Abstract
The association between higher New York Heart Association (NYHA) functional classes and poor outcomes in heart failure (HF) is well known. However, to what extent these associations are confounded by covariates such as age, severity of disease and comorbidity burden are unknown. In the Digitalis Investigation Group trial, 2441 of the 7788 chronic HF patients had NYHA class III–IV symptoms. Propensity scores for NYHA class III–IV were calculated for each patient, and were then used to match 1863 NYHA class III–IV patients with 1863 NYHA class I–II patients. Kaplan-Meier and matched Cox regression analyses were used to estimate associations of NYHA class III–IV with mortality and hospitalizations during 37 months of median follow up. Compared with 34% (641/1863) NYHA class I–II patients (mortality rate, 1175/10,000 person-year of follow-up), 42% (777/1863) NYHA class III–IV patients (rate, 1505/10,000 person-year) died from all causes (hazard ratio {HR} =1.29; 95% confidence interval {CI} =1.14–1.45; P<0.0001). Hospitalizations due to all causes occurred in 66% (1232/1863) NYHA class I–II (hospitalization rate, 3898/10,000 person-year) and 71% (1322/1863) (rate, 4793/10,000 person-year) NYHA class III–IV patients (HR =1.16; 95% CI =1.05–1.28; P=0.003). HR (95%CI) for NYHA class III–IV patients, when compared with NYHA class I–II patients, for other outcomes are: cardiovascular mortality, 1.29 (1.12–1.48; P<0.0001), HF mortality, 1.49 (1.20–1.84; P<0.0001), cardiovascular hospitalization, 1.18 (1.06–1.32; P=0.002), and HF hospitalization, 1.17 (1.03–1.34; P=0.017). Baseline NYHA class is a marker of hospitalization, disease progression, and mortality in a wide spectrum of ambulatory chronic HF patients.
Keywords: heart failure, NYHA class, natural history, outcomes
The association of higher New York Heart Association (NYHA) functional class and poor heart failure (HF) outcomes is well known.1 However, many of these data were derived from advanced HF patients awaiting cardiac transplants, raising concerns for residual confounding by age, severity of disease and comorbidity burden.2, 3 Studies in ambulatory HF patients are limited by small sample size, short-term follow up, and outcome-based multivariable regression analyses.4–6 Outcomes studies based on hospitalized HF patients often lack data on NYHA classification.7–9 The objective of this study was to determine the association between NYHA functional class and broader natural history endpoints such as all-cause and cardiovascular mortality and hospitalizations in ambulatory chronic HF patients using propensity score matching.
Methods
A public use copy of the DIG data sets was used for the current analysis. The DIG trial enrolled 7788 ambulatory chronic HF patients in normal sinus rhythm from 302 clinical centers in the U.S. and Canada during 1991–1993.10, 11 Of these patients, 6800 had left ventricular ejection fraction (LVEF) ≤45% and 988 had LVEF >45%. Participants were classified by DIG investigators into one of the four NYHA classes depending on the severity of HF symptoms and the degree of effort needed to elicit those symptoms: class I (n=1103), class II (n=4244), class III (n=2287), and class IV (n=154). Because of functional similarity between class I and II patients and class III and IV patients, and for the convenience of propensity score matching, we categorized patents as having NYHA class I–II (n=5347) and III–IV (n=2441) symptoms. Primary outcomes of interest were mortality and hospitalizations due to all causes, cardiovascular causes, and worsening HF. Data on vital status were 99% complete.12
Because of significant imbalance in baseline covariates between NYHA class I–II and III–IV patients, propensity scores to have NYHA III–IV symptoms were calculated for each of the 7788 patients using a non-parsimonious multivariable logistic regression model, adjusting for all available baseline covariates (as shown in Table 1), and incorporating significant two-way interaction terms in the model (Figure 1).13, 14 The model calibrated (Hosmer-Lemeshow test: p = 0.303) and discriminated (area under the ROC curve; C = 0.80) well.
Table 1.
