Abstract
Introduction:
The purpose of this study was to examine the impact of smoking in pregnancy on parenting stress. Maternal psychological symptoms and socioeconomic status (SES) were evaluated as potential mediating factors between prenatal cigarette use and later parenting stress.
Method:
The sample included 218 mothers who were recruited at the hospital after birth and completed a 6-month visit with their infants at a university laboratory. Based on the mothers’ responses to interviews at the hospital on tobacco use during pregnancy, the sample included 77 nonsmokers and 141 smokers. Information on sociodemographic variables, prenatal care, and other substance use during pregnancy was collected at the hospital interview. At the 6-month visit, the mothers completed measures of parenting stress and psychological symptoms. Cotinine levels were assessed at both timepoints.
Results:
Regression analysis showed that maternal smoking during pregnancy predicted parenting stress in infancy. Maternal symptoms of psychological distress and SES were evaluated simultaneously to determine whether they functioned as mediating variables between smoking in pregnancy and parenting stress. A multiple mediation analysis (Preacher & Hayes, 2008a) showed that maternal psychological symptoms functioned as a mediating variable but that SES did not.
Conclusions:
Results suggest that mothers who smoke in pregnancy are likely to experience higher levels of psychological symptoms, which, in turn, predict higher levels of parenting stress. Smoking in pregnancy may be a marker for symptoms of psychological distress in mothers.
Introduction
Recent studies have shown a positive relationship between prenatal tobacco exposure and occurrence of behavioral problems in children, but it is uncertain whether these outcomes result from the physiological impact of smoking, related maternal characteristics, genetic risk, or some combination of these variables (e.g., Maughan, Taylor, Caspi, & Moffitt, 2004; Wakschlag, Leventhal, Pine, Pickett, & Carter, 2006). Mothers who smoke during pregnancy and experience more symptoms of psychological distress may have children who are especially at risk for developmental problems. These mothers also may be likely to experience higher levels of parenting stress, defined as stress or discomfort specifically related to the parenting role and to her perception of and interaction with her child (Abidin, 1995). Studies showing that smoking in pregnancy is related to lower levels of nurturing behavior and other negative parenting characteristics (e.g., Fergusson, Woodward, & Horwood, 1998) suggest that these mothers may find the parenting role stressful and provide less optimal caregiving environments for their children. The relationship between smoking in pregnancy and behavioral problems in children may be a function of the mediational role of maternal psychological symptoms, but this issue has not been explored systematically. In addition, mothers who smoke in pregnancy are likely to be from lower socioeconomic status (SES) backgrounds (e.g., Pickett, Wood, Adamson, D’Souza, & Wakschlag, 2008), which also may contribute to stress associated with child rearing. The purpose of this study is to examine the impact of smoking in pregnancy, in combination with maternal psychological symptoms and SES, on parenting stress during infancy.
Overall smoking rates have decreased over time, but a substantial proportion of women continue to smoke while they are pregnant. Despite the widespread availability of information on negative outcomes for infants and societal disapproval, estimates of the number of women who continue to smoke in pregnancy range from 12% to 22% (Centers for Disease Control and Prevention, 2004).
Wakschlag et al. (2003), in an extension of problem behavior theory (Jessor, Donovan, & Costas, 1991), have suggested that persistent smoking in pregnancy may represent one aspect of an extensive pattern of maternal problem behavior likely to affect health-related, adaptive, and interpersonal areas of life. Support for this premise is provided by data from the Wakschlag study above and by a recent analysis of maternal reports of life stressors from a large sample participating in the Millenium Cohort Study in the United Kingdom (Pickett, Wilkinson, & Wakschlag, 2009). This viewpoint suggests that maternal behavior and life circumstances associated with smoking in pregnancy may contribute to parenting stress; it provides a more nuanced explanation for findings linking smoking in pregnancy to negative behavioral outcomes in children. Consistent with this viewpoint, evidence associating smoking in pregnancy with maternal psychological symptoms and socioeconomic disadvantage is examined below.
Pregnant women who smoke are more likely than nonsmokers to meet criteria for externalizing disorders or to report involvement in antisocial behavior (e.g., Flick et al., 2006; Kodl & Wakschlag, 2004; Maughan et al., 2004; Wakschlag et al., 2003). Based on retrospective reports, Kodl and Wakschlag showed an association between maternal externalizing problems in childhood and later smoking during pregnancy. This result is supported by intergenerational research with the Concordia longitudinal study sample (DeGenna, Stack, Serbin, Ledingham, & Schwartzman, 2006, 2007) showing that aggression among school-age girls predicted to smoking in general in adulthood, and smoking in pregnancy in particular, when a subset of the girls was followed up as adults.
