. In conclusion, defects in the intracellular storage and regulated secretion of VWF following ER retention may be a common mechanism underlying VWD with a quantitative deficiency of VWF.
Keywords: Blood Coagulation Factors, Confocal Microscopy, Electron Microscopy (EM), Exocytosis, Genetic Diseases, Hemostasis, Protein Secretion, Subcellular Organelles, Von Willebrand Factor, Weibel-Palade Body
Introduction
The hemostatic protein von Willebrand factor (VWF)2 plays important roles in hemostasis by facilitating platelet adhesion to injured endothelium and by carrying coagulation factor VIII (FVIII) to protect it from rapid proteolytic inactivation (1). A defect in VWF leads to the most common inherited human bleeding disorder, von Willebrand disease (VWD) (2). VWD is classified into 3 types: types 1 and 3 are quantitative VWD characterized by defects that result in a partial (type 1) or virtually complete (type 3) deficiency of VWF in plasma; type 2 VWD is caused by defects that result in qualitatively different VWF with abnormal function (2). Among all the index cases, type 1 VWD is the most common form. Missense mutations compose the majority of mutations causing type 1 VWD (up to 75%), but only a minority of the mutations causing type 3 VWD (3–6). In the latter cases nonsense mutations and deletions predominate.
VWF is synthesized as a precursor protein containing a signal peptide (22-amino acids), an N-terminal propeptide (D1 and D2 domains, 741 amino acids) and a mature peptide comprising multiple domains (D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK, 2050 amino acids) (7). Following its synthesis in the endoplasmic reticulum (ER) proVWF (after cleavage of the signal peptide) dimerizes via formation of intermolecular disulfide bridges in the cysteine knot (CK) domain (1). ProVWF dimers are transported to the Golgi-apparatus; in this compartment propeptide-mediated assembly of multimers occurs through formation of intermolecular disulfide bridges within the D′D3 domains (1). Processing of proVWF presumably by furin occurs in the trans-Golgi network. In the same compartment, VWF is either secreted constitutively or tubulized and packaged into Weibel-Palade bodies (WPBs) for basal and regulated secretion (1, 8, 9). After stimulation, high molecular weight (HMW) VWF multimers released from WPBs rapidly form platelet-catching VWF filaments on the endothelial surface (10). Quantitative or qualitative defects in assembly of HMW VWF multimers contribute to the bleeding tendency in VWD (11–13).
Ectopic expression of VWF in non-endothelial cell lines, like HEK293, leads to the formation of so-called pseudo-WPBs that resemble WPBs in endothelial cells (14–17). Such cell-line models have been proven useful for the study of VWF and e.g. led to the discovery that the non-covalent interaction between the propeptide (D1-D2 domains) and the D′-D3-A1 domains of VWF is essential for tubulation and storage of VWF into WPBs (18, 19).
Missense mutations in VWF have been identified in quantitative as well as qualitative VWF defects. We and others (20–30) have shown that the missense mutations that cause quantitative VWD (type 1 and 3) impair constitutive secretion of VWF. However, the effects of such mutations on WPB formation, VWF tubulation, and regulated WPB secretion remain unknown. Moreover, the effects of type 1/3 VWD-causing VWF mutations on the formation and secretion of WPBs have been neglected in most studies. The aim of our study was to elucidate whether quantitative VWF deficiencies in VWD are due to ER retention and/or lack of WPB formation, or accompanied by the formation of morphologically abnormal WPBs that led to a malfunctioning (regulated) secretion of VWF.
We have studied quantitative VWF missense mutations in HEK293 cells: C1060Y and C1149R in the D3 domain that may affect the non-covalent interaction between the propeptide and mature VWF, and C2739Y and C2754W in the CK-domain that interfere with dimerization of VWF. Based on our results, we propose a common pathogenic mechanism for VWD with quantitative deficiencies of VWF.
EXPERIMENTAL PROCEDURES
Patients and Mutations
The mutations investigated in this study were originally identified in VWD patients. The mutation in exon 24, c.3179G>A, causes a cysteine substitution into tyrosine (p.C1060Y) and was identified in a heterozygous type 1 VWD participant of the MCMDM-1VWD study (31). p.C1149R was identified in heterozygous type 1 VWD patients with moderately severe bleeding tendencies and was reported before (20). p.C2739Y was described in a compound heterozygous type 3 VWD patient with the other allele carrying a premature stop codon (32). p.C2754W was identified in a homozygous type 3 VWD patient (33).
Plasmid Constructs
Recombinant pSVH expression plasmids containing full-length cDNAs encoding either wild-type human VWF (WT-VWF) or C1149R, C2739Y and C2754W VWF variants have been described before (23). The full-length VWF cDNA fragments, obtained by EcoRI restriction of these pSVH-VWF plasmids, were cloned into the pCI-neo mammalian expression vector (Promega, Madison, WI). Mutation C1060Y was introduced into pCI-neo WT-VWF plasmid with the QuikChange XL Site-directed Mutagenesis kit (Stratagene, La Jolla, CA).
Cell Culture and Transfection
HEK293 cells were purchased from the ATCC and cultured in Minimum Essential Medium α Medium (α-MEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum, 50 μg/ml gentamicin (Invitrogen). Human umbilical vein endothelial cells (HUVECs) were obtained as described previously (34) and cultured in EGM-2 medium (Lonza, Breda, The Netherlands) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin (Invitrogen). HEK293 cells were seeded and 24 h later transfected using FuGENE HD transfection reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. For transient transfection, the growth medium was refreshed 24 h after transfection, and cells were grown for another 48 h before analysis (which means 72 h after the actual transfection). All the data in the present study were collected 72 h post-transfection unless otherwise stated. The stably transfected cells were selected by G418 (Invitrogen) for 3∼5 weeks.
