Abstract
Loss of the tumor suppressor merlin is a cause of frequent tumors of the nervous system, such as schwannomas, meningiomas, and ependymomas, which occur spontaneously or as part of neurofibromatosis type 2 (NF2). Because there is medical need for drug therapies for these tumors, our aim is to find therapeutic targets. We have studied the pathobiology of schwannomas, because they are the most common merlin-deficient tumors and are a model for all merlin-deficient tumors. With use of a human schwannoma in vitro model, we previously described strong overexpression/activation of platelet-derived growth factor receptor-β (PDGFR-β) leading to strong, long-lasting activation of extracellular-signal-regulated kinase (ERK1/2) and AKT and increased schwannoma growth, which we successfully inhibited using the PDGFR/Raf inhibitor sorafenib. However, the benign character of schwannomas may require long-term treatment; thus, drug tolerability is an issue. With the use of Western blotting, proliferation assays, viability assays, and a primary human schwannoma cell in vitro model, we tested the PDGFR/c-KIT inhibitors imatinib (Glivec; Novartis) and nilotinib (Tasigna; Novartis). Imatinib and nilotinib inhibited PDGF-DD-mediated ERK1/2 activation, basal and PDGF-DD-mediated activation of PDGFR-β and AKT, and schwannoma proliferation. Nilotinib is more potent than imatinib, exerting its maximal inhibitory effect at concentrations lower than steady-state trough plasma levels. In addition, nilotinib combined with the MEK1/2 inhibitor selumetinib (AZD6244) at low concentrations displayed stronger efficiency toward tumor growth inhibition, compared with nilotinib alone. We suggest that therapy with nilotinib or combinational therapy that simultaneously inhibits PDGFR and the downstream Raf/MEK1/2/ERK1/2 pathway could represent an effective treatment for schwannomas and other merlin-deficient tumors.
Keywords: imatinib/nilotinib/selumetinib, merlin, PDGFR-β/ERK/AKT, proliferation, schwannoma
Loss of a tumor suppressor merlin is a major cause of tumors of the nervous system, such as schwannomas, meningiomas, and ependymomas that occur spontaneously or as part of a hereditary disease neurofibromatosis 2 (NF2), where they are numerous. Current treatments for these tumors are surgery and radiosurgery, which can leave patients with significant morbidity, impaired quality of life, and decreased life expectancy.1 Thus, new therapies are needed. We focused our study on schwannomas, because they are the most common merlin-deficient tumors and are a model for NF2-related tumors.2 With use of our model of human primary schwannoma and Schwann cells,3 we have studied schwannoma pathobiology to define potential therapeutic targets. We have found that schwannoma cells display an enhanced proliferation rate compared to normal Schwann cells as a result of the overexpression/activation of platelet-derived-growth factor receptor (PDGFR) and ErbB2/3, leading to strong, long-lasting activation of extracellular-signal-regulated kinase 1/2 (ERK1/2).4–7 We successfully inhibited schwannoma proliferation, ERK1/2, and AKT activity using the PDGFR/Raf inhibitor sorafenib (Bayer Pharmaceuticals)5 and the ErbB2/3 inhibitor lapatinib (GlaxoSmithKline).7 Schwannoma ERK1/2 activity and proliferation were inhibited by the MEK1/2 inhibitor selumetinib (AZD6244; Astra Zeneca).6
PDGFR is important in schwannoma development because it is strongly overexpressed in human schwannoma primary cells and tissues.5,8 PDGFR overexpression and activation leads to strong and long-lasting activation of ERK1/2 and AKT pathways and increased proliferation of schwannoma cells. A PDGFR/Raf inhibitor, sorafenib, is clearly more effective than either lapatinib or selumetinib (AZD6244) in reducing schwannoma cell proliferation.5,6,8 However, the benign character of these tumors would require long-term therapy; thus, the issue of adverse effects becomes relevant. Therefore, in this study, we evaluated the alternative PDGFR inhibitors imatinib (Gleevec; Novartis) and nilotinib (Tasigna; Novartis) in our in vitro human schwannoma model to assess whether they are as effective as sorafenib.
