Abstract
Reports that iron, zinc and copper homeostasis are in aberrant homeostasis are common for various neurodegenerative diseases, particularly for Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease. Manipulating the levels of these elements in the brain through the application of chelators has been and continues to be tested therapeutically in clinical trials with mixed results. Much of the data indicating that these metals are abnormally concentrated in Alzheimer’s disease and Parkinson’s disease brain tissue was generated through the analysis of post-mortem human tissue which was archived in formalin. In this study, we evaluated the effect of formalin fixation of brain on the levels of three important transition metals (iron, copper, and zinc) by atomic absorption spectroscopy. Paired brain specimens were obtained at autopsy for each case; one was conserved by formalin archival (averaging four years), the other was rapidly frozen. Both white and grey matter samples were analyzed and the concentrations of iron and zinc were found to decrease with fixation. Iron was reduced by 40% (P <0.01), and zinc by 77% (P <0.0001); copper concentrations increased by 37% (P <0.05) by the paired T-test. The increase in copper is likely due to contamination from trace copper in the formalin. These results indicate that transition metal data obtained from fixed tissue may be heavily distorted and care should be taken in interpreting this data.
Keywords: Transition metals, Formalin, Fixation, Alzheimer’s disease, Leaching
Introduction
The measurement of trace metals in human tissue has revealed numerous insights into both normal physiology and disease. Brain iron concentration increases with age, reaching a plateau at about age 55 (Hallgren and Sourander 1958). Disturbances in the levels of various metals including iron in the brain are reported to be associated with many neurodegenerative disorders, including Huntington’s disease, amyotrophic lateral sclerosis, Alzheimer’s disease (AD), and Parkinson’s disease (PD). In these diseases, iron is known to accumulate in the deep grey matter and iron accumulation in the neocortex has been suggested to contribute to the cognitive dysfunction associated with some of these diseases. Abnormal accumulation of iron in the AD brain could account for increased in oxidative injury which is an early finding in AD and increases in brain iron have in fact been reported early in this condition mirroring the oxidative changes (Smith 2010; Nunomura et al. 2001), although several additional studies have failed to detect any abnormal changes in brain iron in AD compared to age-matched controls at various stages of the disease (Hallgren and Sourander 1960; Ward and Mason 1987; Magaki 2007). Additionally, beta-amyloid peptides which aggregate to form senile plaques in Alzheimer’s and Parkinson’s diseases, avidly bind to iron, zinc, and copper. When these metals are bound, the toxicity of beta-amyloid is reported to markedly increase (Rottkamp et al. 2001). Pharmacologically manipulating the levels and/or distribution of these metals in the brain is becoming a clinical reality for AD and PD through the use of chelators and metal ionophores – several of which are in clinical trials (Crapper McLaughlan et al. 1991; Squitti et al. 2002; Lannfelt et al. 2008). Initial results have been mixed; modest improvement in secondary measures like the performance of activities of daily living has been reported, but no agent has yet produced an improvement in cognition.
Reports that iron, zinc, and possibly copper are abnormally concentrated in neocortical brain in Alzheimer’s and that all three metals are deposited in beta-amyloid plaques were primarily generated from analysis of post-mortem, fixed tissue through histochemical and radioanalytical techniques (such as particle induced X-ray emission tomography) (Goodman 1953, Lovell et al. 1998). The reliability of this data set requires that formalin archival of tissue does not disturb the levels of these transition metals. We noted that previous reports of iron concentrations from both normal and diseased brains which utilized fixed specimens appear to report substantially lower concentrations than studies which use never-fixed specimens analyzed with equivalent techniques (Deibel et al. 1996; Lovell et al. 1998). To evaluate whether this observation represents an artifact introduced through the fixation technique, we have evaluated paired brain samples taken from the temporal lobe of AD patients to determine whether samples stored in formalin have equivalent levels of three key transition metals – iron, copper, and zinc.
