Abstract
The prognosis of patients with human high-grade gliomas (HGGs) remains dismal despite major advances in their management, due mainly to the high resistance of these infiltrative tumor cells to programmed cell death (PCD). Most therapeutic strategies for HGGs are aimed to maximize PCD type I, apoptosis or type II, autophagy. These are predominantly distinctive processes, but many studies suggest a cross-talk between the two. A better understanding of the link between PCD types I and II might allow development of more effective therapies for HGGs. In this study, we examined whether there is a common upstream signaling event responsible for both apoptotic and autophagic PCD using 3 chemotherapeutic agents in human HGG cells. Our study shows that each agent caused a significant decrease in cell viability in each of the HGG cell lines tested. The increase rate of apoptosis and autophagy varied among cell lines and chemotherapeutic agents used. Increased expression of cytidine-cytidine-adenosine-adenosine-thymidine (C)/enhancer binding protein (EBP) homologous transcription factor C/EBP homologous protein (CHOP)/growth arrest and DNA damage–inducible gene 153 (GADD153) was documented after use of either pro-autophagic or pro-apoptotic agents. The involvement of CHOP/GADD153 in both type I and type II PCD was confirmed by overexpression and gene-silencing studies. Gene silencing by small-interfering RNA–mediated CHOP/GADD153 resulted in increased cell viability, decreased upregulation of microtubule-associated protein light-chain 3′ type II (LC3II) and cleaved caspase-3, and inhibition of apoptosis and autophagy. Exogenous expression of CHOP/GADD153 triggered apoptosis and autophagy in the absence of other stimuli. The clinical significance of these findings was supported by the evidence that celecoxib, a nonsteroidal anti-inflammatory drug known to induce GADD153-mediated apoptosis, strongly increases both type I and type II PCD in HGG cells when combined with another inducer of GADD153. These data suggest that CHOP/GADD153 should be investigated as a novel targetable signaling step to improve therapies for HGGs.
Keywords: apoptosis, autophagy, CHOP/GADD153, cell viability, gliomas
Gliomas, a heterogeneous group of malignancies that originate and reside within the brain, are the most common primary brain tumors. According to the 2007–2008 report of the Central Brain Tumor Registry of the United States, the national incidence of gliomas is 16.5 cases per 100 000 persons/year.1 This translates to approximately 51 410 newly diagnosed cases in the United States per year.1 High-grade gliomas (HGGs), the most frequent types of gliomas, are associated with poor prognosis. Among these, glioblastoma multiforme (GBM), accounting for 50% of gliomas, is associated with a 5-year survival rate of less than 5% and a median survival rate of approximately 14 months, even after use of aggressive surgical tumor resection and postoperative radiation therapy combined with temozolomide (TMZ) chemotherapy is used.2 This is due mainly to high resistance of these infiltrative tumor cells to programmed cell death (PCD).
HGG cells show only partial sensitivity to therapies that induce apoptosis, i.e., PCD type I.3–6 Recent studies indicate that HGG cells are more sensitive to treatments that induce autophagy, i.e., PCD type II.5,6 A variety of chemical and physical treatments, including ionizing radiation (IR);7 arsenic trioxide, As2O3 (ATO);8,9 TMZ;10 and many others, have been reported to induce autophagy in vitro and in vivo in HGG cells. Additionally, some chemotherapeutics have both pro-apoptotic and pro-autophagic actions. In particular, TMZ, the oral alkylating agent currently considered the standard of care for patients with HGG, has been shown to exert its cytotoxicity by inducing both apoptosis and autophagy.6,10–15
Autophagy and apoptosis cause PCD by two predominantly distinct pathways.5,16,17 Recent studies, however, have suggested a cross-talk between them.18,19 Moretti et al.19 summarized the mechanisms that might connect these two pathways in the setting of IR. IR has been shown to activate unfolded protein response (UPR), which triggers an endoplasmic reticulum (ER) stress response. The UPR consists of a set of adaptive pathways that are triggered by disturbances in normal function of the ER, leading to the production of misfolded proteins. The UPR alleviates ER stress by arresting general translation, upregulation of chaperones and folding enzymes, and degradation of misfolded proteins.20 When the whole ER system is overloaded with misfolded proteins, cells will undergo cell death, typically via apoptosis.21–23 ER stress can also induce autophagic cell death; however, the molecular mechanisms behind it are still not fully elucidated.24,25 A major UPR target is the induction of the glucose-regulated protein (GRP)–78/binding protein (BiP), which plays an important role in protein folding and assembly, targeting misfolded proteins for degradation, ER Ca2+ binding, and control of the activation of transmembrane ER stress sensors.26 Thus, GRP-78 represents a prosurvival arm of the UPR. On the other hand, the cytidine-cytidine-adenosine-adenosine-thymidine (C)/enhancer binding protein (EBP) homologous transcription factor C/EBP homologous protein (CHOP)/growth arrest and DNA damage–inducible gene 153 (GADD153) is one of the critical executioners of the pro-apoptotic arm of the UPR.27 Signals are induced from CHOP that the cell is experiencing ER stress and hence initiating PCD. Microarray studies revealed that CHOP is one of the most highly inducible genes during ER stress.28 Overexpression of CHOP and micro-injection of CHOP protein have been reported to lead to cell-cycle arrest and cell apoptosis.29,30
In our study, we test the hypothesis that in the presence of non-IR–induced ER stress, there is an upstream common signal that links autophagy and apoptosis. Our current experiments show that CHOP/GADD153 serves as an important link between PCD type I and type II in the presence of non-IR–induced ER stress in human HGGs. Finally, to show the potential clinical significance of our results, we investigated the effects of combining two ER stress–inducing agents on HGG PCD. We chose celecoxib (CXB), a nonsteroidal anti-inflammatory drug and COX-2 inhibitor exhibiting a promising chemopreventive activity in experimental animal models of colon cancer and in clinical trials.31–33
Materials and Methods
Cell Lines, Cultures, and Chemotherapeutic Agents
The human HGG cell lines U87, A172, and T98G used for these in vitro experiments were obtained from the American Type Culture Collection. Cells were propagated in monolayer and cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum in a 37°C incubator supplemented with 5% CO2. TMZ was supplied by Schering-Plough, dissolved in dimethyl sulfoxide (DMSO), kept in 50-mM stock solution, and used at a concentration of 100 µmol/L, according to our previous work.10 ATO (MP Biomedicals) was used at a concentration of 4 μM in all experiments, according to our previous work.8,9 Cisplatin (CDDP) (Enzo Life Sciences) was used at a concentration of 5 μg/mL in all experiments.34 Recombinant human DNA damage–inducible transcript 3/GADD153 (Creative BioMart) was used at a concentration of 1 μg/mL. CXB (Toronto Research Chemicals) was used at concentrations of 10 and 20 μM.35
Cell Viability Assay
Cell viability was measured by a 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay kit (Chemicon International). Cells were seeded in 96-well tissue culture plates (1.0 × 103 cells per well) 24 h prior to treatment. Seventy-two hours after treatment, the medium was removed, and fresh medium containing 0.5 mg/mL MTT was added to each well. The cells were incubated at 37°C for 4 h, and then an equal volume of color development solution (0.04 N HCl in isopropanol) was added to each well and mixed thoroughly. The optical density from the plates was read on a micro plate reader at 570 nm.