Imatinib and nilotinib are orally bioavailable, ATP-competitive inhibitors of the BCR-ABL fusion kinase, as well as the DDR, PDGFR, and c-KIT receptor kinases, and have been successfully used for the treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumor (GIST) for many years with well-known tolerability.9–11 We show that both imatinib and nilotinib effectively inhibit PDGF-DD–mediated activation of PDGFRβ, ERK1/2, and AKT, as well as proliferation of human primary schwannoma cells. Basal ERK1/2 is inhibited only by nilotinib, which is more effective overall, because its maximal effects are obtained with concentrations ∼0.5 to 4 times lower than that of plasma observed in patients with CML (1.95 μM) (Novartis) and 6–10 times lower than that of imatinib. The combination of nilotinib (0.25 μM) with the previously tested MEK1/2 inhibitor selumetinib (AZD6244)6 further potentiates the anti-proliferative effects of nilotinib by ∼20% (for PDGF-DD–mediated proliferation) and by ∼50% (for basal proliferation). Our data suggest that nilotinib may be a good candidate for clinical trials on patients with NF2, given alone or combined with an inhibitor of the downstream MEK1/2/ERK1/2 pathway, such as selumetinib (AZD6244).
Materials and Methods
Isolation and Culture of Human Primary Schwann and Schwannoma Cells
Human primary Schwann and schwannoma cells were isolated and cultured in complete medium, as described elsewhere.3 None of the primary cell cultures used were used beyond passage 4. Tissues from 4 to 8 patients were used in each experiment in this study. We mostly used schwannomas from patients with NF2, but we also used some spontaneous schwannomas.
Chemicals
Propidium iodide (PI) and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Sigma, and BrdU and BrdU antibody were obtained from Merck. PDGF-DD was obtained from R&D Systems. Imatinib and nilotinib were kindly supplied by Novartis, and selumetinib (AZD6244 and ARRY-142886) was kindly provided by Astra Zeneca.
Immunoblotting
Cells were cultured on precoated 35-mm plates (Greiner Bio-One), serum starved for 24 hours, stimulated with PDGF-DD, and lysed as described by Utermark et al.12 Sterile water-diluted solutions of imatinib and nilotinib were added 40 minutes before stimulation with PDGF-DD at final concentrations of 1 µM and 10 µM. Immunoblotting was performed as described elsewhere by Kaempchen et al.13 PDGFR, ERK1/2, and AKT activity and phosphorylation was detected by anti-PDGFR β (phospho Y857; 1:1000; Abcam), anti-active–mitogen-activated protein kinase (anti-pThr183-pTyr185-ERK1/2; 1:2000; Promega) and anti-phospho-AKT (Ser473; Cell Signaling Technology) antibodies. Primary antibodies were detected by a goat–anti-rabbit HRP-conjugated secondary antibody (Bio-Rad). The ECL-plus system (Amersham) was used for detection, and RhoGDI was used as a loading control (anti-RhoGDI antibody; 1:500; Santa Cruz Biotechnology), because it is not regulated in our system14 in contrast to standard loading controls. Band densities were quantified using the FluorS-Multi-Imager (Bio-Rad).
Nuclear Staining and BrdUrd Incorporation
Cells were cultivated on precoated 96-well plates (Nunc). Imatinib and nilotinib were added 40 minutes before stimulation with 100 ng/mL PDGF-DD, and cells were cultured for 72 hours (3 days). Because the half-life of imatinib and nilotinib is 18 hours, one-half of the originally added concentrations were added freshly every day. In addition to DAPI staining and determination of the total cell number, we also used the more sensitive and accurate BrdU incorporation method to detect proliferating cells. BrdU incorporation was performed as described by Ammoun et al.5 Total cell amount (DAPI) and number of dividing cells (BrdU-positive) were blindly counted using an inverted fluorescent microscope (Olympus) and 200× magnification. All cells in every well were counted. The total cell number per well differed between various cell batches and was 100–300 cells/well.