Baseline patient characteristics, before and after propensity score matching
| N (%) or mean (±SD) | Before matching* |
NYHA III – IV† (N=1,863) | After matching
|
||
|---|---|---|---|---|---|
| NYHA I – II (N=1,863) | P | P Value | NYHA I – II (N=1,863) | ||
| Age (years) | 63.0 (±10.8) | <0.0001 | 64.7 (±11.0) | 0.734 | 64.8 (±10.5) |
| Age ≥65 years | 992 (49.5%) | 0.012 | 1000 (53.7%) | 0.065 | 1057(56.7%) |
| Female | 434 (23.3%) | 0.002 | 516 (27.7%) | 0.659 | 503 (27.0%) |
| Non-white | 260 (14.0%) | 0.778 | 266 (14.3%) | 0.963 | 268 (14.4%) |
| Body mass index, kg/square meter | 27.4 (±5.03) | 0.923 | 27.4 (±5.8) | 0.786 | 27.4 (±5.8) |
| Duration of HF (months) | 28.6 (±35.4) | 0.022 | 31.3 (±37.7) | 0.474 | 30.1 (±38.8) |
| Primary cause of HF | |||||
| Ischemic | 1274 (68.4%) | 1284 (68.9%) | 1269 (68.1%) | ||
| Hypertensive | 196 (10.5%) | 179 (9.6%) | 199 (10.7%) | ||
| Idiopathic | 266 (14.3%) | 0.830 | 272 (14.6%) | 0.681 | 272 (14.6%) |
| Others | 127 (6.8%) | 128 (6.9%) | 119 (6.4%) | ||
| Prior myocardial infarction | 1158 (62.2%) | 0.660 | 1171 (62.9%) | 1.000 | 1171 (62.9%) |
| Current angina | 431 (23.1%) | <0.0001 | 594 (31.9%) | 0.752 | 604 (32.4%) |
| Hypertension | 876 (47.0%) | 0.922 | 873 (46.9%) | 1.000 | 872 (46.8%) |
| Diabetes | 489 (26.2%) | 0.005 | 567 (30.4%) | 0.377 | 592 (31.8%) |
| Chronic kidney disease | 798 (42.8%) | <0.0001 | 927 (49.8%) | 0.922 | 924 (49.6%) |
| Medications | |||||
| Pre-trial digoxin use | 769 (41.3%) | 0.031 | 835 (44.8%) | 0.947 | 838 (45.0%) |
| Trial use of digoxin | 940 (50.5%) | 0.948 | 937 (50.3%) | 0.646 | 922 (49.5%) |
| ACE inhibitors | 1740 (93.4%) | 0.307 | 1756 (94.3%) | 0.581 | 1747 (93.8%) |
| Hydralazine & nitrates | 23 (1.2%) | 0.882 | 22 (1.2%) | 0.566 | 27 (1.4%) |
| Non-potassium sparing diuretics | 1374 (73.8%) | <0.0001 | 1585 (85.1%) | 0.610 | 1597 (85.7%) |