When indicators of SES are examined, results show that women who smoke in pregnancy are more likely to be from low-income families, to be less well educated, and to live in less-advantaged neighborhoods (e.g., Higgins et al., 2009; Kandel, Griesler, & Schaffran, 2009; Maughan et al., 2004; Pickett et al., 2008; Sellstrom, Arnoldsson, Bremberg, & Hjern, 2008; Weaver, Campbell, Mermelstein, & Wakschlag, 2008). Some studies also show that pregnancy smokers are more likely to be single (Flick et al., 2006; Pickett et al., 2008; Sellstrom et al., 2008; Wakschlag et al., 2003). In their study of levels of stress in relation to smoking status, Weaver et al. reported that stress related to SES was a better predictor of whether women continued to smoke during pregnancy than other potential stress factors.
Given these differences between mothers who smoke during pregnancy and those who do not, it seems likely that child-rearing behavior also will be affected. Fergusson et al. (1998) reported that smoking in pregnancy was related to lower levels of maternal nurturing behavior when children were 3 years of age, more exposure to physical punishment in childhood and adolescence, and greater parental conflict between birth and 5 years of age. Parenting characteristics also have been examined as moderating variables in the relation between prenatal smoking and child behavior problems. For instance, Wakschlag and Hans (2002) reported that the level of maternal responsiveness affected whether boys whose mothers smoked during pregnancy developed symptoms of conduct disorder.
In summary, women who smoke in pregnancy appear to differ from nonsmokers on several dimensions. They are more likely to experience symptoms of psychological distress and to be living in less-advantaged environments. It is likely that prenatal smoking, as mediated by these potential factors, will influence the level of parenting stress the mothers experience and, consequently, the ability of mothers to assume the parenting role. In light of the previous research, the aims of the study are as follows:
To examine the association between prenatal cigarette use and parenting stress at six-month postpartum and
To determine whether maternal psychological symptoms and SES function as mediators in the relationship between prenatal cigarette use and later parenting stress.
Method
Participants
The participants were recruited after birth from two Atlanta hospitals for a study of effects of events during pregnancy on child language development. Recruitment occurred between 2003 and 2006; the sample included 351 mothers. Mothers who smoked during pregnancy and mothers who did not were recruited for the study. For the nonsmoking group, recruiters focused on mothers who were similar demographically to those who smoked. When the infants were 6 months of age, 235 mothers or alternate caregivers and their infants visited the university laborarory for a follow-up visit.
Recruitment and Procedures at Hospital
Mothers were visited by recruiters in the postpartum units, and if interested, they were asked to complete a short screening interview to determine eligibility for the study. Eligible mothers had to be at least eighteen years old; English was required to be the primary language spoken in the household. Mothers were excluded if their infants were less than 34-week gestational age or if the infants had medical complications or conditions (e.g., genetic disorders, visual, or hearing impairments) that would interfere with normative development or language outcomes. Mothers who were eligible and interested completed a consent procedure approved by the Emory University Institutional Review Board and the review boards for the hospitals.
Participating mothers completed an interview with the recruiter on demographic variables, prenatal care, medical history, and use of substances (caffeine, tobacco, alcohol, and other drugs) during pregnancy. Mothers also were asked to respond to another questionnaire on use of illicit drugs during pregnancy, to permit access to medical records for abstraction, and to provide specimens of blood to test for cotinine, the main metabolite of nicotine, and urine to test for use of illicit drugs. Mothers were excluded from the study sample if they reported use of illicit drugs (except marijuana) or if urine screens were positive for use. All mothers received $50 as compensation for completing the hospital data collection visit and small baby gifts.
During the hospital interview, mothers were asked to report the number of cigarettes per day they smoked, on average, during the three months prior to conception and in each of the three trimesters. These four responses were averaged to provide the mean number of cigarettes smoked per day during pregnancy. Validity checks were completed comparing maternal self-reports to cotinine levels as described in detail in Kable, Coles, Lynch, and Carroll (2009).
For analyses of background variables and descriptive statistics on dependent and mediating measures, the participants were assigned to one of three groups: (a) nonsmokers, (b) light smokers (average of ≤14 cigarettes/day), and (c) heavy smokers (average of 15 or more cigarettes/day). In the correlational and mediation analyses, the average computed score was used as a continuous variable.