Cell Toxicity
Cell toxicity during drug treatments was monitored in all experiments by determining cell viability. For short-term drug treatments, which are used for immunoblotting, cell viability was tested by trypan blue staining. For long-term treatments, which are used for determination of cell proliferation, cell viability was tested using PI, which stains only dead cells. DAPI, which stains both dead and living cells, was also used as an additional viability control, because it enables differentiation between the nuclei of living and dead cells on the basis of morphology.
Data Analysis
Student 2-tailed t tests were used for pairwise comparisons and analysis of variance, followed by the Turkey post hoc test for multiple comparisons. All Western blots were performed in at least quadruplicate using independent batches of cells from different individuals. In proliferation assays, cells from 8 and 6 different patients were tested with imatinib and nilotinib, respectively. For cell counting, all cells in the well were counted.
Results
Imatinib and Nilotinib Effectively Inhibit PDGF-DD–Mediated PDGFR-β, ERK1/2, and AKT Phosphorylation and Activity in Schwannoma Cells
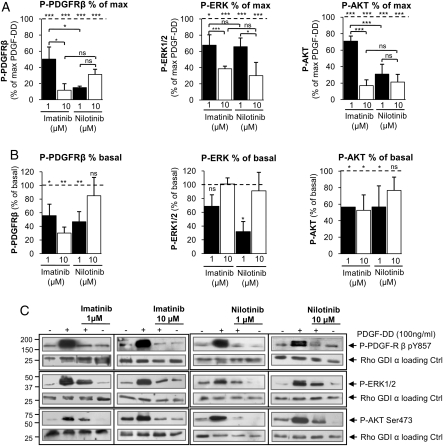
In our previous study, we performed a time-response curve for the PDGFR agonist PDGF-DD and showed that the maximal activity of PDGFR and its downstream kinases ERK1/2 and AKT were 10 minutes after stimulation, declining after 24 hours, likely as a result of receptor desensitation.5 Therefore, 10 minutes was chosen for investigating the efficacy of imatinib and nilotinib in the inhibition of PDGFR, ERK1/2, and AKT activity. The concentration range of the drugs was chosen on the basis of previous in vitro studies that were performed with different cell lines.15–17 Imatinib and nilotinib significantly inhibited the PDGF-DD–mediated (100 ng/mL; 10 minutes) PDGFR-β, ERK1/2, and AKT phosphorylation/activity (Fig. 1A and C). The maximal effect of nilotinib was observed at 1 μM, which inhibited P-PDGFR-β by ∼80% and P-AKT by ∼60%. Imatinib achieved the same efficiency when used at the concentration of 10 μM (Fig. 1A and C). Both inhibitors displayed the same efficiency toward the inhibition of PDGF-DD–mediated (100 ng/mL, 10 minutes) ERK1/2 activity (∼60% maximum inhibition) (Fig. 1A,) which is weaker than the efficiency for P-PDGFR-β inhibition (∼80% maximum inhibition) and P-AKT (∼80% maximum inhibition) (Fig. 1A and C).
Fig. 1.
Effects of imatinib and nilotinib on basal and PDGF-DD–mediated phosphorylation/activation of platelet-derived growth factor receptor-β (PDGFR-β), ERK1/2, and AKT in schwannoma cells (NF2−/−). (A and C) Imatinib and nilotinib significantly inhibited the PDGF-DD–mediated phosphorylation and activation of PDGFR-β, ERK1/2, and AKT in schwannoma cells. (B and C) Basal PDGFR-β and AKT phosphorylation and activity were significantly inhibited by imatinib and nilotinib, and basal ERK1/2 phosphorylation and activity were inhibited only by nilotinib at a lower concentration (1 µM). The cells were serum starved for 24 hours, pre-incubated with imatinib or nilotinib for 40 minutes, stimulated with 100 ng/mL PDGF-DD for 10 minutes, and lysed. Levels of phosphorylated and active PDGFR-β, ERK1/2, and AKT were detected by Western blotting.5,14 In (A), the data are normalized to the maximum PDGF-DD response (100%), and in (B) they are normalized to maximum basal (nonstimulated) response (100%). Data are mean±SEM.