Methods
Post-mortem tissue was donated from the AD Research Center Brain Bank at the University of California, Los Angeles. All patients and/or their surrogates had consented to tissue donation prior to autopsy. The research protocol was approved by the Institutional Review Board of Loma Linda University Medical Center (approval #54174). Both frozen and formalin-archived tissue was collected from the middle temporal gyrus of 4 severe AD brains. Tissue samples were isolated with a diamond blade scalpel and titanium and/or nylon forceps (to avoid contamination) and collected as 30–60 mg specimens of isolated grey or white matter. From fixed tissue, the surface of tissue blocks was dissected away to remove any tissue which might have been exposed to iron-containing instruments through the autopsy procedure.
Tissue was ashed for analysis by standard techniques. Briefly, brain tissue was immersed in concentrated nitric acid (300 μl) overnight, then incubated in a water bath at 80°C for 20 min. The resulting solution was allowed to cool to room temperature and hydrogen peroxide (300 μl, 10 M solution) was added to dissolve lipid components. After 30 min incubation at room temperature, the sample was heated to 70°C in the water bath for 15 min. The resultant solution was allowed to cool for 10 min, then vortexed thoroughly and stored until analysis.
Atomic absorption spectrums were measured with a Varian SpectrAA 220Z atomic absorption spectrometer and processed with SpectrAA software v.4.1. Standard iron and copper curves were produced from 25, 50, 75, and 100 parts per billion solutions of standard iron or copper in nitric acid (Arcos Organics, New Jersey). Standard curve for zinc was produced from 250, 500, 750, and 1000 parts per billion solutions of standard zinc in nitric acid (Solutions Plus Inc, Missouri). The spectrometer was zeroed to a maximum of 0.005 mean absorbance. For total iron measurements samples were diluted 1:40, for zinc measurements samples were diluted 1:10 and for copper samples were diluted 1:20. Standard furnace settings recommended by Varian were used for the analysis. All sample values were the mean of six measurements. Concentrations are calculated to reflect the concentration of the metal in micrograms per gram of native tissue (wet weight). Significance was determined by the two-tailed, paired Student’s t test, with α = 0.05.
Results
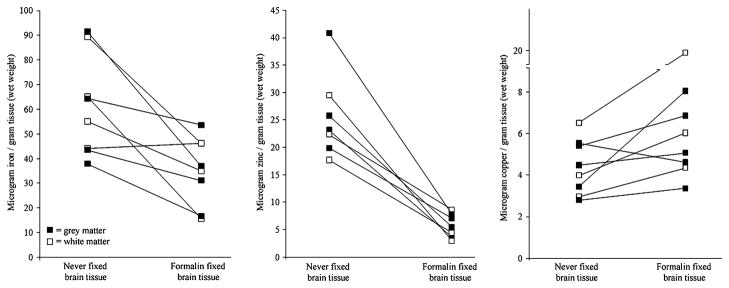
Two samples were analyzed from each of four brains which were severely affected by AD at Braak and Braak stage VI (Braak and Braak 1991). One frozen and one formalin-fixed temporal lobe specimen were obtained from each case for comparison, the mean duration of archival was four years (range 3–6). Each specimen was divided into grey and white matter which were analyzed separately. Six measurements were collected for each sample and the mean of the values was reported in Fig. 1. The mean percent relative standard deviations (%RSD) over these repeated measures were 0.8% for iron, 12.5% for zinc, and 1.8% for copper. The %RSD for zinc was wider because the concentration in fixed specimens approached the threshold of detection for this element (%RSD fixed = 20.8, %RSD frozen = 4.1%).
Fig. 1.
Effect of long-term formalin archival on transition metal levels in paired Alzheimer’s disease brain samples Iron levels were found to decrease by about 40% upon fixation in formalin (P<0.01), zinc levels were shown to decrease with fixation by about 75% (P <0.0001) and copper increased by about 40% (P <0.05). All of these effects appeared to be independent of whether the tissue was collected from grey matter or white matter. Lines between specimens indicate they were taken from the same brain
Iron levels in the fixed tissue were significantly lower than in the frozen specimens (P <0.01 by paired T-test). Seven of the eight specimens examined were found to have decreased iron after fixation, while one remained essentially unchanged. On average, iron concentration was 43% lower in the fixed tissue (mean difference 26.2 μg/g tissue, 95% CI 9.1–43.3 μg/g tissue) and both grey and white matter were affected equally. Brain zinc levels were also found to be notably decreased after fixation (P <0.0001). This change also affected both grey and white matter and resulted in a mean decrease in zinc of 19.8 μg/g tissue (95% CI 13.9–25.8 μg/g tissue). Brain copper was found to be increased in the fixed specimens (P <0.05). This finding was unexpected—seven out of eight specimens contained higher levels of copper than their frozen counterparts and the mean increase in copper was 1.65 μg/g tissue (95% CI 0.21–3.09 μg/g).