Immunofluorescence Analysis
HEK293 cells were seeded on 1% gelatin pre-coated glass coverslips in 24-well plates. Cells were fixed 72 h post-transfection with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) for 30 min on ice. Cells were rinsed with 50 mm Tris-HCl (pH 7.4), and permeabilized with 0.02% saponin (Sigma-Aldrich) in phosphate buffer (pH 7.4) for 20 min at room temperature. Following 10 min blocking with 5% normal goat serum (DAKO, Glostrup, Denmark), cells were incubated with first antibodies and then with fluorescence-conjugated secondary antibodies. Monoclonal antibody CLB-RAg35 was used to visualize VWF (35). Polyclonal rabbit anti-human protein-disulfide isomerase (PDI) antibody A66 (obtained from Prof. I. Braakman, Department of Chemistry, Utrecht University, Utrecht, The Netherlands) was used to visualize the ER (36). Monoclonal anti-GM130 (BD Biosciences, CA) and polyclonal rabbit anti-human TGN46 (Sigma-Aldrich) were used to visualize cis- and trans-Golgi networks, respectively. Monoclonal mouse anti-LAMP1 (mAb BB6; kindly provided by Dr. S. Carlsson, Umeå, Sweden) was used to visualize lysosomes (37). Alexa 488- and Alexa 594-conjugated secondary antibodies were purchased from Invitrogen. Cells were embedded by Aqua-Poly/Mount medium (Polysciences, GmbH, Germany) and analyzed by Leica SL confocal laser scanning microscopy with a 63X/1.40 NA oil objective. To quantify pseudo-WPBs, 150∼300 VWF-positive cells from two independent experiments were randomly selected and analyzed. We used similar morphological criteria as reported by Michaux et al. (17). Briefly, we categorized the VWF-positive but PDI-negative granules as pseudo-WPBs based on the immunofluorescent staining, and the pseudo-WPBs with a ratio of length over diameter <2 as “round pseudo-WPBs” and those with a ratio ≥2 as “elongated pseudo-WPBs.” To quantify the ER retention of VWF, cells were divided into three categories: the cells with all VWF co-stained with PDI as “ER,” the cells with pseudo-WPBs only but no ER retention as “WPB” and the cells with pseudo-WPBs and ER retention as “WPB+ER.”
Electron Microscopy
HEK293 cells were seeded on 35 mm Petri dishes and fixed 72 h post-transfection (overnight at 4 °C) with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 m phosphate buffer (pH 7.4). Cells were then prepared for electron microscopy as previously described (38). Briefly, cells were post-fixed for 60 min with 1% osmium tetroxide and for 30 min with 1% tannic acid. The samples were then dehydrated with serial concentrations of ethanol and embedded in Epon. 70–100 nm sections were cut by means of a Leica Ultracut UC6 microtome and placed on carbon and formvar-coated copper grids (one slot). Sections were stained with uranyl acetate and lead citrate, and then visualized with a Tecnai 12 at 120 kV equipped with a 4kx4k CCD camera (Model Eagle, Fei Company, The Netherlands).
Basal and Regulated Secretion
For the basal secretion, the conditioned media and cell lysates were collected 72 h post-transfection. To study the regulated secretion of VWF, 72 h post-transfection cells were rinsed twice with pre-warmed release medium (OPTIMEM1 medium, 10 mm HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.2% bovine serum albumin, pH 7.4) and incubated for 60 min with the release medium in the absence or presence of phorbol-12-myristate-13-acetate (PMA) (Sigma-Aldrich) at a final concentration of 160 nm. The media were collected for the assay of secreted VWF. To quantify intracellular VWF, cells were rinsed and then lysed overnight at 4 °C in Passive Lysis Buffer (PLB, Promega) supplemented with the protease inhibitor mixture CompleteTM with EDTA (Roche). Cell lysates were collected and vortexed for 10 s. All the media and cell lysates were centrifuged for 5 min at 14,000 × g. The supernatants were supplemented with phenylmethylsulfonyl fluoride (PMSF) (Roche) at a final concentration of 100 μm, and then analyzed immediately or snap-frozen. VWF:Ag was measured by ELISA as previously described (23). To calculate the regulated secretion of VWF during stimulation, secreted VWF was expressed as a fraction of total VWF (% of total = absolute VWF:Ag in the medium/(absolute VWF:Ag in the medium + absolute VWF:Ag in the cell lysate) × 100).
Western Blot Analysis of VWF
Seventy-two hours post-transfection the growth medium was replaced with release medium (see above). Twenty-four hours later, the conditioned media and cell lysates were collected as described above. VWF multimers were analyzed by non-reducing 1.6% agarose gel electrophoresis with sodium dodecyl sulfate (SDS) followed by Western blot as described (23). To analyze subunit composition of VWF, samples were reduced by incubation at 95 °C for 5 min in sample buffer containing 20 mm dithiothreitol and separated on Novex 4% Tris-glycine gel (Invitrogen). Reduced VWF was immunostained with polyclonal rabbit anti-human VWF conjugated to horseradish peroxidase (DAKO) and visualized with Supersignal WestFemto (Thermo Scientific, Rockford, IL).
RESULTS
Basal Secretion of VWF Variants
Basal secretion of VWF variants was investigated in transiently transfected HEK293 cells. The amount of VWF:Ag in medium or lysate is shown in Table 1. Compared with WT-VWF, secretion of C1060Y in the medium was decreased (<65% of WT-VWF), whereas the secretion of the other three variants was more severely impaired (<15% of WT-VWF). There was almost no detectable secretion of the C1149R and C2739Y mutated proteins. The readily detectable presence of C1149R and C2739Y proteins in the cell lysates indicated that those mutants were in fact synthesized but retained intracellularly. The total absolute amount of VWF:Ag measured in medium and lysate, however, was decreased. As no VWF was detectable by immunofluorescent staining in lysosomes or the Golgi apparatus (data not shown), the VWF mutants may have been degraded via the proteasome-associated pathway that has been implicated in the degradation of C1149R in an earlier study (39).
TABLE 1.