Apoptosis and Inhibition Assay
Fluorescence-activated cell sorting (FACS) analysis was used to quantify apoptotic cells after treatment, following labeling with Annexin V–Phycoerythrin (PE) to detect early apoptotic cells and vital dye 7-Amino-Actinomycin (7AAD) to detect late apoptotic and necrotic cells, using an Annexin V kit (BD Biosciences) following manufacturer instructions. Briefly, 2 × 105 HGG cells were seeded in a 6-well plate 24 h prior to treatment. The following day, cells were treated as indicated in the cell viability assay section of this paper, and 72 h later were collected for staining. Gating with appropriate measurement of forward and side scatter was used to exclude cell debris and cell aggregates from the sorting; 5000 to 10 000 cells were counted per analysis. Data from flow cytometry (Becton Dickinson) were further analyzed using the FlowJo software (Treestar). Apoptosis was inhibited with a pan-caspase inhibitor, Z-DEVD-FMK (Z-VAD(OMe)-FMK) (R&D Systems) solubilized in DMSO following manufacturer instructions. Cells were pretreated with 50 μM of Z-DEVD-FMK for 1 h before treatment with each chemotherapeutic. Cell viability was measured by MTT assay 72 h later, as described above.
Autophagy and Inhibition Assay
Autophagy is the process of sequestrating cytoplasmic proteins into the lytic component and is characterized by the formation of acidic vesicular organelles. To detect and quantify these 72 h after treatment, we performed vital staining with acridine orange (AO; Invitrogen), as previously described.10 Briefly, cells were stained with AO at a final concentration of 1 μg/mL for a period of 15 min, removed from the plate with trypsin–ethylenediaminetetraacetic acid, and collected in phenol red–free growth medium. Green (510–530 nm) and red (>650 nm) fluorescence emission from 104 cells illuminated with blue (488 nm) excitation light was measured with FACSCalibur using Flowjo software. To inhibit autophagy, 1 mM 3-methyladenine (Sigma-Aldrich) was added 24 h after treatment with each chemotherapeutic.10 Cell viability was measured by MTT assay 72 h after chemotherapeutic treatment, as described above.
Western Blot Analysis
Briefly, for western blotting experiments, cells were harvested in radioimmunoprecipitation assay buffer (1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS] in phosphate buffered saline) containing a protease inhibitor cocktail (Roche), 50 mM NaF, and 1 mM Na3VO4, homogenized and centrifuged at 12 000 rpm for 10 min at 4°C. The supernatant was used as total cell lysate. Protein lysates (30–50 µg) were denatured in 2% SDS, 10 mM dithiothreitol, 60 mM Tris-HCl (pH 6.8), and 0.1% bromophenol blue and loaded onto a 12% polyacrylamide/SDS gel (National Diagnostics). The separated proteins were then transferred by electroblot (80 mA, 2 h) onto a polyvinylidene fluoride membrane (Roche). The membrane was blocked for 1 h at room temperature (rT) in 0.1 M Tris-HCl (pH 7.6), 1.37 M NaCl, 0.1% Tween 20 (TBS-T) containing 5% nonfat dry milk and incubated overnight at 4°C in TBS-T containing the primary antibody. The membrane was washed in TBS-T, incubated with the secondary antibody conjugated to horseradish peroxidase for 1 h at rT, and then washed in TBS-T. An enhanced chemiluminescence nonradioactive detection system (Amersham Pharmacia Biotech) was utilized to detect the antibody-protein complexes by exposure of the membrane to an X-ray film.
The following primary antibodies were used: rabbit polyclonal LC3B (1:1000) and rabbit polyclonal caspase-3 (1:1000) (Cell Signaling Technology), mouse monoclonal GADD153 (1:500) and goat polyclonal GRP-78 (1:500) (Santa Cruz Biotechnology), and mouse monoclonal actin (1:1000) (Millipore). Cell lysates for the detection of LC3, caspase-3, and GADD153 were prepared 48 h after chemotherapeutic treatment. For GRP-78 detection, cell lysates prepared at 10 h after chemotherapeutic treatment were used.
Transfection with siRNA
CHOP/GADD153 small interfering RNA (siRNA) was purchased from Santa Cruz Biotechnology. The transfection of siRNA was performed according to manufacturer instructions.
Transfection of CHOP/GADD153 Expression Vector
HGG cells were plated in 6-well plates at a density of 3 × 105 cells/mL and incubated at 37°C in a 5% CO2 atmosphere. Cells were transfected with the expression plasmid CHOP/pCMV6-XL5 or empty plasmids (pCMV6-XL5) using Lipofectamine 2000 reagent (Invitrogen). The CHOP expression plasmid CHOP/pCMV6-XL5 was purchased from Origene Technologies.36 Cells were harvested at 72 h after transfection for western blot and FACS analyses.