Discussion
Previous studies have shown that formalin fixation affects the levels of transition metals in various organs, although it has frequently been argued that most transition metals in the brain are not affected by fixation. This was primarily determined either by analysis after a brief fixation period or by indirect means such as determining the concentration of trace elements in the formalin in which the organ was stored for comparison to clean formalin (Andrasi et al. 1990; Bush et al. 1995; Gellein et al. 2008). This approach evaluates transition metal leaching and is obviously sensitive to dilutional variation, evaporation and contamination from other sources. Additionally, because transition metal concentrations in most tissues vary considerably between individuals, a non-paired analysis may mask the effect of formalin. The most sensitive approach is to obtain paired samples from each case, one of which is stored by freezing, the other by formalin archival for several years.
The findings of this study demonstrate that formalin archival of brain samples affects the concentration of several transition metals. Both iron and zinc were found to be significantly depleted after archival in formalin. Copper, however, was found to increase with formalin fixation. This increase in copper may be due to contamination of formalin with copper. While copper levels in formalin are reported to be approximately 2% the level found in brain (0.1 vs. 5 ppm) (Andrasi et al. 1990; Gellein et al. 2008), the AD brain is depleted of copper and over-expresses copper binding proteins such as ceruloplasmin which may increase the tissue’s copper binding capacity and could in theory explain the accumulation of copper in the tissue with fixation (Loeffler et al. 1996). Reports from the literature also indicate that copper levels dramatically increase in AD brain with fixation, a finding which was not noted in control tissue which would support this hypothesis (see Table 1).
Table 1.
Effect of short-term fixation on transition metal concentrations in the amygdala reported in the literature
| Study and method | [Iron] control | [Iron] AD | [Zinc] control | [Zinc] AD | [Copper] control | [Copper] AD |
|---|---|---|---|---|---|---|
| Fixed tissue | 18.9 ± 5.3 | 38.8 ± 9.4 | 22.6 ± 2.8 | 51.4 ± 11.0 | 4.4 ± 1.5 | 19.3 ± 6.3 |
| Lovell et al. 1998—micro-PIXE, 24 h formalin exposure | N = 5 | N = 9 | N = 5 | N = 9 | N = 5 | N = 9 |
| Never-fixed tissue | 49.2 ± 3.3 | 65.5 ± 3.8 | 14.5 ± 0.5 | 18.2 ± 0.7 | 4.1 ± 0.3 | 2.7 ± 0.3 |
| N = 56 | N = 101 | N = 69 | N = 119 | N = 11 | N = 10 | |
| Thompson 1988—INAA | 48.9 ± 3.0 | 60.6 ± 4.9 | 14.1 ± 0.5 | 17.0 ± 0.8 | – | – |
| Samudralwar 1995—INAA | 50.8 ± 3.7 | 70.8 ± 4.0 | 16.7 ± 0.5 | 21.4 ± 0.5 | – | – |
| Deibel 1996—INAA | 48.6 ± 2.2 | 70.8 ± 6.4 | 15.2 ± 0.6 | 19.8 ± 1.0 | 4.1 ± 0.3 | 2.7 ± 0.3 |
| Cornett 1998—INAA | 49.0 ± 4.0 | 64.0 ± 3.0 | 13.6 ± 0.5 | 17.6 ± 0.6 | – | – |
| Rulon 2000—AA | – | – | 15.7 ± 0.5 | 16.6 ± 1.0 | – | – |
| Percent change (Two-tailed t-test) | −61% P <0.0001 |
−41% P <0.0001 |
+56% P <0.0001 |
+182% P <0.0001 |
+7.3% P = 0.52 |
+615% P <0.0001 |
All six studies listed were published from the same laboratory at the University of Kentucky. One study analyzed fixed tissue; the others analyzed never-fixed tissue – all used quantitative analytical methods. The never-fixed results are pooled in the second row and listed individually below. Measurements represent microgram metal per gram tissue, wet weight (results from Deibel et al. 1996 were converted from dry weight measurements to wet weight). Errors listed are standard deviation
AD Alzheimer’s disease, PIXE particle induced x-ray emission, INAA instrumental neutron activation analysis