Expression of VWF variants in transfected HEK293 cells
Each value represents the mean ± S.E. of three independent experiments in duplicate. Co-transfections were in a 1:1 ratio with WT-VWF cDNA.
| Variant | Transfection | VWF:Aga medium | VWF:Aga lysate | ||
|---|---|---|---|---|---|
| mU | % | mU | % | ||
| WT-VWF | 92.5 ± 3.7 | 100 | 38.2 ± 5.6 | 100 | |
| C1060Y | Single transfection | 57.4 ± 1.8 | 62.3 ± 4.2 | 37.9 ± 10.5 | 96.3 ± 13.7 |
| Co-transfection | 80.7 ± 7.2 | 88.0 ± 10.9 | 39.1 ± 2.0 | 104.9 ± 8.8 | |
| C1149R | Single transfection | 2.7 ± 1.5 | 3.1 ± 1.6 | 51.2 ± 10.8 | 139.1 ± 38.3 |
| Co-transfection | 39.7 ± 2.0 | 43.2 ± 3.9 | 56.3 ± 2.7 | 151.6 ± 16.3 | |
| C2739Y | Single transfection | 0.5 ± 0.3 | 0.7 ± 0.4 | 34.1 ± 6.5 | 88.8 ± 8.2 |
| Co-transfection | 52.1 ± 2.8 | 56.8 ± 5.4 | 39.1 ± 3.2 | 104.5 ± 9.5 | |
| C2754W | Single transfection | 11.4 ± 1.3 | 12.3 ± 1.0 | 43.0 ± 6.5 | 113.2 ± 9.4 |
| Co-transfection | 58.4 ± 6.2 | 63.9 ± 9.5 | 47.9 ± 1.7 | 129.4 ± 14.4 | |
a VWF:Ag (mU) was produced by about 7 × 105 cells in the medium or lysate. In parallel, VWF:Ag is expressed as percentage relative to the amount of VWF:Ag in the medium and lysate of cells expressing WT-VWF.
To mimic heterozygosity, co-transfections were performed with WT-VWF (Table 1). The secretion of VWF after C1060Y/WT co-transfection was close to WT-VWF only, and in the case of C1149R/WT, C2739Y/WT, and C2754W/WT co-transfections yielded levels of about 40–65% of WT-VWF.
Intracellular Distribution of VWF in Transfected HEK293 Cells
The intracellular localization of VWF in transfected HEK293 cells was analyzed with confocal immunofluorescence microscopy by co-staining of VWF and the ER marker PDI. Upon transfection HEK293 cells formed VWF storage vesicles, so-called pseudo-WPBs that resemble WPBs in endothelial cells (Fig. 1A and 2, A and B) (17). Seventy-two hours post-transfection almost all cells that expressed WT-VWF stored VWF in elongated pseudo-WPBs (Fig. 1A). Granular staining (non-overlapping with PDI) resembling pseudo-WPBs was observed to a varying extent for the four VWF mutants C1060Y, C1149R, C2739Y, and C2754W (Fig. 1, B–E). The pseudo-WPBs formed by C1060Y were indistinguishable from those formed by WT-VWF (Fig. 1B). The pseudo-WPBs formed by the other mutants, C1149R, C2739Y, and C2754W were shorter and often round (Fig. 1, C–E). Compared with WT-VWF and C1060Y, mutations C1149R, C2739Y, and C2754W led to retention of much of the produced VWF in the ER (Fig. 1, A–E). Upon co-transfection with WT-VWF (Fig. 1, F–I), the impaired storage of VWF variants was corrected to a variable extent with the majority of cells containing VWF in both pseudo-WPBs and ER.
FIGURE 1.
Intracellular distribution of WT-VWF and variants in transfected cells. HEK293 cells were fixed for immunofluorescence imaging 72 h after single transfection (A–E) or co-transfection of VWF variants with WT-VWF (F–I). Fixed cells were stained for VWF (green channel, left panel) and for PDI (ER marker, red channel, middle panel). In the right panel (merge of green and red channels), the pseudo-WPBs show up in green (VWF staining only), and the ER containing VWF shows up in yellow as a result of double staining with ER marker PDI. Scale bars, 5 μm.
These observations were further confirmed by transmission electron microscopy (TEM). In WT-VWF-transfected cells, elongated and electron dense pseudo-WPBs were observed with internal striations, indistinguishable from those found in endothelial cells (Fig. 2, A and B). Pseudo-WPBs were also observed in the cells following expression of each of the VWF mutants (Fig. 2, C–E) except for mutant C2739Y for which we were not able to visualize with certainty organelles with internal tubules. In accordance with the light microscopical data, most of the pseudo-WPBs observed in the cells expressing mutants were much shorter than those in WT-VWF-transfected cells. Strikingly, in the C2754W-transfected cells the majority of VWF storage vesicles visualized by TEM were rather round instead of elongated (74 of 80 pseudo-WPBs are round, i.e. 93%) (Fig. 2E and supplemental Fig. S1C). In contrast, 4 out of 47 pseudo-WPBs (i.e. 9%) were round for WT-VWF. Those round vesicles reflect pseudo-WPBs as they contain disorganized tubular structures which suggest the storage of VWF tubules, as shown by tilting the sample (Fig. 2F, i—i″). Similar round-shaped pseudo-WPBs were also observed in C1149R-transfected cells, although to a lesser extent (7 of 24, i.e. 29%). Furthermore, grossly dilated ER was observed in many cells expressing the VWF mutants, although rarely for C1060Y (Fig. 3).
FIGURE 2.
(Pseudo-) WPBs visualized by TEM. HUVECs were fixed and analyzed with TEM (A). HEK293 cells were fixed 72 h post-transfection with WT-VWF (B), C1060Y (C), C1149R (D), or C2754W (E and F, i-i″) and analyzed with TEM. In C2754W-transfected cells, the majority of storage vesicles are round (E). The presence of tubular structures inside the round vesicles was confirmed by tilting the sections (F). In panel F, HEK293 cells transiently expressing C2754W or stably expressing C1149R were analyzed with TEM. The sample is 100 nm thick and visualized at 0° tilt (i, ii), minus 30° tilt (i′, ii′) or plus 30° tilt (i″, ii″). Note in C2754W one short hinged tubule indicated by arrows (i), two transverse tubules (hollow rings, i′), and two short longitudinal (vertical) tubules (i″). In C1149R, the transversally sectioned tubules (rings) are indicated by arrows in the right pseudo-WPB (ii) and in the left pseudo-WPB (ii′). By tilting the stage +30°, two short longitudinal tubules appeared in the right pseudo-WPB and some longer tubules in the left one (indicated by arrows in ii″). The relatively fewer and shorter tubules in the round pseudo-WPB suggest the storage of VWF in disarray. In panels A–E, scale bars, 200 nm; in panel F, scale bars, 100 nm.