Basal PDGFR-β and AKT Phosphorylation and Activity are Inhibited by Both Imatinib and Nilotinib, Whereas Basal ERK1/2 Phosphorylation and Activity are Inhibited Only by Nilotinib
Both inhibitors inhibited basal PDGFR-β and AKT activity, with maximal efficiency (∼40% inhibition) obtained at the concentration of 1 μM (Fig. 1B and C). Basal ERK1/2 activity was inhibited only by nilotinib at 1 μM (∼60% inhibition); imatinib was ineffective at any concentration (Fig. 1B and C). A higher concentration of nilotinib (10 μM) was ineffective in inhibiting the basal activity of PDGFR-β and ERK1/2, and inhibition of AKT activity was not statistically significant (Fig. 1B and C), in contrast to the PDGF-DD–mediated activity.
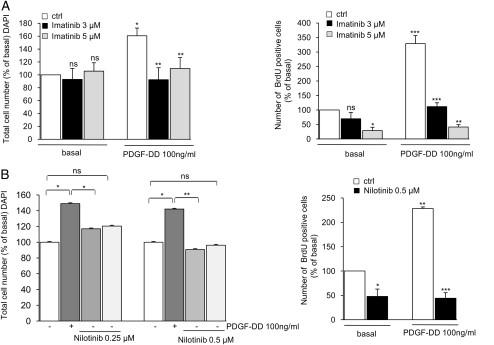
Basal and PDGF-DD–Mediated Proliferation of Schwannoma Cells is Significantly Inhibited by Imatinib and Nilotinib
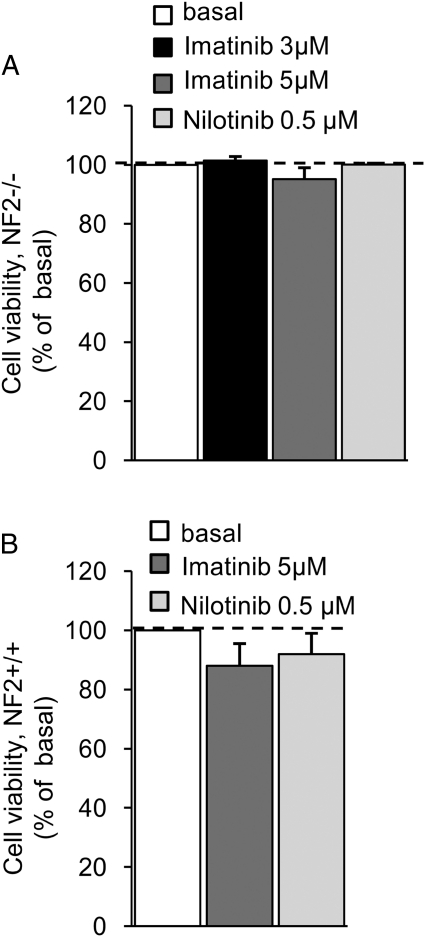
For the proliferation assays, we initially used 10 µM of imatinib. This concentration, however, appeared to be toxic after 72 hours of incubation. Therefore, we used lower concentrations of imatinib (ie, 3 µM and 5 µM). For nilotinib, we titrated down the concentration from 1 µM, which yielded maximal effectiveness in short-term experiments, to 0.25 µM (1 µM, 0.5 µM, and 0.25 µM) to find the lowest, most-effective concentration of the drug. PDGF-DD–mediated (100 ng/mL, 72 hours) schwannoma proliferation was strongly reduced to basal levels with 3 μM of imatinib. Nilotinib at 1 μM was also very effective at completely inhibiting PDGF-DD–mediated schwannoma proliferation to less than the basal levels (data not shown). We therefore performed further titration of the nilotinib concentration and found that the lowest and most effective concentration was 0.5 µM, because nilotinib at this concentration inhibited both PDGF-DD–mediated (Fig. 2B) and basal proliferation (Fig. 2). Nilotinib at 0.25 µM displayed only a partial inhibitory effect (Fig. 2B). Thus, our data show that nilotinib exhibits a maximal effect at a concentration 6-fold lower than that of imatinib (Fig. 2A and B). To investigate the effects of imatinib and nilotinib on basal proliferation, we also used a more sensitive method to monitor proliferation—namely, BrdU incorporation. Basal proliferation was significantly inhibited by both inhibitors. Nilotinib (0.5 μM, yielding ∼50% inhibition) yielded 10-fold higher efficiency than imatinib (5 μM, giving ∼50% inhibition) (Fig. 2A and B). The viability of schwannoma and Schwann cells was not affected by imatinib, either at 3 μM (schwannoma cells) or 5 μM (Schwann and schwannoma cells), nor was it affected by nilotinib at 0.5 μM (Schwann and schwannoma cells) (Fig. 3A and B)—concentrations below serum trough levels in patients with CML after administration of a daily dose of nilotinib (1.95 μM) and imatinib (1.98 μM).