The effect of fixation on brain iron has not been previously well-documented, although we noted that studies which analyzed fixed brain reported different levels of metals than those analyzing never-fixed brain (Table 1). Iron levels detected using a micro-particle induced X-ray emission (PIXE) analysis of formalin fixed AD amygdala were 38.8 μg/g wet tissue while other studies from the same laboratory using quantitative analyses of never-fixed AD amygdala found a mean iron concentration of 65.5 μg/g wet tissue (Cornett et al. 1998, Deibel et al. 1996; Lovell et al. 1998; Samudralwar et al. 1995; Thompson et al. 1988). These studies showed that fixed tissue from the same brain region contained 41% less iron than comparable never-fixed tissue, which is strikingly consistent with the 43% reduction we found in the paired samples in this study. Additionally, iron concentration in control tissue was found to decrease by even more (never-fixed 48.7 μg/g versus fixed 18.9 μg/g—a 61% reduction, P <0.0001). The effects on copper levels in these studies were more severe in AD brain than what we report here. While fixed and frozen amygdala tissue from control brain was not significantly affected (4.1 vs. 4.4 μg/g, P = 0.52), AD tissue was reported to contain 2.7 μg copper/g in never-fixed tissue and 19.3 μg/g in fixed tissue—a 615% increase (Deibel et al. 1996; Lovell et al. 1998). Finally, zinc levels were also found to be altered between the studies, although they did not match the results of our study. Fixation increased zinc levels by 54% in control tissue and by 179% in AD tissue (Cornett et al. 1998; Deibel et al. 1996; Lovell et al. 1998; Rulon et al. 2000; Samudralwar et al. 1995; Thompson et al. 1988). Our results found that zinc was depleted by 75% in temporal lobe tissue from AD patients. The discrepancy in these results may indicate that effects of fixation differ between brain regions, or with the length of fixation. Additionally, we noted a wide variance in the effect of fixation on tissue concentrations of all three metals, which makes it difficult to simply calculate a correction for fixation. Regardless, formalin fixation (even briefly) appears to destabilize the concentration of multiple metal species and may affect normal and diseased brain differently. From these observations, we feel it is appropriate to cautiously interpret transition metal data derived from fixed tissue and future studies should be limited to fresh or frozen specimens.
Altered homeostasis of iron, zinc and/or copper has been suggested to be involved in many neurodegenerative diseases; however, reports of metals concentration in these tissues have been remarkably disparate in their conclusions. Before seriously considering the use of chelators or other compounds to manipulate the homeostasis of these essential biometals, it will be necessary to determine whether and to what degree they are truly dysregulated. This requires a critical evaluation of the available data. Because a significant portion of that data was generated from fixed tissue, it may be necessary to re-examine the fundamental assumptions about the role of metals in various neurodegenerative diseases.
Acknowledgments
This research was funded by the National Institutes of Health (AG20948). Harry V. Vinters is supported in part by P01 AG12435, P50 AG16570 and the Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine.
Footnotes
None of the authors have real or potential conflicts of interest related to this work.
Contributor Information
Matthew Schrag, Email: mschrag@llu.edu, Neurosurgery Center for Research, Loma Linda, University, 11175 Campus Street, Coleman Pavilion Suite, 11113, Loma Linda, CA 92350, USA.
April Dickson, Neurosurgery Center for Research, Loma Linda, University, 11175 Campus Street, Coleman Pavilion Suite, 11113, Loma Linda, CA 92350, USA.