FIGURE 3.
ER visualized by TEM. HEK293 cells were fixed and analyzed as in Fig. 2. The morphology of the ER is normal in mock (A) or WT-VWF transfected cells (B), but dilated ER was observed in the cells following expression of each of the five mutants (C–F). Note the grossly dilated ER with thin tails in panel F. The arrows, arrowheads, and stars indicate the pseudo-WPBs, ER and mitochondria, respectively (panels A–F). Scale bars, 500 nm.
Impaired WPB Formation and Increased Intracellular Retention
We randomly selected confocal microscopy fields and analyzed 150∼300 cells from each sample for the quantification of VWF storage patterns. First, we analyzed the formation of pseudo-WPBs per se following expression of WT-VWF or VWF variants. The proportion of cells containing more than 30 pseudo-WPBs per cell was over 70% in WT-VWF-transfected cells, which was much higher than for the VWF variants (Fig. 4A).
FIGURE 4.
Defects in the pseudo-WPBs formation. Transfected HEK293 cells were fixed and stained as described in Fig. 2, 72 h after the single transfection (A, B) or co-transfection (C, D). Images were randomly taken by the confocal microscopy and all transfected cells in the images were selected for quantitative analysis. The selected cells were divided into three categories according to the number of pseudo-WPBs per cell (A, C): more than 30; from 11 to 30; fewer than 10 pseudo-WPBs per cell; or according to the number of elongated pseudo-WPBs per cell (B, D): more than 4; from 1 to 4; none elongated pseudo-WPBs per cell. The graph represents the percentage of cells in each category. 150∼300 cells expressing VWF variants were analyzed for each bar. Each bar represents the mean of two independent experiments.
To further characterize the effects of the mutations on the elongation of pseudo-WPBs, cells were categorized according to the number of elongated pseudo-WPBs per cell. The proportion of cells containing more than 4 elongated pseudo-WPBs was decreased for all mutants (Fig. 4B). Interestingly, the number of pseudo-WPBs formed by C1060Y per cell was comparable to C2754W (Fig. 4A), but the proportion of cells containing more than 4 elongated pseudo-WPBs was much lower for the latter (68% for C1060Y versus 18% for C2754W), indicating that the majority of the pseudo-WPBs formed by C2754W were rather round (Fig. 4B). Upon co-transfection with WT-VWF, the defects in the number and elongation of the pseudo-WPBs caused by the mutations were partly corrected (Fig. 4, C and D).
Finally, the retention of VWF variants in the ER was quantified. As shown in Fig. 5B, only a small population of cells expressing WT-VWF (less than 10%) showed that part of the VWF was located in the ER, which may be due to protein overexpression in HEK293 cells. The proportion of cells with VWF located in the ER was slightly increased by C1060Y compared with that by WT-VWF, whereas it was drastically increased by C1149R, C2739Y, and C2754W. It indicates the latter three mutations caused a marked retention of VWF in the ER with less VWF stored in the pseudo-WPBs (Fig. 5B).
FIGURE 5.
Retention of VWF in the ER. According to the intracellular location of VWF in transfected HEK293 cells, the selected cells were classified into 3 categories (A): WPB represents cells with all intracellular VWF stored in pseudo-WPBs (green); WPB+ER represents cells with intracellular VWF located in both ER (yellow) and pseudo-WPBs (green); and ER represents cells with all intracellular VWF located in ER (yellow). Scale bars, 5 μm. B and C, graph represents the percentage of cells in each category. 150∼300 cells expressing VWF variants were analyzed for each bar. Each bar represents the mean of two independent experiments.
The defects in VWF storage caused by the mutations were a net effect of impaired pseudo-WPBs formation, lack of elongation of pseudo-WPBs, and increased ER retention of VWF. All three aspects were corrected to different extents by co-transfection of these variants with WT-VWF (Figs. 4, C and D and 5C).
Regulated Secretion of VWF
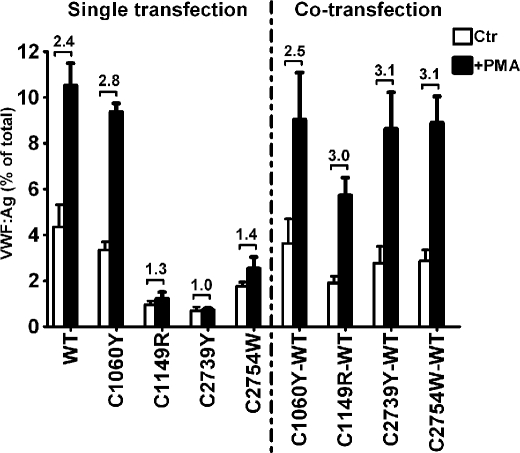
Release of pseudo-WPBs from HEK293 cells can be induced by secretagogues such as PMA (17). Incubation of HEK293 cells for 60 min with PMA increased the secretion of WT-VWF from 4.3% (Ctr) to 10.5% (+PMA), a 2.4 times increase (Fig. 6). The induced secretion under stimulation was slightly lower with C1060Y, but much less with the other three mutations. No detectable induced release was observed by stimulation of C1149R- or C2739Y-transfected cells. In co-transfections of all four variants with WT-VWF at a 1:1 ratio, PMA stimulation resulted in a 2.5 to 3.1 times increase of secretion over unstimulated cells, comparable to WT-VWF. However, the percentage of total VWF that was secreted, basal (Ctr) as well as stimulated (+PMA), was only partly restored in co-transfections of C1149R.
FIGURE 6.
