Abstract
Phosphoinositide 3-kinases (PI3Ks) are considered promising drug targets in oncology. In this issue of Cancer Cell, Schmid et al. demonstrate that the PI3Kγ isoform is required for inflammatory myeloid cells to traffic to tumors. Though tumor cells do not express PI3Kγ selective inhibition of this isoform suppresses tumor growth and angiogenesis.
Entry of inflammatory cells into the tumor microenvironment is now considered a key feature of tumorigenesis, promoting hallmarks of cancer including angiogenesis and invasion (Hanahan and Weinberg, 2011; Mantovani and Sica, 2010). Consequently, agents targeting inflammatory cell recruitment might have therapeutic benefit in cancer while avoiding the intrinsic propensity of cancer cells to become drug-resistant. The signals that attract myeloid cells from the blood to enter tumor tissue are complex, and act through multiple distinct receptor subtypes (Figure 1). This presents a challenge for identifying a suitable target for drug development. A paper in the current issue (Schmid et al., 2011) demonstrates that diverse extracellular factors converge upon a single signaling enzyme (PI3Kγ) to trigger myeloid cell adhesion to endothelial cells (ECs). Specific blockade of PI3Kγ is sufficient to reduce tumor inflammation and angiogenesis, indirectly suppressing the growth of tumors in vivo.
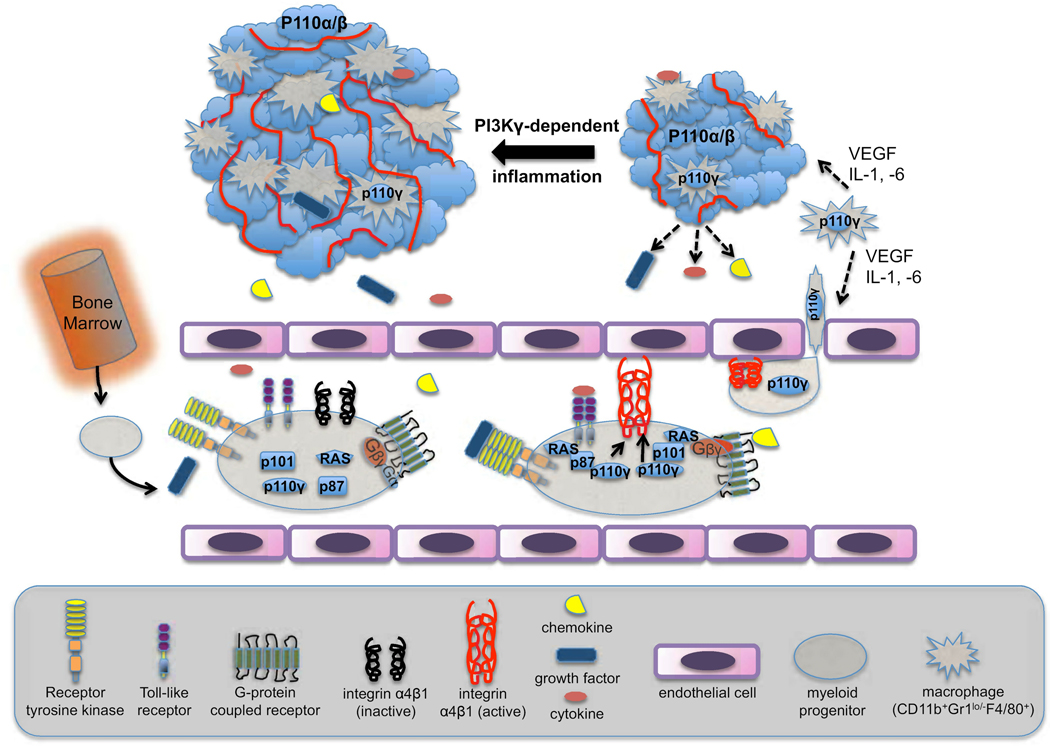
Figure 1. p110γ in myeloid cells mediates adhesion to endothelial cells, driving tumor inflammation and progression.