| Potassium diuretics | 134 (7.2%) | 0.707 | 141 (7.6%) | 0.542 | 151 (8.1%) |
| Potassium supplement | 474 (25.4%) | <0.0001 | 602 (32.3%) | 0.360 | 575 (30.9%) |
| Symptoms and signs of heart failure | |||||
| Dyspnea at rest | 265 (14.2%) | <0.0001 | 583 (31.3%) | 0.723 | 572 (30.7%) |
| Dyspnea on exertion | 1263 (67.8%) | <0.0001 | 1706 (91.6%) | 1.000 | 1705 (91.5%) |
| Jugular venous distension | 170 (9.1%) | <0.0001 | 327 (17.6%) | 0.384 | 307 (16.5%) |
| Third heart sound | 359 (19.3%) | <0.0001 | 546 (29.3%) | 0.971 | 547 (29.4%) |
| Pulmonary râles | 223 (12.0%) | <0.0001 | 401 (21.5%) | 0.749 | 392 (21.0%) |
| Lower extremity edema | 281 (15.1%) | <0.0001 | 488 (26.2%) | 0.881 | 483 (25.9%) |
| Heart rate (/minute), | 77.4 (±12.5) | <0.0001 | 79.5 (±12.4) | 0.931 | 79.6 (±12.5) |
| Blood pressure (mm Hg) | |||||
| Systolic | 128.0 (±19.9) | 0.002 | 125.9 (±21.2) | 0.109 | 127.0 (±19.7) |
| Diastolic | 75.6 (±11.1) | 0.007 | 74.5 (±12.0) | 0.565 | 74.8 (±11.1) |
| Chest radiograph findings | |||||
| Pulmonary congestion | 206 (11.1%) | <0.0001 | 334 (17.9%) | 0.966 | 332 (17.8%) |
| Cardiothoracic ratio >0.5 | 1037 (55.7%) | <0.0001 | 1231 (66.1%) | 0.152 | 1273 (68.3%) |
| Serum creatinine (mg/dL) | 1.26 (±0.35) | <0.0001 | 1.30 (±0.38) | 0.455 | 1.31 (±0.39) |
| Ejection fraction (%) | 33.2 (±12.0) | <0.0001 | 30.3 (±12.7) | 0.717 | 30.4 (±11.6) |
| Ejection fraction >45% | 248 (13.3%) | 0.015 | 199(10.7%) | 0.329 | 180 (9.7%) |
Of the 5347 NYHA I–II patients, 1863 were randomly chosen for pre-match comparison with 1863 NYHA III and IV. This was done to have similar pre-match and post-match sample sizes, and to avoid overestimation of significant p values from a larger sample size.
Of the 2441 NYHA III–IV patients, 1863 (76%) were matched with 1863 NYHA I–II patients with similar propensity scores
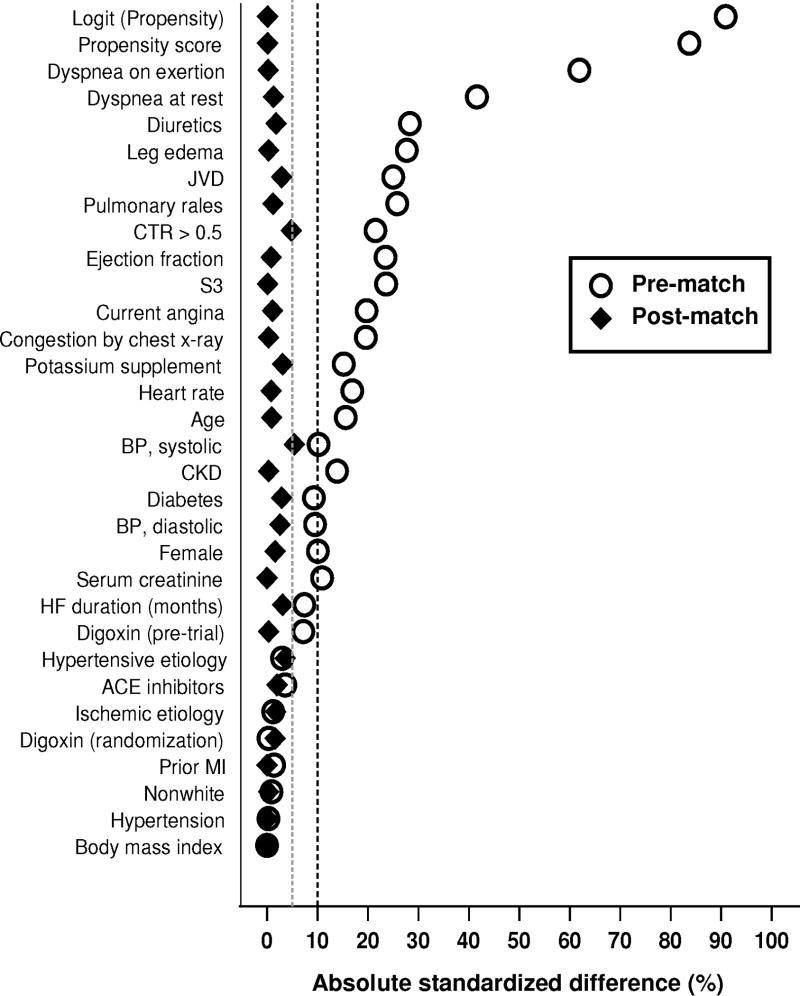
Figure 1.