Effects of quantitative mutations on the regulated secretion of VWF. Seventy-two hours post-transfection, HEK293 cells were rinsed twice with the release medium and incubated at 37 °C for 60 min in the release medium without (Ctr) or with 160 nm PMA (+PMA). Then the release media and cell lysates were collected as described under “Experimental Procedures.” Each bar represents VWF secreted into the release medium as a fraction of total VWF (secreted plus intracellular) ± S.E. (three independent experiments in duplicate). The numbers above the bars indicate the fold increase of secreted VWF comparing the stimulated (+PMA) and control (Ctr) samples.
Multimeric Analysis of VWF
The full range of VWF multimers was present in both the conditioned medium and the lysate of WT-VWF-transfected HEK293 cells (Fig. 7, A and B). The pattern was comparable to the multimers obtained from HUVECs (Fig. 7B), confirming the validity of the HEK293 system for the study of synthesis and storage of HMW multimers.
FIGURE 7.
Electrophoretic characteristics of VWF in the media and lysates. Seventy-two hours after the single transfection (A, B, E) or co-transfection (C, D), HEK293 cells were rinsed and incubated with the release medium at 37 °C. After 24 h, media (A, C) and lysates (B, D) were collected and analyzed under non-reducing conditions. Normal pooled plasma (NPP) was used as control for the multimer analysis. The arrowheads in panels A, B, and C indicate the odd-numbered multimers. In panels A and B the parts separated by the line between C1060Y and C1149R were from the same gel. In panels A and C, the lanes for C2754W are from another experiment showing more clearly the odd-numbered multimers. In panel E, VWF was analyzed under reducing conditions. Lanes 1 (from a different gel) and 2 show WT-VWF in the conditioned medium and lysate, respectively; lanes 3–6 show C1060Y, C1149R, C2739Y, and C2754W in the lysate, respectively.
Compared with WT-VWF, C1060Y led to the loss of the largest multimers in secreted VWF (Fig. 7A). C1149R and C2754W showed more severe defects in the VWF multimerization with mainly dimers for C1149R and abundant monomers and dimers as well as additional trimers and tetramers for C2754W. The VWF levels in media were too low to visualize multimers for C2739Y (Fig. 7A). The multimer patterns of VWF in the lysates were comparable to the patterns in conditioned media for all the VWF variants, except for C2739Y, which could not be visualized in medium and showed mainly monomers in the cell lysate (Fig. 7B).
Co-transfection of each of the variants with WT-VWF yielded a wide range of multimers (Fig. 7, C and D). Interestingly, C2754W/WT-VWF yielded additional odd-numbered multimers in the medium (Fig. 7C). As shown before, trimers and higher odd-numbered multimers in C2754W result from N-terminal disulfide bond formation of the excess of monomers (40). The appearance of the larger odd-numbered multimers is clear evidence for the actual formation of heterodimers and heteromultimers in the co-transfections.
To confirm correct processing of VWF in HEK293 cells, we performed reducing SDS-PAGE. The secreted VWF is mainly mature VWF, while in the cell lysates mature VWF as well as uncleaved proVWF was shown for WT-VWF and C1060Y (Fig. 7E). The other mutants showed primarily uncleaved proVWF in the cell lysates (Fig. 7E). This is consistent with the observation that VWF is mainly stored in pseudo-WPBs for WT-VWF and C1060Y but retained in the ER for the other three mutants (Fig. 5B).
DISCUSSION
In this study, we analyzed the intracellular storage and regulated secretion of VWF. Our findings highlight the detailed effects of VWF mutations on the formation of WPBs and on the basal and regulated secretion of VWF. Through these mechanisms the pathogenic nature of those mutations in VWD are unraveled. Because of the lack of WPB formation in most expression systems that have been extensively applied (21, 41), the current concept of the pathogenesis of VWF mutations identified in VWD are mainly based on the expression data on the constitutive secretion, synthesis, and multimer patterns of VWF. However, the main source of VWF in the circulation are the endothelial cells that synthesize and store VWF into WPBs and secrete VWF into the circulation through basal and regulated pathways (8, 9). We therefore believe that the analysis of VWF mutations in an expression system, like HEK293 cells, that can form WPB like storage organelles will yield additional insight into the pathogenic nature of VWF mutations (17).
We confirmed the validity of the HEK293 cell model. Upon expression of WT-VWF the HEK293 cells formed intracellular storage organelles, pseudo-WPBs, which were indistinguishable from endothelial WPBs. Furthermore, these pseudo-WPBs stored multimeric VWF, displayed an elongated shape with a size of 100∼200 nm by 1∼5 μm, contained internal striations representing VWF tubules, and actively secreted stored VWF upon PMA-stimulation. By expression of the four VWF mutants in HEK293 cells, we found that intracellular accumulation of VWF mutants was associated with ER retention, dilation of the ER, and disturbed WPB formation (fewer and morphologically abnormal pseudo-WPBs). These defects in VWF routing were accompanied by reduced basal and regulated secretion. The combined effects are summarized in Table 2.
TABLE 2.
Summary of defects caused by VWF mutants compared to WT-VWF
| Domain | Variant | VWD type | ER retention | Pseudo-WPB formation |
Secretion of VWF |
Secreted multimers | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | Elongated shaped | Tubular storagee | Basal | Stimulated | |||||
| D3 | C1060Y | 1a | ↑ | ↓ | ↓ | Normal | ↓ | Normal/↓ | Lack of HMW multimers |
| CK | C2754W | 3b | ↑↑ | ↓ | ↓↓ | Severely affected | ↓↓ | ↓↓ | Mainly LMW multimersf |
| D3 | C1149R | 1a | ↑↑↑ | ↓↓↓ | ↓↓ | Normal but shorter | ↓↓↓ | ↓↓↓ | Mainly dimers |
| CK | C2739Y | 3c | ↑↑↑ | ↓↓↓ | ↓↓↓ | Not confirmed | ↓↓↓ | ↓↓↓ | Not detectable |
a Patient heterozygous.
b Patient homozygous.
c Patient compound heterozygous for a prematrure stop codon on the other allele.
d Based on the immunofluorescent analysis.
e Based on the TEM analysis.
f Showed odd-numbered multimers.