Statistical Analysis
All studies were repeated 3 times using triplicate samples for cell counting analysis. The statistical significance of the experimental results was evaluated using unpaired Student's t-tests. The data are expressed as means ± standard deviations and are considered significant when P < .05.
Results
TMZ, ATO, and CDDP Induce Decreased Cell Viability and Increased Apoptosis and Autophagy in Human HGGs
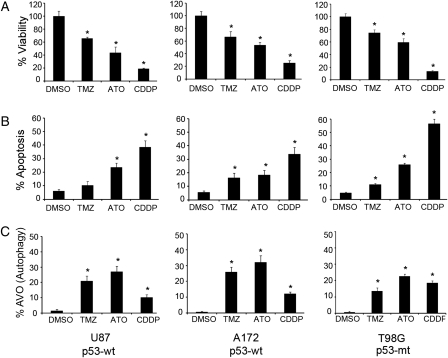
The pro-apoptotic and pro-autophagic effects of TMZ (100 μM), ATO (4 μM), and CDDP (5 μg/mL) were assessed on 3 HGG cell lines: U87, A172, and T98G. Each treatment caused a significant decrease in cell viability in each of the tested cell lines (P < .05, Student's t-test) compared with the nontreated controls independent of p53 status (Fig. 1A). CDDP had the most profound effects in all cell lines.
Fig. 1.
Cell viability, apoptosis, and autophagy rate in human HGG after treatment with TMZ, ATO, or CDDP. (A) A significant decrease in cell viability was found after each treatment in all 3 HGG cell lines. (B) Apoptotic rate quantified by FACS analysis showed a significant increase after ATO or CDDP in all 3 cell lines. TMZ caused a significant increase in apoptotic rate in A172 and T98G cells. (C) Rate of autophagic acidic vesicular organelles quantified by FACS analysis showed a significant increase in all 3 cell lines after treatment with TMZ, ATO, or CDDP. Data shown as bar graph representing mean ± SD of 3 experiments; each experiment done in triplicate; *(P < .05, Student's t-test compared with DMSO control); p53-wt = wild type, p53-mt = mutant.
A significant increase in apoptotic rate was seen in all 3 cell lines after treatment with ATO and CDDP compared with control cells (P < .05). TMZ caused a significant increase in apoptotic rate in T98G and A172 cells (Fig. 1B). CDDP had the most robust pro-apoptotic effect in all cell lines.
To explore the possible induction of autophagy, we quantified the presence of acidic vesicular organelles, which are characteristic of this process and can be detected by flow cytometry with AO staining. A significant increase in autophagy rate (P < .05) was found in all 3 cell lines after treatment with each of the 3 agents (Fig. 1C).
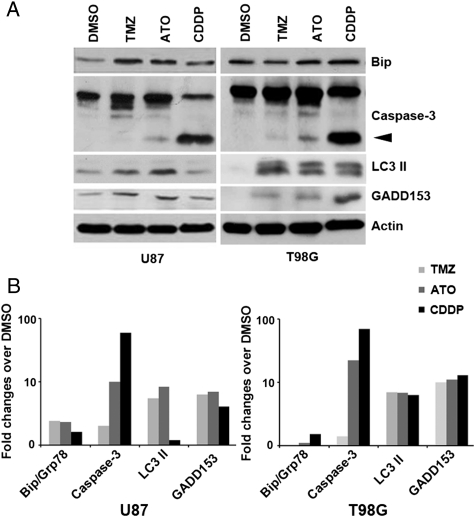
To confirm the presence of apoptosis and autophagy signaling and the induction of ER stress, western blotting was used to detect GRP-78/BiP, an ER stress marker; microtubule-associated protein light-chain 3′ type II (LC3II; autophagy); and cleaved caspase-3 (apoptosis). These results confirmed the presence of expected signaling in U87 and T98G cells after each treatment (Fig. 2). The activation of CHOP/GADD153 was seen after each treatment, suggesting that it could be a linking signal for both apoptosis and autophagy.
Fig. 2.
CHOP/GADD153 links apoptosis and autophagy. (A) Western blotting showing expression of ER stress (GRP-78/BiP), apoptosis (cleaved caspase-3), autophagy (LC3II) markers, and CHOP/GADD153 expression in U87 and T98G cells after each treatment. Arrowhead: cleaved caspase-3. Pan-actin used as a loading control. (B) Bar graphs of densitometric evaluation of western blot analyses in U87 and T98G cells.
Effects of Inhibition of Apoptosis or Autophagy on Cell Viability After TMZ, ATO, or CDDP Treatment of Human HGGs
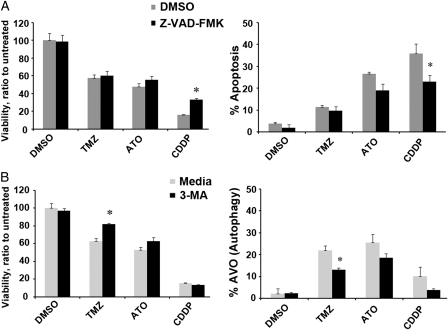
To determine the predominant pathway of PCD after treatment with each chemotherapeutic agent, we conducted inhibition experiments and assessed cell viability. Apoptosis was inhibited by the pan-caspase inhibitor Z-VAD-FMK and autophagy by 3-methyladenine, an autophagy inhibitor acting on the activity of phosphatidyl-inositol-3 kinase with halted formation of autophagosome and autophagic vacuoles. Lack of significant change in cell viability after blockage of apoptosis (Fig. 3A) or autophagy (Fig. 3B) was seen only after treatment with ATO in U87 HGG cells. After treatment with CDDP, inhibition of apoptosis caused significant increased viability. However, inhibition of autophagy did not have any significant effect. On the contrary, after treatment with TMZ, inhibition of autophagy caused significant increased viability. However, inhibition of apoptosis did not have any significant effect. These results confirm that TMZ causes cell demise mostly by autophagy and CDDP mostly by apoptosis. Similar effects were seen in T98G HGG cells (data not shown).