Bone marrow-derived myeloid cells enter tumor sites by first adhering to endothelial cells in the local vasculature. Adhesion is driven by extracellular signals that increase avidity of the integrin α4β1. The extracellular signaling molecules are produced by both the tumor cells and local macrophages and include chemokines, growth factors and cytokines. Receptors for these diverse ligands each stimulate PI3Kγ, linking to the catalytic subunit p110γ through either the p87 or p101 regulatory subunits and through the Ras GTPase. Myeloid cells entering the tumor mature into macrophages (CD11b+Gr-1loF4/80+) that secrete a partially overlapping set of chemotactic and angiogenic factors. The inflammation associated with progressive recruitment of myeloid cells enhances tumor growth and angiogenesis.
The study starts by addressing the question of which integrins mediate the attachment of myeloid cells to ECs and invasion into tumors. In the experimental models used, monocyte-derived cells rather than neutrophils are the major subset of myeloid cells that populate and persist in tumor tissue (Figure 1). The tumor cells as well as resident myeloid cells secrete soluble factors that recruit additional myeloid cells, amplifying inflammation. These factors include chemokines that signal through G protein-coupled receptors (GPCRs), VEGF that activates receptor tyrosine kinases (RTKs), and cytokines such as IL-1β that signal via the TLR/IL-1R family of receptors that are coupled to intracellular tyrosine kinases (TKs). Surprisingly, each of these diverse stimuli promoted adhesion to EC through activation of the same integrin molecule, α4β1. This led the investigators to hypothesize that a common signaling pathway is triggered by diverse receptors on myeloid cells to increase the avidity of α4β1, a process called “inside-out” signaling. Testing of a large panel of chemical inhibitors indicated that PI3Ks might be key intermediates in the inside-out signaling pathway.
PI3Ks are a family of lipid kinases that phosphorylate phosphatidylinositol (PI) and its derivatives to generate 3-phosphorylated phosphoinositides (Vanhaesebroeck et al., 2010). The main product of class I PI3Ks, phosphatidylinositol-3,4,5-trisphosphate (PIP3), initiates signaling pathways essential for cell growth, proliferation, survival and migration downstream of growth factors and oncoproteins. Two PI3K enzymes (PI3Kα and PI3Kδ) are primarily activated by TK-based signals. A third isoform, PI3Kβ, can be activated either by TKs or by GPCRs. Genetic analysis of human cancer has shown that the PI3Kα isoform plays a dominant role (Figure 1). Gain-of-function mutations in the PIK3CA gene, which encodes the catalytic subunit PI3Kα, are found in a broad spectrum of tumors, with incidence of 30–40% in some cancer subtypes (Samuels and Ericson, 2006). PI3Kβ and PI3Kδ activity might also be important in some tumors (Ciraolo et al., 2008; Jia et al., 2008; Lannutti et al., 2011). The fourth member of the class I PI3K subgroup, PI3Kγ, has received less attention as a drug target in oncology for two main reasons. First, PI3Kγ is activated by GPCRs but has not been demonstrated to function in signals emanating from TKs, which are the dominant drivers of the cancer phenotype. Second, PI3Kγ is mainly found in leukocytes, with few tumors showing prominent expression or function of this isoform. Hence, one would not expect selective inhibitors of PI3Kγ to suppress directly the proliferation or survival of most cancer cells. Yet what if PI3Kγ is integral for the function of other cells in the tumor microenvironment?
Schmid et al. provide abundant evidence to support this idea. The team used a variety of experimental approaches to assess the role of PI3Kγ: knockout mice; knock-in mice with a kinase-dead mutation in PI3Kγ; selective inhibitors of PI3Kγ and other isoforms; and RNAi-mediated knockdown. In each case, interfering with PI3Kγ function strongly blocked the activation of integrin α4β1. In addition, receptor-mediated increases in PIP3 and phosphorylation of the PI3K effector AKT were absolutely dependent on PI3Kγ and not other isoforms. The results were comparable whether the cells were stimulated through GPCRs (with the chemokines SDF-1α or C5a), through RTKs (with growth factors VEGF or CSF-1) or via receptors linked to cytoplasmic TKs (with cytokines IL-1, IL-6 or TNFα). Further, only PI3Kγ knockdown prevented myeloid cells from trafficking to tumors in mice. This indicates that whatever the mix of chemotactic factors present in vivo, the signal to invade the tumor requires only the PI3Kγ isoform.