The ER retention and dilated ER are most likely caused by the VWF mutations per se as dilated ER was neither observed in mock-transfected cells nor in cells expressing WT-VWF (Fig. 3). Dilated ER is unlikely to be the result of delayed exiting of mutant VWF from the ER, as prolonged culturing of cells after transient transfection for 72 h, or even for a much longer period of up to 6 days for stably transfected cells, did not result in reduction of ER retention nor in more pseudo-WPB formation (supplemental Fig. S2).
The retention of VWF mutants in the ER also impaired storage into WPBs and trafficking of VWF into the regulated secretion pathway. Compared with WT-VWF, the mutations strongly decreased the number of pseudo-WPBs (C1149R and C2739Y) as well as the length of these organelles (C1149R, C2739Y, and C2754W) (Fig. 4 and Table 2). Diminished post-Golgi storage of these mutants was also confirmed by the presence of abundant uncleaved proVWF in the cell lysates (Fig. 7E). Because pseudo-WPBs resemble endothelial WPBs and likely serve as the releasable pool of VWF upon stimulation (11, 17), decrease in the size of this releasable pool caused by the VWF mutations explains the attenuated regulated secretion of VWF (Fig. 6 and Table 2). The impaired elongation of pseudo-WPBs may also attenuate the regulated secretion of VWF. It has been shown that rounding of WPBs by disturbing intracellular pH environment with monensin does not affect the regulated secretion of VWF (18), however, the effects of the disturbance in elongation of WPBs by specific mutations of VWF are currently unclear. We speculate that round shaped WPBs caused by VWF mutations may be functionally different from the shape changes induced by modulation of intra-organellular pH.
We observed that a substantial number of pseudo-WPBs formed by C1149R were shorter and/or round (Figs. 2, D and Fii-ii″ and 4C and supplemental Fig. S2). The short pseudo-WPBs may reflect the formation of shorter or less rigid VWF tubules by this mutant. VWF C1149R might have disrupted tubulation and storage of VWF in the cells due to the decreased binding of mature VWF C1149R to the propeptide (42), which has been shown to be essential for the unique storage of VWF in compacted tubules, resulting in the elongated shape of WPBs (18, 19).
The elongation of WPBs depends on the correct tubulation of VWF. Expression of truncated VWF constructs has shown that the D1D2D′D3A1 domains are sufficient to form elongated pseudo-WPBs (18) and the remaining part of VWF is supposed to form the matrix between tubules (19). In this study, we found that two natural mutations in the CK domain of VWF, C2739Y, and C2754W, severely disturbed the elongation of pseudo-WPBs. In particular, C2754W formed numerous round pseudo-WPBs (93% of total pseudo-WPBs observed by TEM) with tubules in disarray (Fig. 2, E, Fi-i″, and supplemental Fig. S1C). The defects in elongation of these pseudo-WPBs may be partially due to relatively low concentrations of these variants available for tubulation in the trans-Golgi network as abundant VWF was retained in the ER (Figs. 1, D and E, 3, E and F, and 5B). The round pseudo-WPBs in C2754W-transfected cells are not just explained by immaturity of pseudo-WPBs as immature pseudo-WPBs in WT-VWF-transfected cells showed regular and parallel tubules (supplemental Fig. S1, A and B). Interestingly, round pseudo-WPBs formed by C2754W showed disorganized storage of VWF tubules, which were different from the transversely cut WPBs (38, 43, 44) or VWF subunit-containing structures (44). The disordered VWF tubules suggest that C2754W deteriorates the matrix between VWF tubules, and further disturbs the rigidity of VWF tubules. As proposed, the rigidity of VWF tubules is responsible for the elongation of WPBs (45), therefore, we argue that disturbance of the matrix by mutations in the CK domain, in addition to the D1D2D′D3A1 domains, might also limit the length of WPBs.
HMW VWF multimers are released from WPBs at sites of vascular damage in response to secretion stimuli like thrombin, stress, vasopressin, or its synthetic analog desmopressin (DDAVP), which is used in the treatment of VWD. The formation and exocytosis of WPBs are therefore important in determining the VWD phenotype as well as treatment. The expression data predict that patients heterozygous for C1060Y or C1149R (mimicked by co-transfections with WT-VWF) have a mild reduction in numbers of endothelial WPBs and an intermediate response to DDAVP (46), whereas the patients who are compound heterozygous for C2739Y or homozygous for C2754W will have severely reduced numbers and aberrant WPBs and will display a poor response to DDAVP. The VWF level detected in the medium in co-transfections is higher than may be expected based on the plasma VWF:Ag levels observed in the patients, but this is explained by the fact that in the expression system there is no contribution of clearance. Increased clearance has been implicated in several type 1 VWD patients before and specifically has been shown for C1149R (42). The increased clearance contributes to the dominant-negative effects of the mutations.
As shown in other expression systems (20, 23, 47), missense mutations in VWF lead to a structural change of the protein which may result in multimer abnormality in single transfections as seen in the present study. However, the range of VWF multimers in the type 1 VWD patients heterozygous for C1060Y and C1149R, respectively, was recapitulated in the co-transfections with only a marginal decrease of the largest multimers that mimic the heterozygous states in the patients (Fig. 7C). Mutations C2739Y and C2754W are known to disrupt the dimerization of VWF and result in abnormal multimerization (23), while the extremely low levels of VWF secretion corresponded to the phenotype of VWD type 3 patients (32, 33). Thus, even though the single transfections show abnormalities at the multimer level, the main effect of the mutations on the phenotype is quantitative.
In conclusion, our results show that disrupted trafficking of VWF to WPBs (and/or formation of aberrant WPBs) and consequently a decreased regulated secretion of VWF is a potentially common pathogenic mechanism in some VWD subtypes with quantitative deficiencies of VWF. Although we have focused on mutations involving cysteines, also non-cysteine mutations may be implicated in similar mechanisms as was suggested for R273W (17) and R854W (48). Accompanying effects on WPB secretion and VWF string formation may also contribute to the clinical phenotype, and therefore the effects of VWF mutations on these aspects need further study. Analysis of the WPB formation and function can be a promising way to evaluate the pathogenic nature of VWF mutations and may bring more new insights into the mechanisms underlying VWD.