Fig. 3.
Effects of autophagy or apoptosis blockage on U87 cells after treatement with TMZ, ATO, or CDDP. (A) Bar graph representation of cell viability (left panel) and apoptosis (right panel) 3 days after each treatment with apoptosis blockade by Z-VAD-FMK (pan-caspase inhibitor). Note that an increase in cell viability after apoptosis blockage was seen with only CDDP treatment. (B) Bar graph representation of cell viability (left panel) and autophagy (right panel) 3 days after each treatment with autophagy blockade by 3-methyladenine (inhibitor of formation of acidic vesicular organelles). Note that an increase in cell viability after autophagy blockage was seen only with TMZ treatment. Results are normalized to DMSO. Data are shown as mean ± SD of 3 independent experiments. *P < .05, Student's t-test compared with DMSO.
Together, these results support the concept that the cytopathic effects of ATO on human HGGs are caused by both apoptosis and autophagy. Thus, ATO was used in our next experiments to test our hypothesis that CHOP/GADD153 serves as an upstream link between apoptosis and autophagy.
CHOP/GADD153 Links Apoptosis and Autophagy in Human HGGs
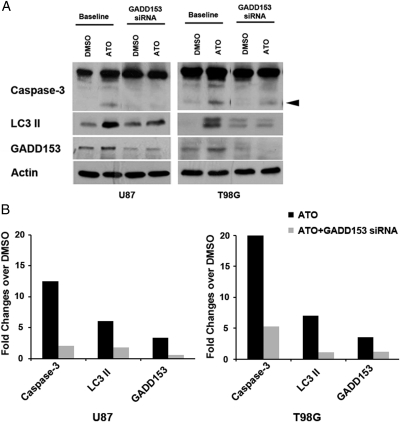
To elucidate the potential role of CHOP/GADD153 in autophagic and apoptotic PCD in human HGGs, we performed western blot analysis following silencing of CHOP/GADD153 by siRNA (Fig. 4). Inhibition of CHOP/GADD153 resulted in decreased expression of apoptosis (cleaved caspase-3) and autophagy (LC3II) markers in U87 and T98G HGG cells. These results suggest that CHOP/GADD153 serves as an upstream link between apoptosis and autophagy.
Fig. 4.
CHOP/GADD153 links apoptosis and autophagy in human HGG. (A) Western blotting at baseline and after blocking of CHOP/GADD153 by GADD153 siRNA in the presence or absence (DMSO) of ATO in U87 and T98G cells showing the expression of caspase-3, LC3II, and GADD153. Arrowhead: cleaved caspase-3. Actin was used as a loading control. (B) Bar graphs showing semi-quantitative densitometry values normalized to actin in U87 and T98G cells. Values expressed as fold changes compared to DMSO at baseline. Note the markedly decreased induction of cleaved caspase-3 and LC3 II in both HGG cells following silencing of CHOP/GADD153. Control siRNA resulted in expression similar to baseline (data not shown).
To confirm the role of CHOP/GADD153 in linking PCD types I and II, we performed cell viability assays and quantification of autophagy and apoptosis after siRNA blockage of CHOP/GADD153. Cell viability was significantly increased after CHOP/GADD153 blockage in U87 (Fig. 5A) and T98G (Fig. 5B) HGG cells. Apoptosis and autophagy were also significantly reduced after CHOP/GADD153 silencing in both HGG cells.
Fig. 5.
Effects of CHOP/GADD153 siRNA on cell viability, apoptosis, and autophagy after ATO treatment. Bar graphs showing a significant increase in cell viability after GADD153 silencing in U87 (A) and T98G (B) HGG cells (left panel). A significant decrease in apoptotic (middle panel) and autophagic (right panel) rate was also seen. Data are shown as mean ± SD of 3 independent experiments. Viability data are shown as normalized to DMSO (no ATO treatment); *P < .05, Student's t-test compared with control siRNA.
Overexpression of CHOP/GADD153 Decreases Cell Viability by Inducing Apoptosis and Autophagy in Human HGGs
To substantiate the significance of GADD153 on apoptosis, autophagy, and cell viability, an expression plasmid containing the full-length complementary DNA of human GADD153 was transiently transfected into HGG cells. GADD153 overexpression significantly decreased cell viability by inducing apoptotic as well as autophagic cell death (Fig. 6). Overexpression of GADD153 and LC3 and cleavage of caspase-3 were confirmed by western blot analysis (Fig. 6D and H). Similar effects were seen when commercially available recombinant human DNA damage–inducible transcript 3/GADD153 was added to the human HGG cells (data not shown). Taken together, these results indicate that ectopic expression of GADD153 in human HGG cells results in increased PCD type I and type II in the absence of other apoptotic/autophagic signals.
Fig. 6.
Cell viability, apoptosis, and autophagy rate in human HGG cells after GADD153 complementary DNA overexpression. Bar graphs showing a significant decrease in cell viability after overexpression of GADD153 complementary DNA in U87 (A) and T98G (E) HGG cells. A significant increase in apoptotic (B and F) and autophagic (C and G) rate was also seen. *P < .05, Student's t-test compared with vector. Western blotting showing increased expression of CHOP/GADD153, apoptosis (cleaved caspase-3), and autophagy (LC3II) markers in U87 (D) and T98G (H) cells after transient transfection of CHOP/GADD153.