Six-Month Procedures and Final Sample
Mothers or primary caregivers and their infants were invited to the university laboratory at six months. Mothers were asked to complete questionnaire measures at this visit, including the measures of parenting stress and psychological symptoms examined in this study. Urine samples were collected from mothers and infants for assessment of cotinine levels.
Of the 235 participants who completed this visit, 1 who did not complete the questionnaire measures, 10 who had used illicit drugs other than marijuana during pregnancy, and the 6 alternate caregivers were excluded from the present analysis. The final sample includes the 218 biological mothers who completed the six-month follow-up interviews: 77 who did not smoke in pregnancy and 141 who did (99 light and 42 heavy smokers). The average number of cigarettes smoked per day for the smokers ranged from 0.5 to 37.5 with means of 8.2 (SD = 3.8) for the light smokers and 19.4 (SD = 6.0) for the heavy smokers.
Analyses previously reported (Kable et al., 2009) comparing participants who completed the six-month follow-up visit with those who did not showed no differences on sociodemographic variables. Mothers who were lost to follow-up were likely to report smoking more cigarettes prior to pregnancy (but not during pregnancy) and had higher cotinine levels when tested at birth than those who continued. Mothers who continued with the study reported using more ounces of absolute alcohol per week during pregnancy than those who did not.
Measures
Both measures collected at the hospital (interview responses on demographic variables and prenatal substance use and blood for cotinine assessment) and at the six-month visit (measures of maternal psychological symptoms and parenting stress and urine for cotinine assessment) are included in the present analyses.
Demographic and Other Prenatal Substance Use Variables
Questions on parental age and education, relationship status of the mother, maternal ethnicity, household income, and sources of public assistance accessed by the family were included in the maternal interview completed at the hospital.
In addition to tobacco use, mothers also were interviewed at the hospital concerning their use of alcohol, caffeine, and marijuana during the pregnancy. Mothers were asked about the amount and frequency of drinking specific types of alcohol for the three months prior to pregnancy and each trimester. This information was used to calculate the average number of ounces of absolute alcohol per week (AA per week) for each time interval.
Socioeconomic Status
This variable was developed in an earlier analysis of socioenvironmental variables from this sample (Kable et al., 2009). The socioenvironmental variables were included in a factor analysis using a principal-components procedure with varimax rotation. Factor loadings for all included variables were standardized to Z-scores and output for later analyses. The socioeconomic status factor (SES-Factor) that emerged from this analysis was included as the measure of SES in the present study. The SES-Factor was positively related to parental age, education, and household income; it was negatively related to whether the family received public assistance (e.g., Medicaid) and Women, Infants, and Children's services.
Maternal Psychological Symptoms
The Symptom Checklist-90-R (SCL-90-R; Derogatis, 1994) was administered at the six-month visit to measure this variable. It is a well-validated self-report measure; respondents are asked to rate the severity of each of 90-symptom items for the past seven days. Ten symptom dimensions (e.g., depression, anxiety, psychoticism) can be scored from responses. The Global Severity Index, a summary measure based on number and intensity of symptoms reported, is also provided.
Parenting Stress
Mothers completed the short form of the Parenting Stress Index (PSI; Abidin, 1995) at the six-month visit. The PSI is a reliable self-report measure of stress occurring in the context of the parent–child relationship. It includes 36 items rated on a 5-point scale from ”strongly agree” to “strongly disagree.” The measure yields a Total Stress score as well as scores on three stress subscales: Parental Distress, Parent–Child Dysfunctional Interaction, and Difficult Child. Items focus on stress related to the parenting role and perception of child's behavior and temperament. The PSI also includes a Defensive Responding subscale; no group mean scores were in the defensive range (≤10).
Results
Demographic and Background Characteristics of Participants
Demographic and exposure variables for mothers in the nonsmoking, light, and heavy smoking groups are displayed in Table 1. Although the groups were similar on many variables, women who smoked in pregnancy were significantly less likely to be well educated or to be married or living with a partner and more likely to have lower household incomes than those in the nonsmoking groups. There was no significant difference among groups in the amount of alcohol consumed in the third trimester, but mothers in the smoking groups were more likely to use marijuana and reported higher caffeine intake during pregnancy than nonsmokers. However, as a small number of mothers reported prenatal use of marijuana (n = 19) and the mean amounts of caffeine use were not high, these variables were not incorporated into the analysis.
Table 1.