Supplementary Material
Acknowledgments
We thank members of the Dept. of Molecular Cell Biology, Leiden University of Medical Center, Erik Bos, Karen A. Jansen, Jos J. M. Onderwater, Frank G. A. Faas, Jan C. M. Slats, Annelies van der Laan for expert technical assistance and Jack A. Valentijn for helpful discussion and help with the confocal imaging. We thank Hans Vos from the Dept. of Thrombosis and Hemostasis for helpful suggestions to make plasmids. We thank Annemarie van Oeveren-Rietdijk from the Dept. of Nephrology, Leiden University of Medical Center, for providing the HUVECs.
This work was supported by a grant from the China Scholarship Council (2007U21083) and in part, by a grant from the Netherlands Organization for Scientific Research (NWO), Grant No. 91209006.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- VWF
- von Willebrand factor
- WPB
- Weibel-Palade body
- VWD
- von Willebrand disease
- ER
- endoplasmic reticulum
- PMA
- phorbol-12-myristate-13-acetate
- DDAVP
- desmopressin (1-deamino-8-d-arginine vasopressin).
REFERENCES
- 1. Sadler J. E. (1998) Annu. Rev. Biochem. 67, 395–424 [DOI] [PubMed] [Google Scholar]
- 2. Sadler J. E., Budde U., Eikenboom J. C., Favaloro E. J., Hill F. G., Holmberg L., Ingerslev J., Lee C. A., Lillicrap D., Mannucci P. M., Mazurier C., Meyer D., Nichols W. L., Nishino M., Peake I. R., Rodeghiero F., Schneppenheim R., Ruggeri Z. M., Srivastava A., Montgomery R. R., Federici A. B. (2006) J. Thromb. Haemost. 4, 2103–2114 [DOI] [PubMed] [Google Scholar]
- 3. Cumming A., Grundy P., Keeney S., Lester W., Enayat S., Guilliatt A., Bowen D., Pasi J., Keeling D., Hill F., Bolton-Maggs P. H., Hay C., Collins P. (2006) Thromb. Haemost. 96, 630–641 [PubMed] [Google Scholar]
- 4. Goodeve A., Eikenboom J., Castaman G., Rodeghiero F., Federici A. B., Batlle J., Meyer D., Mazurier C., Goudemand J., Schneppenheim R., Budde U., Ingerslev J., Habart D., Vorlova Z., Holmberg L., Lethagen S., Pasi J., Hill F., Hashemi S. M., Baronciani L., Hallden C., Guilliatt A., Lester W., Peake I. (2007) Blood. 109, 112–121 [DOI] [PubMed] [Google Scholar]
- 5. James P. D., Notley C., Hegadorn C., Leggo J., Tuttle A., Tinlin S., Brown C., Andrews C., Labelle A., Chirinian Y., O'Brien L., Othman M., Rivard G., Rapson D., Hough C., Lillicrap D. (2007) Blood. 109, 145–154 [DOI] [PubMed] [Google Scholar]
- 6. Eikenboom J. C. (2001) Best. Pract. Res. Clin. Haematol. 14, 365–379 [DOI] [PubMed] [Google Scholar]
- 7. Verweij C. L., Diergaarde P. J., Hart M., Pannekoek H. (1986) EMBO J. 5, 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayadas T., Wagner D. D., Simpson P. J. (1989) Blood. 73, 706–711 [PubMed] [Google Scholar]
- 9. Giblin J. P., Hewlett L. J., Hannah M. J. (2008) Blood. 112, 957–964 [DOI] [PubMed] [Google Scholar]
- 10. Dong J. F., Moake J. L., Nolasco L., Bernardo A., Arceneaux W., Shrimpton C. N., Schade A. J., McIntire L. V., Fujikawa K., López J. A. (2002) Blood. 100, 4033–4039 [DOI] [PubMed] [Google Scholar]
- 11. Sporn L. A., Marder V. J., Wagner D. D. (1986) Cell. 46, 185–190 [DOI] [PubMed] [Google Scholar]
- 12. Sporn L. A., Marder V. J., Wagner D. D. (1987) Blood. 69, 1531–1534 [PubMed] [Google Scholar]
- 13. Ewenstein B. M., Warhol M. J., Handin R. I., Pober J. S. (1987) J. Cell Biol. 104, 1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hop C., Guilliatt A., Daly M., de Leeuw H. P., Brinkman H. J., Peake I. R., van Mourik J. A., Pannekoek H. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1763–1768 [DOI] [PubMed] [Google Scholar]
- 15. Voorberg J., Fontijn R., Calafat J., Janssen H., van Mourik J. A., Pannekoek H. (1993) EMBO J. 12, 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner D. D., Saffaripour S., Bonfanti R., Sadler J. E., Cramer E. M., Chapman B., Mayadas T. N. (1991) Cell 64, 403–413 [DOI] [PubMed] [Google Scholar]
- 17. Michaux G., Hewlett L. J., Messenger S. L., Goodeve A. C., Peake I. R., Daly M. E., Cutler D. F. (2003) Blood. 102, 2452–2458 [DOI] [PubMed] [Google Scholar]
- 18. Michaux G., Abbitt K. B., Collinson L. M., Haberichter S. L., Norman K. E., Cutler D. F. (2006) Dev. Cell. 10, 223–232 [DOI] [PubMed] [Google Scholar]
- 19. Huang R. H., Wang Y., Roth R., Yu X., Purvis A. R., Heuser J. E., Egelman E. H., Sadler J. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 482–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eikenboom J. C., Matsushita T., Reitsma P. H., Tuley E. A., Castaman G., Briët E., Sadler J. E. (1996) Blood. 88, 2433–2441 [PubMed] [Google Scholar]