Combination of ATO with CXB Significantly Enhances PCD in Human HGG Cells by Inducing ER Stress
In view of the differential ER stress–inducing effects generated by ATO and CXB,30,37,38 we next examined the effects of the combination of these stressors on human HGG cell growth and survival. We combined ATO with CXB at subtoxic concentration (Fig. 7). Combined ATO and CXB treatment resulted in significant decreased cell viability, with additive effects in U87 cells, whereas treatment with CXB alone did not. To elucidate the basis for this decrease, apoptosis and autophagy were analyzed. Combined ATO and CXB treatment resulted in a significant increase in autophagy and apoptosis in both U87 and T98G human HGG cells, whereas individual drugs did not. We next investigated the potential contribution of ER stress to the above-presented combination drug effects. U87 and T98G cells were treated with ATO and CXB as single treatment and in combination, and levels of CHOP (ER stress), and apoptosis and autophagy markers (cleaved caspase-3 and LC3II) were analyzed. ER stress protein CHOP was strongly elevated by combination treatments of ATO and CXB (Fig. 7G and H) in both U87 and T98G cells compared with single drug treatment. The apoptosis executioner caspase-3 and autophagy marker LC3II were more strongly activated by combination drug treatments than by individual drug treatment. Taken together, these results indicate that ATO, when combined with CXB, caused stronger ER stress induction and increased PCD compared with single drug treatment.
Fig. 7.
Combination of ATO with CXB significantly enhances PCD in human HGG cells. Cell viability, apoptosis, and autophagy rate in human HGGs after treatment with ATO and/or CXB. Bar graph showing significant decrease in cell viability after combined treatment with ATO and CXB in both U87 (A) and T98G (B) HGG cell lines. Apoptotic and autophagic rates were significantly increased after ATO and CXB combination treatment in U87 (C and E) and T98G (D and F) cell lines. Mean ± SD of 3 experiments; each experiment done in triplicate. *P < .05 compared with DMSO, #P < .05 compared with ATO, †P < .05 compared with CXB 10 μM, $P < .05 compared with CXB 20 μM. Western blot analyses of CHOP/GADD153, apoptosis (cleaved caspase-3), and autophagy (LC3II) markers in U87 (G) and T98G (H) cells after single and combination treatment with ATO and CXB. Combination of ATO and CXB resulted in enhanced expressions of all markers. Arrowhead: cleaved caspase-3. Lower panels to G and H, bar graphs of densitometric evaluation of western blot analyses.
Discussion
In this study we confirm the pro-apoptotic and pro-autophagic effects of TMZ, ATO, and CDDP on human HGG cells. We show that each treatment induces ER stress and increases expression of CHOP/GADD153. Additionally, we show that GADD153 is critical for ATO-induced apoptosis and autophagy of human HGG cells. Two lines of evidence support this conclusion. First, silencing of the GADD153 gene by siRNA blocked ATO-induced apoptosis and autophagy in HGG cells. Second, increased concentration of GADD153 by adding ectopic recombinant protein or overexpression of CHOP/GADD153 complementary DNA was sufficient to induce decreased cell viability by inducing apoptosis and autophagy in HGG cells in the absence of additional apoptotic/autophagy stimuli. Finally, we show that combining 2 known ER stressors, ATO and CXB, strongly increases PCD types I and II in HGG cells. These results suggest that CHOP/GADD153 plays an important role in linking non-IR ER stress–induced apoptosis and autophagy in HGGs. This link should be further investigated as a potential target for adjuvant therapy of human HGGs, which remains a therapeutic challenge.
Patients with HGGs have a poor prognosis, in spite of state-of-the-art maximal surgical tumor resection and postoperative IR combined with TMZ.2 This is due mainly to the high resistance of these infiltrative tumor cells to apoptosis, i.e., PCD type I. Recent evidence shows that HGG cells might be more sensitive to autophagy, i.e., PCD type II.5,6 Since both IR and TMZ have been shown to induce apoptosis and autophagy,7,10,11,14,19 it is reasonable to postulate that if the upstream signal(s) linking PCD type I and type II is identified, this could then be targeted to potentiate PCD. In this study we confirmed that each of the three chemotherapy agents tested (ATO, TMZ, and CDDP) induced decreased cell viability by causing apoptosis and autophagy. When apoptosis or autophagy was blocked, however, a lack of significant change in cell viability was seen after treatment with only ATO, indicating that cytotoxic effects of ATO on human HGGs are caused by both apoptosis and autophagy. With TMZ treatment there was a significant effect on cell viability only after blocking autophagy, and with CDDP only after blocking apoptosis. These results support the concept that TMZ causes tumor cell demise mostly by autophagy, as we and others previously reported.10,11 Conversely, the primary cytopathic effect of CDDP is via apoptosis.34,39
Recent studies highlight the complex interplay between apoptosis and autophagy. When the ER is subjected to stress, both apoptosis and autophagy are affected. The link between these 2 PCDs is, however, still not fully characterized. To identify such link in this study, we used ATO as a non-IR ER stress inducer known to cause apoptosis and autophagy. We tested the hypothesis that CHOP/GADD153 serves as an upstream link for apoptosis and autophagy in HGGs. CHOP/GADD153 is a leucine zipper transcription factor present at low levels under normal conditions but highly upregulated in response to a variety of stresses and agents, especially ER stress.40,41 In tumor cells, the GADD153 promoter has been found to be responsive to a broad spectrum of genotoxic agents. The CHOP gene can be activated through the ER stress response elements in response to cellular stress,42,43 amino acid response elements in response to amino acid starvation,44–46 and the C/EBP–activating transcription factor composite site, a part of the amino acid response elements–1, in response to phosphatidylcholine depletion and ER stress.47 Recently, Pyrko et al.40 showed induction of CHOP in glioma cell lines following treatment with TMZ. Induction of CHOP/GADD153 after treatment with ATO has been shown previously in neutrophils in glioma, pancreatic, colorectal, and breast cancers.37,48,49 CDDP has been shown to induce GADD153 in head/neck and ovarian cancer, xenografted melanoma, and squamous cell carcinoma.50–52 In our study, we showed robust increased expression of CHOP/GADD153 after treatment with TMZ, ATO, or CDDP in U87 and T98G HGG cells. Additionally, suppression of CHOP/GADD153 by siRNA caused a significant decrease in apoptotic and autophagic rates in HGG cells, as indicated by decreased expressions of cleaved caspase-3 and LC3II and decreased formation of acidic vesicular organelles. These findings suggest that CHOP/GADD153 plays a key role in linking non-IR ER stress–induced apoptosis and autophagy. GADD153 expression has been associated with apoptosis in response to a number of stress stimuli, including anticancer agents, retinoic acid, and nutrient deprivation.45,53–56 In addition to its function as a transcription factor, GADD153 has been shown to mediate apoptosis through a nontranscriptional pathway.53,57 Recent studies demonstrated that knockdown of CHOP using CHOP-specific siRNA significantly suppressed apoptosis induction and activation of effector caspase-3.48,58–60 Recent studies also suggest that UPR-triggered CHOP promotes the activation of the autophagic process through the transcriptional control of ATG5 and LC3B.61 A recent study by Santos-Gomez et al.62 showed that dopamine treatment triggers autophagy in neuroblastoma cells, which is associated with GADD153 induction. Additionally, Ke et al.61 recently showed that knockdown of CHOP by siRNA duplexes greatly downregulated the expression of LC3B-II. Additional studies are needed to elucidate the mechanism(s) of CHOP-regulated expression of LC3.