Absolute standardized differences before and after propensity score matching comparing covariate values for patients with New York Heart Association class I–II versus III–IV
ACE=angiotensin-converting enzyme; BP=blood pressure; CKD=chronic kidney disease; CTR=cardiothoracic ratio; JVD=jugular venous distention; MI=myocardial infarction; S3=third heart sound
Using a SPSS macro, we matched each NYHA III–IV patient with another patient, who had NYHA class I–II symptoms, but had similar propensity score for NYHA III–IV symptoms.15–19 Overall, 76% (1863/2441) NYHA III–IV patients were matched with 1863 of NYHA I–II patients with similar propensity scores. To assemble a comparable sized pre-match cohort, we randomly selected 1863 NYHA I–II patients from the pre-match file and were paired with 1863 NYHA III–IV in the matched file.
Absolute standardized difference in propensity scores between NYHA I–II and NYHA III–IV patients before and after matching were respectively 84% and 0.1% (Figure 2). Absolute standardized difference after matching between NYHA I–II versus III–IV patients in all measured covariates were <5% (Figure 2). An absolute standardized difference of <10% is considered acceptable reduction of bias.16–21
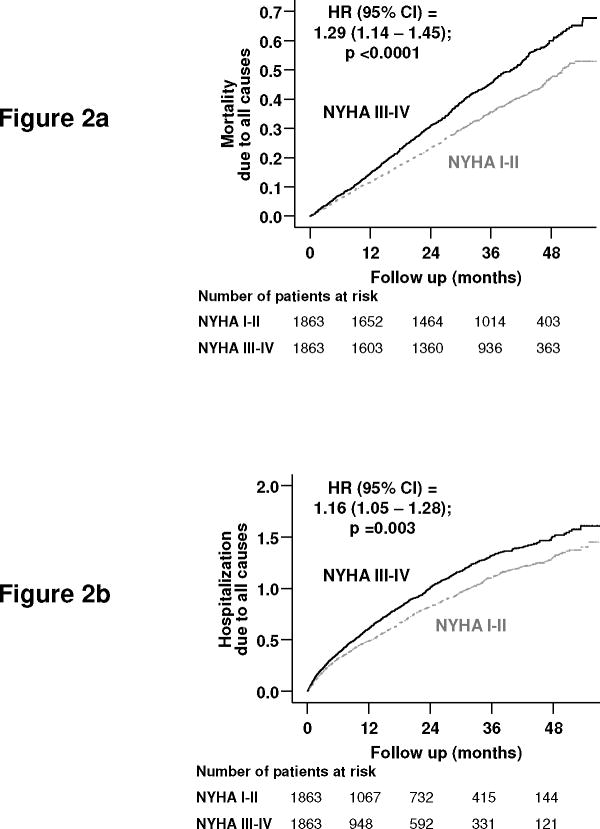
Figure 2.
Kaplan-Meier plots for (a) all-cause mortality and (b) all-cause hospitalization
HR=hazard ratio; CI=confidence interval
Baseline characteristics of HF patients with NYHA I–II versus III–IV symptoms were compared using Pearson chi-square and Wilcoxon rank-sum tests. Kaplan-Meier analysis and matched Cox regression analyses were used to determine association of NYHA III–IV (relative to class I or II) and various outcomes. Subgroup analyses and first-order interaction were used to test heterogeneity of the association between NYHA class and mortality. All statistical tests were done using SPSS for Windows (Release 14), and two-tailed 95% confidence levels; a p <0.05 was required to reject the null hypothesis.