Demographic Characteristics and Prenatal Substance Use of Mothers (N = 218a)
| Variables | Groups |
||||
| 1. Control (n = 77) | 2. Light smoking (≤14 cigarettes/day; n = 99) | 3. Heavy smoking (15 or more cigarettes/day; n = 42) | Statistics | p value | |
| Age at birth, M (SD) | 26.6 (4.7) | 25.0 (5.4) | 26.2 (5.8) | F(2, 215) = 2.22 | ns |
| Ethnic background, n (%) | = 4.17 | ns | |||
| Caucasian | 53 (68.8) | 65 (65.7) | 34 (81.0) | ||
| Black | 21 (27.3) | 31 (31.3) | 8 (19.0) | ||
| Other | 3 (3.9) | 3 (3.0) | 0 (0) | ||
| Education, n (%) | = 19.18 | <.001 | |||
| High-school graduate or less | 18 (23.4) | 52 (52.5) | 24 (57.1) | ||
| Education beyond high school | 59 (76.6) | 47 (47.5) | 18 (42.9) | ||
| Current annual income, n (%), n = 213 | = 12.71 | .002 | |||
| <50,000 | 33 (44.0) | 68 (70.8) | 26 (61.9) | ||
| ≥50,000 | 42 (56.0) | 28 (29.2) | 16 (38.1) | ||
| Marital status, n (%) | = 25.28 | <.001 | |||
| Married or living with partner | 60 (77.9) | 43 (43.4) | 17 (40.5) | ||
| Single, divorced, separated, widowed | 17 (22.1) | 56 (56.6) | 25 (59.5) | ||
| Cigarettes per day (computed average), M (SD) | 0 (0) | 8.2 (3.8) | 19.4 (6.0) | F(2, 215) = 387.03 | <.001b |
| Maternal blood cotinine value—birth, M (SD), n = 143 | .47 (3.5) | 17.3 (35.9) | 25.5 (37.9) | F(2, 140) = 8.75 | <.001c |
| Maternal urine cotinine value—6 months, M (SD), n = 196 | 30.0 (132.2) | 857.6 (915.7) | 1172.8 (1270.2) | F(2, 193) = 29.35 | <.001c |
| Third trimester alcohol use—absolute alcohol/week in ml), M (SD), n = 208 | 124.2 (182.5) | 122.0 (162.1) | 123.3 (193.1) | F(2, 205) =.003 | ns |
| Marijuana; n (%) used | 1 (1.3) | 11 (11.1) | 7 (16.7) | = 9.38 | .009 |
| No. of caffeinated beverages per day, M (SD), n = 217 | 1.4 (1.6) | 3.0 (2.9) | 3.8 (3.8) | F(2, 214) = 11.73 | <.001c |
Note. ns = nonsignificant.
If data for a variable are not available for some participants, the n used for the analysis is noted next to the variable name.
Post-hoc Tukey B shows control < light < heavy smoking group.
Post-hoc Tukey B shows that the control group is significantly lower than the light and heavy smoking groups.
Relation Between Self-Report of Smoking in Pregnancy and Cotinine Levels
Maternal blood cotinine level at birth and maternal urine cotinine level at six months were significantly related to smoking group. Patterns of means for both variables showed a linear dose–response relationship and provide support for the validity of the self-report measure of cigarette use. Correlations were calculated between the log transformation of the maternal blood and urine cotinine measures (used to correct for the skewness of the cotinine distributions) and the self-report estimates of smoking (overall computed average and estimates for the four separate time periods). Correlations between the log transformation of maternal blood cotinine at birth and self-reports of cigarettes per day were all significant (.307 ≤ r ≤ .498, ps < .001). Results were similar for the correlations between the log transformation of maternal urine cotinine at six months and the same self-reports of smoking (.416 ≤ r ≤ .610, ps < .001).
Analyses of Smoking in Pregnancy and Parenting Stress
Average number of cigarettes per day during pregnancy, described in detail in the “Methods” section, was used as the primary independent variable of interest. The continuous measure of maternal smoking was necessary for regression and mediational analyses and allowed for the examination of dose–response relationships. Analyses by group (nonsmoking/light/heavy) were completed to provide descriptive information for the dependent and mediating measures (see Table 2).
Table 2.