Among all chemotherapeutic agents available, platinum-based drugs, such as CDDP and carboplatin, have played the most important role in the treatment of solid tumors.63 The major mechanism underlying CDDP activity is the induction of DNA damage and induction of apoptosis.39 Its use as adjuvant treatment of HGGs has been limited by its toxicity after systemic administration and poor brain penetration due to the blood–brain barrier. Recently, interest in this drug has resurged, as it has been shown to induce autophagy in the U251 HGG cell line.64 Additionally, it has been shown that it can be delivered intraparenchymally by convection-enhanced delivery, with increased survival in F98 glioma-bearing rats.65 Our study confirms the pro-apoptotic and pro-autophagic effects of CDDP. These occur by induction of ER stress and activation of CHOP/GADD153 signaling. However, since suppression of CHOP/GADD153 by siRNA after CDDP results in a significant decrease in only apoptotic rate (data not shown), and only treatment with a pan-caspase inhibitor results in significant increased viability, as shown in Fig. 3, we suggest that most of the cell demise after CDDP occurs secondary to PCD type I. These results taken together with those reported above support the concept that although CHOP/GADD153 plays a key role in linking non-IR ER stress–induced apoptosis and autophagy, additional signaling pathways might be involved. Further studies to clarify this aspect are needed.
In the present study, we show that overexpression of CHOP/GADD153 resulted in decreased cell viability via apoptosis and autophagy in human HGG cells in the absence of additional apoptotic/autophagy stimuli. This observation suggests that CHOP itself may be more efficient to sensitize cells to other ER stress agents. Indeed, we showed that when ATO and CXB were combined, enhanced cytotoxicity occurred. These data provide the evidence that the combination of 2 ER stress inducers, at clinically achievable concentrations, exerts greatly increased cytotoxicity on HGG cells. Each drug can independently induce ER stress,37,38 and their combination further aggravates ER stress to the point where the protective components of this system are overwhelmed and the pro-apoptotic/pro-autophagic constituents gain dominance to PCD. Although further in vivo animal studies are required to support the clinical application of ATO/CXB as a co-treatment, with the potential of CXB to bypass the blood–brain barrier,66 our findings support clinical translation of combined ATO and CXB in HGG patients.
Acknowledgment
This work was partially supported by NIH/NCI R01 CA1129489-01A1.
Conflict of interest statement. None declared.
References
- 1.CBTRUS, Statistical report: Primary Brain Tumors in the United States, 2000–2004. 2008.
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Eng J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. doi:10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler DS, Kung AL, Kieran MW. Anti-apoptosis mechanisms in malignant gliomas. J Clin Oncol. 2008;26:493–500. doi: 10.1200/JCO.2007.13.9717. doi:10.1200/JCO.2007.13.9717. [DOI] [PubMed] [Google Scholar]
- 5.Lefranc F, Kiss R. Autophagy, the Trojan horse to combat glioblastomas. Neurosurg Focus. 2006;20:E7. doi: 10.3171/foc.2006.20.4.4. doi:10.3171/foc.2006.20.4.4. [DOI] [PubMed] [Google Scholar]
- 6.Lefranc F, Facchini V, Kiss R. Proautophagic drugs: A novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395–1403. doi: 10.1634/theoncologist.12-12-1395. doi:10.1634/theoncologist.12-12-1395. [DOI] [PubMed] [Google Scholar]
- 7.Paglin S, Hollister T, Delohery T, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 8.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63(9):2103–2108. [PubMed] [Google Scholar]
- 9.Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24:980–991. doi: 10.1038/sj.onc.1208095. doi:10.1038/sj.onc.1208095. [DOI] [PubMed] [Google Scholar]
- 10.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11(4):448–457. doi: 10.1038/sj.cdd.4401359. doi:10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 11.Ulasov IV, Sonabend AM, Nandi S, Khramstov A, Han Y, Lesniak MS. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagetic and apoptotic cell death in vivo. British Journal of Cancer. 2009;100:1154–1164. doi: 10.1038/sj.bjc.6604969. doi:10.1038/sj.bjc.6604969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaliberova LN, Krendelchtchikova V, Harmon DK, et al. CRAdRGDflt – IL24 virotherapy in combination with chemotherapy of experimental glioma. Cancer gene Therapy. 2009;16:794–805. doi: 10.1038/cgt.2009.23. doi:10.1038/cgt.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das A, Banik NL, Ray SK. Modulatory Effects of Acetazolomide and Dexamethasone on Temozolomide Mediated Apoptosis in Human Glioblastoma T98G and U87MG Cells. Cancer Invest. 2008;26:352–358. doi: 10.1080/07357900701788080. doi:10.1080/07357900701788080. [DOI] [PubMed] [Google Scholar]
- 14.Roos WP, Batista LFZ, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6 – methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. doi:10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama T, Iwado E, Kondo Y, et al. Autophagy- inducing agents augment the antitumor effect of telomerase- selective oncolytic adenovirous OBP-405 on glioblastoma cells. Gene therapy. 2008;15:1233–1239. doi: 10.1038/gt.2008.98. doi:10.1038/gt.2008.98. [DOI] [PubMed] [Google Scholar]