Results
Overall, patients had a mean age of 65 years, 28% were female, and 14% were nonwhites. Baseline characteristics of patient with NYHA I–II versus III–IV symptoms, before and after matching are displayed in Table 1. There were significant differences in covariates before matching, which was absent from the matched cohort. Quantitative measures of biases before and after matching, and reduction of bias after matching are displayed in Figure 1. Values of absolute standardized differences for all covariates were <5%, suggesting considerable reduction of bias.16, 17, 20, 21
Mortality
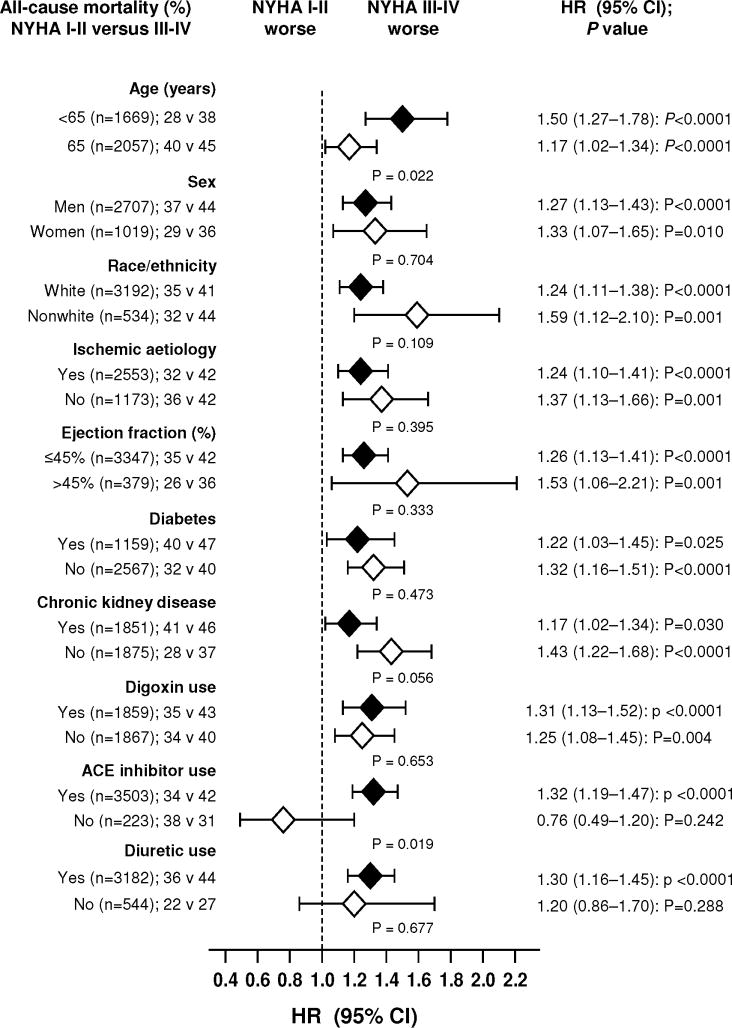
Overall, 1418 patients (38%) died, including 1114 (30%) deaths from cardiovascular causes and 518 due to HF during the median follow up of 37 months. Kaplan-Meier plots for death due to all causes are displayed in Figure 2a. Compared with 641 deaths from all causes in NYHA I–II patients during 5455 years of follow up (rate, 1175/10,000 person-year), there were 777 death in NYHA III–IV patients during 5162 years (rate, 1505/10,000 person-year; Table 2). NYHA III–IV symptoms were associated with a significant 29% increase in all-cause mortality (hazard ratio, 1.29, 95% confidence interval, 1.14– 1.45; p <0.0001; Table 2). NYHA III–IV was associated with similar increase in mortality due to cardiovascular causes and HF (Table 2). The association between NYHA class III–IV and all-cause mortality were observed across various subgroups of patients, (Figure 3).
Table 2.