Group Means, SDs, and Significance Tests for Parenting Stress Scales and Potential Mediating Variables (N = 218a)
| Variables | Groups |
||||
| 1. Control (n = 77) | 2. Light smoking (≤14 cigarettes/day; n = 99) | 3. Heavy smoking (15 or more cigarettes/day; n = 42) | Statistics | p value | |
| PSI total score, M (SD) n = 217 | 58.4 (13.1) | 59.8 (13.5) | 67.4 (18.5) | F(2, 214) = 5.61 | .004b |
| PSI Parental Distress subscale, M (SD) | 23.7 (7.4) | 25.8 (8.0) | 29.7 (9.8) | F(2, 215) = 7.17 | .001b |
| PSI Parent–Child Dysfunctional Interaction subscale, M (SD) | 15.6 (4.3) | 15.0 (3.9) | 17.4 (5.8) | F(2, 215) = 4.24 | .016b |
| PSI Difficult Child subscale, M (SD) n = 217 | 19.1 (5.6) | 19.1 (5.1) | 21.0 (7.8) | F(2, 214) = 1.84 | ns |
| PSI Defensive Responding subscale, M (SD) | 14.0 (4.7) | 15.1 (4.7) | 17.5 (5.7) | F(2, 215) = 6.98 | .001b |
| SCL-90-R GSI T-Score, M (SD) | 50.4 (10.1) | 53.5 (11.7) | 58.4 (12.4) | F(2, 215) = 6.83 | .001b |
| Socioeconomic status factor score, M (SD) | 0.499 (0.985) | −0.258 (.960) | −0.140 (0.949) | F(2, 215) = 14.11 | <.001c |
Note. GSI = Global Severity Index; PSI = Parenting Stress Index; SCL-90-R = Symptom Checklist-90-R.
If data are not available for some participants, the n used in the analysis is noted next to the variable name.
Post-hoc Tukey B shows that the heavy smoking group score is significantly higher than the other two groups.
Post-hoc Tukey B test shows that the control group is significantly higher than the other two groups.
The PSI Total Stress Index was used as the primary dependent variable of interest. Individual stress subscales were all highly intercorrelated (.38 ≤ r ≤ .528, ps < .001); therefore, the Total Stress Index was used as the primary dependent variable of interest. Scores were calculated as item-rating sum totals from the 36 items and ranged from 36 to 115.
Two mediating variables are proposed in this study: maternal psychological symptoms and SES. For maternal psychological symptoms, all subscales from the SCL-90-R were highly intercorrelated (.434 ≤ r ≤ .805, ps < .001); therefore, the Global Severity Index was used. Scores were reported as T-scores (M = 50, SD = 10) and ranged from 30 to 81. SES, as measured by the SES-Factor score, also was hypothesized as a mediating variable and has been described in the “Methods” section.
Basic parametric analyses were conducted to examine relationships among the variables of interest. Parenting stress (PSI Total Stress Index) was positively correlated with the average number of cigarettes per day during pregnancy, r (217) = .18, p = .008. Examining specific stress subscales revealed that prenatal smoking was significantly associated with Parental Distress; r (218) = .223, p = .001; and Parent–Child Dysfunctional Interaction; r (218) = .193, p = .004; but not with the Difficult Child subscale. Parenting stress was also negatively correlated with the SES-Factor, r (217) = −.194, p = .004, and positively correlated with maternal psychological symptoms as measured by the Global Severity Index for the SCL-90-R, r (217) = .495, p < .001. The average number of cigarettes per day in pregnancy also was negatively correlated with the SES-Factor; r (218) = −.248, p <.001; and positively correlated with maternal psychological symptoms; r (218) = .187, p = .006.
Regression analysis was conducted to examine whether maternal smoking during pregnancy predicts parenting stress at six months postpartum. Simple linear regression confirmed that maternal smoking during pregnancy predicted parenting stress (b = .35, SEb = .13, β = .18, p = .008). The association was positive; higher smoking during pregnancy was associated with higher parenting stress at six months postpartum.
It was predicted that maternal psychological symptoms (Global Severity Index of the SCL-90-R) and SES (SES-Factor) would mediate the relationship between maternal smoking during pregnancy and parenting stress at six months postpartum. To test this hypothesis, a multiple mediation analysis was completed as described by Preacher and Hayes (2008a). This analysis provides a means for evaluating the indirect effects of two or more mediating variables simultaneously. According to these authors, the indirect effect of a specific mediator is “conditional” on the other mediators in the model. The significance of each mediator is evaluated in the context of the full model, including other proposed mediators.