- 16.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. doi:10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 17.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. doi:10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16(7):966–975. doi: 10.1038/cdd.2009.33. doi:10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 19.Moretti L, Cha YI, Nierman KJ, Lu B. Switch between Apoptosis and Autophagy. Cell Cycle. 2007;6:793–798. doi: 10.4161/cc.6.7.4036. doi:10.4161/cc.6.7.4036. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. doi:10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 21.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. doi:10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari D, Pinton P, Szabadkai G, et al. Endoplasmic reticulum, Bcl–2 and Ca2+ handling in apoptosis. Cell Calcium. 2002;32:413–420. doi: 10.1016/s0143416002002014. doi:10.1016/S0143416002002014. [DOI] [PubMed] [Google Scholar]
- 23.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. doi:10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 24.Ogata M, Hino S, Saito A, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. doi:10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. doi:10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. doi:10.1016/S0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 27.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. doi:10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez AM, Martínez-Botas J, Malagarie-Cazenave S, et al. Induction of the endoplasmic reticulum stress protein GADD153/CHOP by capsaicin in prostate PC-3 cells: a microarray study. Biochem Biophys Res Commun. 2008;372:785–791. doi: 10.1016/j.bbrc.2008.05.138. doi:10.1016/j.bbrc.2008.05.138. [DOI] [PubMed] [Google Scholar]
- 29.Blaschke F, Bruemmer D, Yin F, et al. C-reactive protein induces apoptosis in human coronary vascular smooth muscle cells. Circulation. 2004;110:579–587. doi: 10.1161/01.CIR.0000136999.77584.A2. doi:10.1161/01.CIR.0000136999.77584.A2. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Hwang CI, Juhnn YS, Lee JH, Park WY, Song YS. GADD153 mediates celecoxib-induced apoptosis in cervical cancer cells. Carcinogenesis. 2007;28:223–231. doi: 10.1093/carcin/bgl227. doi:10.1093/carcin/bgl227. [DOI] [PubMed] [Google Scholar]
- 31.Koki AT, Masferrer JL. Celecoxib: a specific COX-2 inhibitor with anticancer properties. Cancer Control. 2002;9:28–35. doi: 10.1177/107327480200902S04. [DOI] [PubMed] [Google Scholar]
- 32.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase- 2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. doi:10.1016/S1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 33.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. doi:10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa T, Kubota T, Ido K, Sakuma T, Matsuda K. The combined effects of multiple chemotherapeutic agents for malignant glioma cells. J Neurooncol. 2007;84(1):31–37. doi: 10.1007/s11060-007-9357-8. doi:10.1007/s11060-007-9357-8. [DOI] [PubMed] [Google Scholar]
- 35.Kang KB, Zhu C, Yong SK, Gao Q, Wong MC. Enhanced sensitivity of celecoxib in human glioblastoma cells: Induction of DNA damage leading to p53-dependent G1 cell cycle arrest and autophagy. Mol Cancer. 2009;8:66. doi: 10.1186/1476-4598-8-66. doi:10.1186/1476-4598-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang YY, Zhong M, Zhang LP, et al. Tribble 3, a novel oxidized low-density lipoprotein-inducible gene, is induced via the activating transcription factor 4-C/EBP homologous protein pathway. Clin Exp Pharmacol Physiol. 2010;37:51–55. doi: 10.1111/j.1440-1681.2009.05229.x. doi:10.1111/j.1440-1681.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- 37.Binet F, Chiasson S, Girard D. Arsenic trioxide induces endoplasmic reticulum stress-related events in neutrophils. Int Immunopharmacol. 2010;10:508–512. doi: 10.1016/j.intimp.2010.01.013. doi:10.1016/j.intimp.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Pyrko P, Kardosh A, Liu YT, et al. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther. 2007;6:1262–1275. doi: 10.1158/1535-7163.MCT-06-0629. doi:10.1158/1535-7163.MCT-06-0629. [DOI] [PubMed] [Google Scholar]
- 39.Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990;2:275–280. [PubMed] [Google Scholar]
- 40.Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67(20):9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. doi:10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 41.Wang XZ, Lawson B, Brewer JW, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luethy JD, Holbrook NJ. Activation of the gadd153 promoter by genotoxic agents: a rapid and specific response to DNA damage. Cancer Res. 1992;52:5–10. [PubMed] [Google Scholar]
- 43.Ubeda M, Habener JF. CHOP gene expression in response to endoplasmic-reticular stress requires NFY interaction with different domains of a conserved DNA-binding element. Nucleic Acids Res. 2000;28:4987–4997. doi: 10.1093/nar/28.24.4987. doi:10.1093/nar/28.24.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida H, Okada T, Haze K, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. doi:10.1128/MCB.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruhat A, Jousse C, Wang XZ, Ron D, Ferrara M, Fafournoux P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem. 1997;272:17588–17593. doi: 10.1074/jbc.272.28.17588. doi:10.1074/jbc.272.28.17588. [DOI] [PubMed] [Google Scholar]