Mortality and hospitalizations by causes in heart failure patients before and after matching by propensity scores for NYHA class III–IV
| NYHA I–II (N=1,863) | NYHA III–IV (N=1,863) | Absolute difference* (per 10,000 person-years of follow up) | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Mortality Rate, per 10,000 person-years of follow up (Events/total follow up years) | |||||
| Mortality | |||||
| All-cause | 1,175 (641/5,455) | 1,505 (777/5,162) | + 330 | 1.29 (1.14–1.45) | <0.0001 |
| Cardiovascular | 924 (504/5,455) | 1,182 (610/5,162) | + 258 | 1.29 (1.12–1.48) | <0.0001 |
| Worsening heart failure | 409 (223/5,455) | 571 (295/5,162) | + 162 | 1.49 (1.20–1.84) | <0.0001 |
| Hospitalization** | |||||
| All-cause | 3,898 (1,232/3,161) | 4,793 (1,322/2,758) | + 895 | 1.16 (1.05–1.28) | 0.003 |
| Cardiovascular | 2,580 (965/3,741) | 3,227 (1,075/3,331) | + 647 | 1.18 (1.06–1.32) | 0.002 |
| Worsening heart failure | 1,348 (613/4,547) | 1,592 (665/4,175) | + 244 | 1.17 (1.03–1.34) | 0.017 |
| Number of total hospitalizations | 12,129 | 15,105 | + 2,976 | ||
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the NYHA class III–IV group from the event rates in the NYHA class I–II group (before values were rounded)
Data shown include the first hospitalization of each patient due to each cause.
Figure 3.
Hazard ratio and 95% confidence interval (CI) for all-cause mortality in subgroups of heart failure patients matched by propensity scores for New York Heart Association (NYHA) class III–IV
(ACE=angiotensin-converting enzyme; chronic kidney disease=estimated glomerular filtration rate <60 ml/min/1.73m2)
Hospitalization
Overall, 2554 patients (69%) were hospitalized due to all causes, including 2040 (55%) due to cardiovascular causes and 1278 (34%) due to worsening HF. Kaplan-Meier plots for hospitalizations due to all causes are displayed in Figure 2b. Compared with 1232 hospitalizations from all causes in NYHA I–II patients during 3161 years of follow up (rate, 3898/10,000 person-year), 1322 NYHA III–IV patients were hospitalized during 2758 years of follow up (rate, 4793/10,000 person-year; Table 2). NYHA III–IV was associated with a significant 16% increase in all-cause hospitalization (hazard ratio, 1.16, 95% confidence interval, 1.05– 1.28; p <0.0001; Table 2). NYHA III–IV was associated with similar increase in hospitalizations due to cardiovascular causes and worsening HF (Table 2).
Discussion
These findings suggest that subjective determination of baseline NYHA classes based on functional capacity and HF symptoms can serve as a marker of important natural history endpoints in a wide spectrum of chronic systolic and diastolic HF patients. This is the first demonstration of a significant association between NYHA class and long-term outcomes in HF using propensity score analysis. NYHA classification can be easily obtained by clinicians and may be used to identify high risk HF patients for appropriate interventions.
NYHA functional classification, although subjective and may vary over time, is a clinical measure of overall symptom burden in HF.22 However, the findings of the current analysis suggest that higher symptom burden in HF may represent more than worsening clinical symptoms related to noncompliance with medications or salt or fluid. Instead, this may be a marker of disease progression, hospitalization and mortality. NYHA class III–IV HF patients are more likely to be elderly, women, have longer duration of HF, current angina, diabetes, and chronic kidney disease (Table 1). They were also more likely to have symptoms and signs of HF and be receiving diuretics. However, these are unlikely to explain our findings as our propensity score matching reduced bias to <5% absolute standardized differences for all these covariates.16, 17, 21, 23
The results of the current study are consistent with those for prior studies. However, most of those studies are based on advanced systolic HF patients awaiting cardiac transplants.2, 3 Studies of ambulatory HF patients suggesting association between higher NYHA functional class and poor short-term outcomes are limited by small sample size, shorter follow up and residual confounding.4–6, 24–26 Studies of predictors of HF outcomes based on HF registries typically do not report data on NYHA classification.7–9 Large sample size, long follow up, cause- specific outcomes and use of propensity score matching distinguishes the current study from previous studies. In particular, as illustrated in Table 1 and Figure 1, propensity score matching allows a more quantitatively assessment of bias reduction.