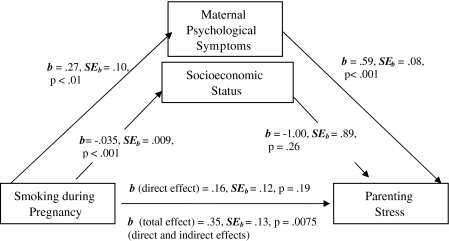
The analysis was completed using the SPSS macro developed by Preacher and Hayes (2008a, 2008b). This program uses a bootstrapping resampling strategy to evaluate significance of the model and effects of mediators; for this analysis, 5,000 bootstrap samples were used. The model tested included prenatal tobacco use as the independent variable, total parenting stress at six months as the dependent variable, and both maternal psychological symptoms and SES as the mediating variables (see Figure 1). Coefficients indicating relationships between the independent variable, each mediating variable, and the dependent variable were computed. In addition, the total effect (sum of direct effect and mediated effects); the direct effect (effect of the independent variable minus the effects of the mediating variables); and the significance levels of total, direct, and specific indirect (mediating variables) effects were calculated.
Figure 1.
Results of mediational model showing associations among pregnancy smoking, the proposed mediating variables (maternal psychological symptoms and socioeconomic status), and parenting stress.
Results showed that maternal psychological symptoms functioned as a mediator between prenatal tobacco use and parenting stress, but SES did not. The coefficient for the total effect (sum of the direct effect and the mediated or indirect effects) of prenatal tobacco use on parenting stress was significant (b = 0.3520, SE = 0.1304, t = 2.6991, p = .0075). Coefficients for relations among the independent, mediating, and dependent variables are displayed in Figure 1.
Tests of specific indirect effects were completed with product of coefficients tests (also known as the Sobel test) as well as CIs produced by the bootstrapping analysis. Both results showed that the total indirect effects and the specific effect for maternal psychological symptoms were significant, but the specific effect for SES was not. Results for indirect effects are displayed in Table 3. The test for the direct effect of prenatal tobacco use (with mediated indirect effects removed) was not significant, providing additional evidence for the mediation model. The overall model was significant (R2 = .2587, F(3, 213) = 24.78, p < .001).
Table 3.
Results for Tests of Indirect Effects in Mediation Model
| Indirect effect | Point estimate | Product of coefficients tests (Sobel test) |
Bootstrapping (95% CIs) a |
|||
| SE | Z | p value | Lower | Upper | ||
| Total | 0.1932 | 0.0714 | 2.7037 | .0069 | 0.0398 | 0.3718 |
| Maternal psychological symptoms | 0.1581 | 0.0640 | 2.4697 | .0135 | 0.0218 | 0.3208 |
| Socioeconomic status | 0.0351 | 0.0323 | 1.0857 | .2776 | −0.0165 | 0.1149 |
Note.
Bias corrected and accelerated CIs.
Discussion
Results of the analyses showed that (a) maternal smoking during pregnancy predicted maternal report of parenting stress at six months postpartum and (b) maternal psychological symptoms mediated the relationship between smoking during pregnancy and mothers’ experience of parenting stress, but SES did not.
Recent studies have linked prenatal cigarette use to higher levels of behavioral and psychiatric problems in offspring; however, the potential contributions of this exposure, maternal characteristics, and the caregiving environment have been unclear. A primary contribution of this study is the clarification of the role of maternal psychological symptoms in mediating the effect of smoking in pregnancy on maternal caregiving potential. Many studies have shown correlations between smoking in pregnancy and maternal psychopathology (e.g., Flick et al., 2006; Kodl & Wakschlag, 2004; Maughan et al., 2004); however, the process through which these factors might together affect the child-rearing environment, and thus childhood outcomes, has not been clearly delineated. Findings from the current study show that mothers who smoked during pregnancy were likely to experience higher levels of psychological symptoms, which in turn predicted higher levels of parenting stress; thus, a mediational model was confirmed. Mediation was further supported by timing of the measures collected, with smoking during pregnancy assessed prior to mothers’ psychological symptoms and parenting stress. We suspect, however, that, given prior literature, differences in level of maternal psychological symptoms existed prior to the pregnancy and that this variable represents an enduring characteristic of the mother. Furthermore, the association between cigarette smoking and psychiatric disorders in general has been documented (e.g., Degenhardt & Hall, 2001; Kahler et al., 2009; Saban & Flisher, 2010) and lends support to the interpretation that smoking in pregnancy, a risky behavior likely to affect infant health, may be a marker for psychological distress in mothers, particularly for those who smoke more heavily. This interpretation is consistent with but more focused than the suggestion by Pickett et al. (2008) that smoking in pregnancy may be a marker for psychosocial difficulties of these mothers or that of Hutchinson, Pickett, Green, and Wakschlag (2010) that it may be a marker for “intergenerational processes associated with both the tendency to smoke and to have offspring with behavioural and cognitive problems … . (p. 479).”