- 46.Cherasse Y, Maurin AC, Chaveroux C, et al. The p300/CBP-associated factor (PCAF) is a cofactor of ATF4 for amino acid-regulated transcription of CHOP. Nucleic Acids Res. 2007;35:5954–5965. doi: 10.1093/nar/gkm642. doi:10.1093/nar/gkm642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Sanden MH, Meems H, Houweling M, Helms JB, Vaandrager AB. Induction of CCAAT/enhancer-binding protein (C/EBP)-homologous protein/growth arrest and DNA damage-inducible protein 153 expression during inhibition of phosphatidylcholine synthesis is mediated via activation of a C/EBP-activating transcription factor-responsive element. J Biol Chem. 2004;279:52007–52015. doi: 10.1074/jbc.M405577200. doi:10.1074/jbc.M405577200. [DOI] [PubMed] [Google Scholar]
- 48.Kim EH, Yoon MJ, Kim SU, Kwon TK, Sohn S, Choi KS. Arsenic trioxide sensitizes human glioma cells, but not normal astrocytes, to TRAIL-induced apoptosis via CCAAT/enhancer-binding protein homologous protein-dependent DR5 up-regulation. Cancer Res. 2008;68(1):266–275. doi: 10.1158/0008-5472.CAN-07-2444. doi:10.1158/0008-5472.CAN-07-2444. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Ding X, Adrian TE. Arsenic trioxide causes redistribution of cell cycle, caspase activation, and GADD expression in human colonic, breast, and pancreatic cancer cells. Cancer Invest. 2004;22:389–400. doi: 10.1081/cnv-200029068. doi:10.1081/CNV-200029068. [DOI] [PubMed] [Google Scholar]
- 50.Los G, Benbatoul K, Gately DP, et al. Quantitation of the change in GADD153 messenger RNA level as a molecular marker of tumor response in head and neck cancer. Clin Cancer Res. 1999;5:1610–1618. [PubMed] [Google Scholar]
- 51.Gately DP, Jones JA, Christen R, Barton RM, Los G, Howell SB. Induction of the growth arrest and DNA damage-inducible gene GADD153 by cisplatin in vitro and in vivo. Br J Cancer. 1994;70:1102–1106. doi: 10.1038/bjc.1994.455. doi:10.1038/bjc.1994.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnsson A, Strand C, Los G. Expression of GADD153 in tumor cells and stromal cells from xenografted tumors in nude mice treated with cisplatin: correlations with cisplatin-DNA adducts. Cancer Chemother Pharmacol. 1999;43:348–352. doi: 10.1007/s002800050906. doi:10.1007/s002800050906. [DOI] [PubMed] [Google Scholar]
- 53.Kim DG, You KR, Liu MJ, Choi YK, Won YS. GADD153-mediated anticancer effects of N-(4-hydroxyphenyl)retinamide on human hepatoma cells. J Biol Chem. 2002;277:38930–38938. doi: 10.1074/jbc.M205941200. doi:10.1074/jbc.M205941200. [DOI] [PubMed] [Google Scholar]
- 54.Carlson SG, Fawcett TW, Bartlett JD, Bernier M, Holbrook NJ. Regulation of the C/EBP-related gene gadd153 by glucose deprivation. Mol Cell Biol. 1993;13:4736–4744. doi: 10.1128/mcb.13.8.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eymin B, Dubrez L, Allouche M, Solary E. Increased gadd153 messenger RNA level is associated with apoptosis in human leukemic cells treated with etoposide. Cancer Res. 1997;57:686–695. [PubMed] [Google Scholar]
- 56.Gately DP, Howell SB. Paclitaxel activation of the GADD153 promoter through a cellular injury response element containing an essential Sp1 binding site. J Biol Chem. 1996;271:20588–20593. doi: 10.1074/jbc.271.34.20588. doi:10.1074/jbc.271.34.20588. [DOI] [PubMed] [Google Scholar]
- 57.Maytin EV, Ubeda M, Lin JC, Habener JF. Stress-inducible transcription factor CHOP/gadd153 induces apoptosis in mammalian cells via p38 kinase-dependent and -independent mechanisms. Exp Cell Res. 2001;267:193–204. doi: 10.1006/excr.2001.5248. doi:10.1006/excr.2001.5248. [DOI] [PubMed] [Google Scholar]
- 58.Ma J, Qiu Y, Yang L, et al. Desipramine induces apoptosis in rat glioma cells via endoplasmic reticulum stress-dependent CHOP pathway. J Neurooncol. 2011;101(1):41–48. doi: 10.1007/s11060-010-0237-2. doi:10.1007/s11060-010-0237-2. [DOI] [PubMed] [Google Scholar]
- 59.Pino SC, O'Sullivan-Murphy B, Lidstone EA, et al. CHOP mediates endoplasmic reticulum stress-induced apoptosis in Gimap5-deficient T cells. PLoS One. 2009;4:e5468. doi: 10.1371/journal.pone.0005468. doi:10.1371/journal.pone.0005468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng X, Leyva ML, Jenny M, et al. A ruthenium-containing organometallic compound reduces tumor growth through induction of the endoplasmic reticulum stress gene CHOP. Cancer Res. 2009;69:5458–5466. doi: 10.1158/0008-5472.CAN-08-4408. doi:10.1158/0008-5472.CAN-08-4408. [DOI] [PubMed] [Google Scholar]
- 61.Ke PY, Chen SS. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate anti-viral immunity in vitro. J Clin Invest. 2011;121:37–56. doi: 10.1172/JCI41474. doi:10.1172/JCI41474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gómez-Santos C, Ferrer I, Santidrián AF, Barrachina M, Gil J, Ambrosio S. Dopamine induces autophagic cell death and alpha-synuclein increase in human neuroblastoma SH-SY5Y cells. J Neurosci Res. 2003;73:341–350. doi: 10.1002/jnr.10663. doi:10.1002/jnr.10663. [DOI] [PubMed] [Google Scholar]
- 63.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–584. doi: 10.1038/nrc2167. doi:10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 64.Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevocic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumor cells. J Cell Mol Med. 2009;13(9B):3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. doi:10.1111/j.1582-4934.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rousseau J, Barth RF, Fernandez M, et al. Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J Neurooncol. 2010;98:287–295. doi: 10.1007/s11060-009-0074-3. doi:10.1007/s11060-009-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409–415. doi: 10.1097/00000542-200502000-00026. doi:10.1097/00000542-200502000-00026. [DOI] [PubMed] [Google Scholar]