These results challenge the paradigm that PI3Kγ functions exclusively in GPCR signaling. To lend weight to this observation, the investigators undertook a major effort to understand the biochemical mechanisms that allow the PI3Kγ isoform to couple to TK-based signals. They show that treatment of myeloid cells with VEGF-A causes the cognate RTK (VEGFR1) to physically associate with PI3Kγ but not other class I isoforms. They define a pathway in which RTK engagement promotes activation of the Ras GTPase, which recruits and activates PI3Kγ via its regulatory subunit p87 (Figure 1). A distinct regulatory subunit, p101, plays a complementary role in activating PI3Kγ downstream of GPCRs and Ras in this system. It will be interesting to see whether this division of labor is common to other leukocyte subsets. A key experiment showed that knockdown of p87 and p101 had additive effects on suppressing myeloid cell trafficking to tumors in mice. This indicates that chemotactic factors in vivo likely include distinct stimuli acting through TKs and GPCRs.
Suppressing PI3Kγ clearly prevents accumulation of certain myeloid populations in tumors; does this have any impact on tumor growth? Schmid et al. demonstrate that transplanted and spontaneous tumors develop more slowly in PI3Kγ-deficient mice. In each model, suppression of PI3Kγ resulted in impaired angiogenesis. This probably results from the absence of myeloid cells that secrete angiogenic factors such as VEGF (Figure 1), rather than a defect in ECs. Indeed, bone marrow chimera experiments showed that the defective cell type in the tumor microenvironment is of hematopoietic origin. Treating mice with selective PI3Kγ inhibitors also reduced tumor growth, even though the compounds had no direct impact on cancer cell proliferation in vitro. A key experiment showed that PI3Kγ inhibitors do not further reduce tumor growth in wild-type mice bearing PI3Kγ-deficient blood cells. These results demonstrate that PI3Kγ inhibitors act via a cancer cell-extrinsic manner to suppress tumor-associated inflammation and angiogenesis.
Drug discovery pipelines include a growing number of PI3K inhibitors in clinical trials for oncology, autoimmunity, allergy and other disease states (Workman et al., 2010). Until now, PI3Kγ has been considered mainly as a target for inflammatory diseases such as arthritis (Ruckle et al., 2006), and the development of PI3Kγ-selective compounds has been slower than other target profiles. Although the conclusions of Schmid et al. need to be validated in other models, the current data support the novel conclusion that the anti-inflammatory potential of PI3Kγ inhibitors might be harnessed to disrupt the tumor microenvironment and slow the progression of cancer. The fact that most solid tumor cells do not express PI3Kγ should limit the development of resistance to PI3Kγ inhibitors. These considerations should increase momentum for PI3Kγ inhibitor programs. To be sure, there remain challenges to developing treatments targeting cancer inflammation. Should such agents be given as preventive therapy, or will they be effective in treating established malignancies? These questions can be addressed initially through additional preclinical studies. If PI3Kγ-targeted agents can limit the growth of established tumors, an important implication is that drugs targeting all class I PI3Ks (or just PI3Kα and PI3Kγ) should be more effective than selective PI3Kα inhibitors, even in patients whose tumors are driven by PI3KCA mutations.
Acknowledgement
We thank Christian Rommel for helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, et al. Phosphoinositide 3-kinase p110{beta} activity: Key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an 'aspirin of the 21st century'? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, Acevedo LM, Manglicmot JR, Song X, Wrasidlo W, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kg, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011 doi: 10.1016/j.ccr.2011.04.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Workman P, Clarke PA, Raynaud FI, van Montfort RL. Drugging the PI3 Kinome: From Chemical Tools to Drugs in the Clinic. Cancer Res. 2010;70:2146–2157. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]