An alternative interpretation of these data might be that the prenatal exposure to nicotine alone leads to behavioral differences in the infants (e.g., higher irritability, more difficult temperament), thus predicting higher levels of parenting stress. However, the analyses of the stress subscale results for the PSI do not support this interpretation. While amount of smoking in pregnancy was positively correlated with the Parental Distress and Parent–Child Dysfunctional Interaction subscales, it was not significantly correlated with the Difficult Child subscale, which measures the mother's perception of the child's behavior as challenging to manage or temperamentally demanding.
While SES was significantly correlated with prenatal tobacco use and parenting stress, it did not function as a mediator between these variables. Due to the correlation between SES and maternal psychological symptoms, r (218) = −.221, p = .001, it is possible that the impact of this variable is reduced in the multiple mediation analysis. Preacher and Hayes (2008a) allude to this issue in their discussion of collinearity in these analyses. SES may be too distal from the mother–child relationship to function as an effective mediator as compared with the more proximal symptom variable.
One important study characteristic that limits the interpretation is that all data are based on maternal report. Self-report of smoking during pregnancy may be minimized due to the negative stigma associated with this behavior. The self-report measure, however, also was significantly correlated with maternal cotinine level at birth and six months, which supports its validity. Reports of maternal psychological symptoms, variables related to SES, and parenting stress also come from the mother. There are no independent evaluations of psychiatric status or observations of stress in dealing with the infant.
In summary, the results suggest that children whose mothers smoke during pregnancy and experience more psychological symptoms may be at increased risk for developing behavior problems. Although the means for the smoking groups were not in the clinical range, the symptoms still seem to impact the mother's experience of parenting stress. It is plausible to hypothesize that higher levels of psychological symptoms and parenting stress in smoking mothers will have a negative impact on caregiving potential of the mother and the child-rearing environment she can provide. Future research is needed on the relation between smoking in pregnancy and child-rearing behaviors in these mothers. While many studies have shown that pregnancy smoking is related to behavioral problems in children, the process of influence is far from clear. Mother–child interaction, especially maternal responsiveness, should be examined as a function of smoking and maternal psychological symptoms.
Funding
This work was supported by National Institute of Child Health and Human Development grant (# R01 HD041203) to CDC.
Declaration of Interests
None declared.
Acknowledgments
The authors would like to acknowledge the cooperation of the many families who agreed to participate in the study.
References
- Abidin RR. Parenting stress index. 3rd ed. Lutz, FL: Psychological Assessment Resources; 1995. Professional Manual. [Google Scholar]
- Centers for Disease Control and Prevention. 2004 Surgeon General's report-The health consequences of smoking: Smoking among adults in the United States: Reproductive health. 2004. Retrieved from http://www.cdc.gov/tobacco/data_statistics/sgr/2004/highlights/reproductive/index.htm. [Google Scholar]
- Degenhardt L, Hall W. The relationship between tobacco use, substance-use disorders and mental health: Results from the National Survey of Mental Health and Well-being. Nicotine & Tobacco Research. 2001;3:225–234. doi: 10.1080/14622200110050457. doi:10.1080/14622200 110050457. [DOI] [PubMed] [Google Scholar]
- DeGenna NM, Stack DM, Serbin LA, Ledingham JE, Schwartzman AE. From risky behavior to health risk: Continuity across two generations. Developmental and Behavioral Pediatrics. 2006;27:297–309. doi: 10.1097/00004703-200608000-00004. Retrieved from http://journals.lww.com/jrnldbp/ [DOI] [PubMed] [Google Scholar]
- DeGenna NM, Stack DM, Serbin LA, Ledingham JE, Schwartzman AE. Maternal and child health problems: The inter-generational consequences of early maternal aggression and withdrawal. Social Science and Medicine. 2007;64:2417–2426. doi: 10.1016/j.socscimed.2007.03.001. doi:10.1016/j.socscimed.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R administration, scoring & procedures manual. Minneapolis, MN: NCS Pearson; 1994. [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. Retrieved from http://archpsyc.ama-assn.org. [DOI] [PubMed] [Google Scholar]