- 21. Eikenboom J., Hilbert L., Ribba A. S., Hommais A., Habart D., Messenger S., Al-Buhairan A., Guilliatt A., Lester W., Mazurier C., Meyer D., Fressinaud E., Budde U., Will K., Schneppenheim R., Obser T., Marggraf O., Eckert E., Castaman G., Rodeghiero F., Federici A. B., Batlle J., Goudemand J., Ingerslev J., Lethagen S., Hill F., Peake I., Goodeve A. (2009) J. Thromb. Haemost. 7, 1304–1312 [DOI] [PubMed] [Google Scholar]
- 22. Tjernberg P., Castaman G., Vos H. L., Bertina R. M., Eikenboom J. C. (2006) Br. J. Haematol. 133, 409–418 [DOI] [PubMed] [Google Scholar]
- 23. Tjernberg P., Vos H. L., Castaman G., Bertina R. M., Eikenboom J. C. (2004) J. Thromb Haemost 2, 257–265 [DOI] [PubMed] [Google Scholar]
- 24. Allen S., Abuzenadah A. M., Hinks J., Blagg J. L., Gursel T., Ingerslev J., Goodeve A. C., Peake I. R., Daly M. E. (2000) Blood 96, 560–568 [PubMed] [Google Scholar]
- 25. Allen S., Abuzenadah A. M., Blagg J. L., Hinks J., Nesbitt I. M., Goodeve A. C., Gursel T., Ingerslev J., Peake I. R., Daly M. E. (2000) Blood. 95, 2000–2007 [PubMed] [Google Scholar]
- 26. Casonato A., Cattini M. G., Soldera C., Marcato S., Sartorello F., Pontara E., Pagnan A. (2004) J. Lab. Clin. Med. 144, 254–259 [DOI] [PubMed] [Google Scholar]
- 27. Casonato A., Sartorello F., Cattini M. G., Pontara E., Soldera C., Bertomoro A., Girolami A. (2003) Blood. 101, 151–156 [DOI] [PubMed] [Google Scholar]
- 28. Casonato A., Sartorello F., Pontara E., Gallinaro L., Bertomoro A., Grazia C. M., Daidone V., Szukowska M., Pagnan A. (2007) Thromb Haemost 98, 1182–1187 [DOI] [PubMed] [Google Scholar]
- 29. Hommais A., Stépanian A., Fressinaud E., Mazurier C., Meyer D., Girma J. P., Ribba A. S. (2006) J. Thromb. Haemost. 4, 148–157 [DOI] [PubMed] [Google Scholar]
- 30. Rosenberg J. B., Haberichter S. L., Jozwiak M. A., Vokac E. A., Kroner P. A., Fahs S. A., Kawai Y., Montgomery R. R. (2002) Blood. 100, 1699–1706 [DOI] [PubMed] [Google Scholar]
- 31. Hampshire D. J., Burghel G. J., Goudemand J., Bouvet L. C., Eikenboom J. C., Schneppenheim R., Budde U., Peake I. R., Goodeve A. C. (2010) Haematologica. 95, 2163–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Z. P., Blombäck M., Egberg N., Falk G., Anvret M. (1994) Genomics. 21, 188–193 [DOI] [PubMed] [Google Scholar]
- 33. Schneppenheim R., Budde U., Obser T., Brassard J., Mainusch K., Ruggeri Z. M., Schneppenheim S., Schwaab R., Oldenburg J. (2001) Blood. 97, 2059–2066 [DOI] [PubMed] [Google Scholar]
- 34. Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. (1973) J. Clin. Invest. 52, 2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romani de, Wit T., Rondaij M. G., Hordijk P. L., Voorberg J., van Mourik J. A. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 755–761 [DOI] [PubMed] [Google Scholar]
- 36. Benham A. M., Cabibbo A., Fassio A., Bulleid N., Sitia R., Braakman I. (2000) EMBO J. 19, 4493–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mommaas A. M., Mulder A. A., Out C. J., Girolomoni G., Koerten H. K., Vermeer B. J., Koning F. (1995) Eur. J. Immunol. 25, 520–525 [DOI] [PubMed] [Google Scholar]
- 38. Valentijn K. M., Valentijn J. A., Jansen K. A., Koster A. J. (2008) J. Struct. Biol. 161, 447–458 [DOI] [PubMed] [Google Scholar]
- 39. Bodó I., Katsumi A., Tuley E. A., Eikenboom J. C., Dong Z., Sadler J. E. (2001) Blood 98, 2973–2979 [DOI] [PubMed] [Google Scholar]
- 40. Tjernberg P., Vos H. L., Spaargaren-van Riel C. C., Luken B. M., Voorberg J., Bertina R. M., Eikenboom J. C. (2006) Thromb. Haemost. 96, 717–724 [DOI] [PubMed] [Google Scholar]
- 41. Wang J. W., Eikenboom J. (2010) Hamostaseologie. 30, 150–155 [PubMed] [Google Scholar]
- 42. Schooten C. J., Tjernberg P., Westein E., Terraube V., Castaman G., Mourik J. A., Hollestelle M. J., Vos H. L., Bertina R. M., Berg H. M., Eikenboom J. C., Lenting P. J., Denis C. V. (2005) J. Thromb. Haemost. 3, 2228–2237 [DOI] [PubMed] [Google Scholar]
- 43. Lui-Roberts W. W., Collinson L. M., Hewlett L. J., Michaux G., Cutler D. F. (2005) J. Cell Biol. 170, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zenner H. L., Collinson L. M., Michaux G., Cutler D. F. (2007) J. Cell Sci. 120, 2117–2125 [DOI] [PubMed] [Google Scholar]
- 45. Berriman J. A., Li S., Hewlett L. J., Wasilewski S., Kiskin F. N., Carter T., Hannah M. J., Rosenthal P. B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17407–17412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Federici A. B. (2008) Haemophilia 14, Suppl. 1, 5–14 [DOI] [PubMed] [Google Scholar]
- 47. Schneppenheim R., Michiels J. J., Obser T., Oyen F., Pieconka A., Schneppenheim S., Will K., Zieger B., Budde U. (2010) Blood. 115, 4894–4901 [DOI] [PubMed] [Google Scholar]
- 48. Castaman G., Giacomelli S. H., Jacobi P., Obser T., Budde U., Rodeghiero F., Haberichter S. L., Schneppenheim R. (2010) J. Thromb. Haemost. 8, 2011–2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.