Arshad Jiffry, Neurosurgery Center for Research, Loma Linda, University, 11175 Campus Street, Coleman Pavilion Suite, 11113, Loma Linda, CA 92350, USA.
David Kirsch, Neurosurgery Center for Research, Loma Linda, University, 11175 Campus Street, Coleman Pavilion Suite, 11113, Loma Linda, CA 92350, USA.
Harry V. Vinters, Department of Pathology and Laboratory Medicine, University of California Los Angeles, Los Angeles, CA, USA
Wolff Kirsch, Neurosurgery Center for Research, Loma Linda, University, 11175 Campus Street, Coleman Pavilion Suite, 11113, Loma Linda, CA 92350, USA.
References
- Andrasi E, Nadasdi J, Molznar Z, Bezur L, et al. Determination of main and trace element contents of human brain by NAA and ICP-AES methods. Biol Trace Elem Res. 1990;26(7):691–698. doi: 10.1007/BF02992725. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bush V, Moyer T, Batts K, Parisi J. Essential and toxic element concentrations in fresh and formalin-fixed human autopsy tissues. Clin Chem. 1995;41(2):284–294. [PubMed] [Google Scholar]
- Cornett C, Markesbery W, Ehmann W. Imbalances of trace elements related to oxidative damage in AD brain. Neurotoxicology. 1998;19:339–345. [PubMed] [Google Scholar]
- Crapper McLaughlan D, Dalton A, Kruck T, Bell M, et al. Intramusclar desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- Deibel M, Ehmann W, Markesbery W. Copper, iron and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J Neurol Sci. 1996;143:137–142. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- Gellein K, Flaten T, Erikson K, Aschner M, Syverson T. Leaching of trace elements from biological tissue by formalyn. Biol Trace Elem Res. 2008;121:221–225. doi: 10.1007/s12011-007-8051-1. [DOI] [PubMed] [Google Scholar]
- Goodman L. Alzheimer’s disease; a clinico-pathologic analysis of 23 cases with a theory on pathogenesis. J Nerv Ment Dis. 1953;118:97–130. [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The non-haemin iron in the cerebral cortex in Alzheimer’s disease. J Neurochem. 1960;5:307–310. doi: 10.1111/j.1471-4159.1960.tb13369.x. [DOI] [PubMed] [Google Scholar]
- Lannfelt L, Blennow K, Zetterberg H, Batsman S, et al. Safety, efficacy and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease; a phase IIa, double blind randomized placebo controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- Loeffler D, LeWitt P, Juneau P, Sima A, et al. Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Res. 1996;738:265–274. doi: 10.1016/s0006-8993(96)00782-2. [DOI] [PubMed] [Google Scholar]
- Lovell M, Robertson J, Teesdale W, Campbell J, Markesbery W. Copper, iron and zinc in AD senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirei K, et al. Oxidative damage is the earliest eventin Alzheimer Disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Rottkamp C, Raina A, Zhu X, Gaier E, et al. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001;30:447–450. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- Rulon L, Robertson J, Lovell M, Deibel M, et al. Serum zinc levels and Alzheimer’s disease. Biol Trace Elem Res. 2000;75:79–85. doi: 10.1385/bter:75:1-3:79. [DOI] [PubMed] [Google Scholar]
- Samudralwar D, Diprete C, Ni B, Ehmann W, Markesbery W. Elemental imbalances in the olfactory pathway in Alzheimer’s disease. J Neurol Sci. 1995;130:139–145. doi: 10.1016/0022-510x(95)00018-w. [DOI] [PubMed] [Google Scholar]
- Squitti R, Rossini P, Cassetta E, Moffa F, et al. D-penicillamine reduces serum oxidatitive stress in AD patients. Eur J Clin Inv. 2002;32:51–59. doi: 10.1046/j.1365-2362.2002.00933.x. [DOI] [PubMed] [Google Scholar]
- Thompson C, Markesbery W, Ehmann W, Mao Y, Vance D. Regional brain trace-element studies in Alzheimer’s disease. Neurotoxicology. 1988;9:1–8. [PubMed] [Google Scholar]