- Flick LH, Cook CA, Homan SM, McSweeney M, Campbell C, Parnell L. Persistent tobacco use during pregnancy and the likelihood of psychiatric disorders. American Journal of Public Health. 2006;96:1799–1807. doi: 10.2105/AJPH.2004.057851. Retrieved from http://www.ajph.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug and Alcohol Dependence. 2009;104S:S100–S105. doi: 10.1016/j.drugalcdep.2009.03.013. doi:10.1016/j.drugalcdep.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J, Pickett KE, Green J, Wakschlag LS. Smoking in pregnancy and disruptive behaviour in 3-year-old boys and girls: An analysis of the UK Millennium Cohort Study. Journal of Epidemiological Community Health. 2010;64:82–88. doi: 10.1136/jech.2009.089334. doi:10.1136/jech.2009.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa FM. Beyond adolescence: Problem behavior and young adult development. New York: Academic Press; 1991. [Google Scholar]
- Kable JA, Coles CD, Lynch ME, Carroll J. The impact of maternal smoking on fast auditory brainstem responses. Neurotoxicology and Teratology. 2009;31:216–224. doi: 10.1016/j.ntt.2009.02.002. doi:10.1016/j.nnt.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Daughters SB, Leventhal AM, Rogers ML, Clark MA, Colby SM, et al. Personality, psychiatric disorders, and smoking in middle-aged adults. Nicotine & Tobacco Research. 2009;11:833–841. doi: 10.1093/ntr/ntp073. doi:10.1093/ntr/ntp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: Risk factors and consequences for offspring. Drug and Alcohol Dependence. 2009;104S:S24–S33. doi: 10.1016/j.drugalcdep.2008.12.005. doi:10.1016/j.drugalcdep.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl MM, Wakschlag LS. Does a childhood history of externalizing problems predict smoking during pregnancy? Addictive Behaviors. 2004;29:273–279. doi: 10.1016/j.addbeh.2003.08.003. doi:10.1016/j.add.beh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems: Testing genetic and environmental explanations of the association. Archives of General Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. Retrieved from http://archpsyc.ama-assn.org. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Wilkinson RG, Wakschlag LS. The psychosocial context of pregnancy smoking and quitting in the Millennium Cohort Study. Journal of Epidemiological Community Health. 2009;63:474–480. doi: 10.1136/jech.2008.082594. doi:10.1136/jech.2008.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Wood C, Adamson J, D’Souza L, Wakschlag LS. Meaningful differences in maternal smoking behavior during pregnancy: Implications for infant behavioural vulnerability. Journal of Epidemiological Community Health. 2008;62:318–324. doi: 10.1136/jech.2006.058768. doi:10.1136/jech.2006.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008a;40:879–891. doi: 10.3758/brm.40.3.879. doi:10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS macro for multiple mediation. The Ohio State University; 2008b. Written by Andrew F. Hayes. Retrieved from http://www.afhayes.com. [Google Scholar]
- Saban A, Flisher AJ. The association between psychopathology and substance use in young people: A review of the literature. Journal of Psychoactive Drugs. 2010;42:37–47. doi: 10.1080/02791072.2010.10399784. Retrieved from http://www.journalofpsychoactivedrugs.com. [DOI] [PubMed] [Google Scholar]
- Sellstrom E, Arnoldsson G, Bremberg S, Hjern A. The neighbourhood they live in: Does it matter to women's smoking habits during pregnancy? Health Place. 2008;14:155–166. doi: 10.1016/j.healthplace.2007.05.007. doi:10.1016/j.healthplace.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Development and Psychopathology. 2002;14:351–369. doi: 10.1017/s0954579402002092. Retrieved from http://journals.cambridge.org. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Leventhal BL, Pine DS, Pickett KE, Carter AS. Elucidating early mechanisms of developmental psychopathology: The case of prenatal smoking and disruptive behavior. Child Development. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. Retrieved from http://www3.interscience.wiley.com. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Middlecamp MK, Walton LL, Tenzer P, Leventhal BL. Pregnant smokers who quit, pregnant smokers who don’t: Does history of problem behavior make a difference? Social Science and Medicine. 2003;56:2449–2460. doi: 10.1016/s0277-9536(02)00248-4. Retrieved from http://www.elsevier.com. [DOI] [PubMed] [Google Scholar]
- Weaver K, Campbell R, Mermelstein R, Wakschlag L. Pregnancy smoking in context: The influence of multiple levels of stress. Nicotine & Tobacco Research. 2008;10:1065–1073. doi: 10.1080/14622200802087564. doi:10.1080/14622200802087564. [DOI] [PMC free article] [PubMed] [Google Scholar]