This study has several limitations. While propensity score technique can account for imbalances in all measured covariates, it may or may not balance unmeasured covariates. However, for such an unmeasured confounder to explain away our finding it must be strongly associated with both NYHA class and outcomes, and be not strongly associated with any of the many baseline covariates in the DIG trial.11, 16–19 We did not have data on NYHA status during the follow up. It is possible that some patients with NYHA class I–II became III–IV due to disease progression or noncompliance with therapy, and vice-versa. However, such misclassification is likely to be random and could only have underestimated the association observed in our analysis. Finally, the results of this study are based on relatively young male HF patients in normal sinus rhythm from a pre-beta-blocker era and their relevance to contemporary HF patients is unknown.
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100).
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup ML, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) American College of Cardiology; [Accessed on August 17, 2005]. 2005. Web Site. Available at: http://www.acc.org/clinical/guidelines/failure//index.pdf. [DOI] [PubMed] [Google Scholar]
- 2.Campana C, Gavazzi A, Berzuini C, Larizza C, Marioni R, D’Armini A, Pederzolli N, Martinelli L, Vigano M. Predictors of prognosis in patients awaiting heart transplantation. J Heart Lung Transplant. 1993;12:756–765. [PubMed] [Google Scholar]
- 3.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 4.Scrutinio D, Lagioia R, Ricci A, Clemente M, Boni L, Rizzon P. Prediction of mortality in mild to moderately symptomatic patients with left ventricular dysfunction. The role of the New York Heart Association classification, cardiopulmonary exercise testing, two-dimensional echocardiography and Holter monitoring. Eur Heart J. 1994;15:1089–1095. doi: 10.1093/oxfordjournals.eurheartj.a060633. [DOI] [PubMed] [Google Scholar]
- 5.Madsen BK, Hansen JF, Stokholm KH, Brons J, Husum D, Mortensen LS. Chronic congestive heart failure. Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J. 1994;15:303–310. doi: 10.1093/oxfordjournals.eurheartj.a060495. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444–450. doi: 10.1016/j.ahj.2005.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162:1689–1694. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. Jama. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 10.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 11.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 12.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 14.Rubin DB. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 15.Levesque R. Macro. In: Levesque R, editor. SPSS® Programming and Data Management, 2nd Edition. A Guide for SPSS® and SAS® Users. 2. Chicago, IL: SPSS Inc; [Last access date: June 4, 2005]. Available online at: http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 16.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed A, Husain A, Love TE, Gambassi G, Dell’italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A, Ali M, Lefante CM, Mullick MS, Kinney FC. Geriatric heart failure, depression, and nursing home admission: an observational study using propensity score analysis. Am J Geriatr Psychiatry. 2006;14:867–875. doi: 10.1097/01.JGP.0000209639.30899.72. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006;152:956–966. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25:2084–2106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 21.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 22.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JG, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, Goff DC, Grover FL, Malenka DJ, Peterson ED, Redberg RF. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Failure Society of America. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, Blazing MA, Davenport C, Califf RM, Krishnan RR, O’Connor CM. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 25.Muntwyler J, Abetel G, Gruner C, Follath F. One-year mortality among unselected outpatients with heart failure. Eur Heart J. 2002;23:1861–1866. doi: 10.1053/euhj.2002.3282. [DOI] [PubMed] [Google Scholar]
- 26.Bouvy ML, Heerdink ER, Leufkens HG, Hoes AW. Predicting mortality in patients with heart failure: a pragmatic approach. Heart. 2003;89:605–609. doi: 10.1136/heart.89.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]