Fig. 2.
Basal and PDGF-DD–mediated proliferation of schwannoma cells during treatment with imatinib and nilotinib. (A and B) Imatinib and nilotinib significantly inhibited basal and PDGF-DD–mediated proliferation of schwannoma cells. Nilotinib displayed higher efficiency than imatinib, partly inhibiting PDGF-DD–mediated proliferation at a concentration of 0.25 µM (B; left panel) and reaching a maximal effect in the inhibition of both basal and PDGF-DD–mediated proliferation at a concentration of 0.5 µM (B; left and right panels). The maximum effects of imatinib were reached at 3 µM for the inhibition of PDGF-DD–mediated proliferation and 5 µM for basal proliferation. Cells were cultured in serum-free medium for 72 hours in the presence or absence of imatinib, nilotinib, and 100 ng/mL PDGF-DD. The inhibitors were added 40 minutes before stimulation with PDGF-DD. Cell proliferation was monitored by 4′,6-diamidino-2-phenylindole staining and BrdUrd incorporation. The data are normalized to basal (nonstimulated) levels and given as % of basal (100%). Data are mean±SEM.
Fig. 3.
Viability of schwannoma (NF2−/−) and Schwann (NF2+/+) cells during treatment with imatinib and nilotinib. Imatinib at 3 µM (NF2−/−) and 5 µM (NF2−/− and NF2+/+) and nilotinib at 0.5 µM (NF2−/− and NF2+/+) were not toxic for either schwannoma (NF2−/−) (A) or Schwann (NF2+/+) (B) cells. Cells were cultured in serum-free medium for 72 hours in the presence or absence of imatinib or nilotinib. Cell viability was monitored by propidium iodide staining. The data are normalized to the basal levels and given as a percentage of basal. Data are mean±SEM.
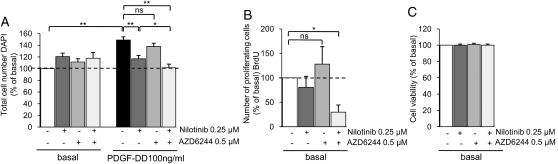
The Combination of Nilotinib and the MEK1/2 Inhibitor Selumetinib (AZD6244) Displayed Increased Efficiency Toward Inhibition of Schwannoma Proliferation
Because nilotinib, being more effective than imatinib, was slightly less effective than the previously tested agent sorafenib,5 we tested whether this result was due to the inhibitory effect of sorafenib on the Raf/MEK1/2/ERK1/2 pathway, in addition to PDGFR. Consequently, we treated human primary schwannoma cells with low concentrations of nilotinib (0.25 μM) in combination with a low concentration of the MEK1/2 inhibitor selumetinib (AZD6244; 0.5 μM) for 72 hours. We demonstrated that the combination of these 2 drugs was more effective than either drug alone, completely inhibiting both basal and PDGFR-mediated proliferation of schwannoma cells (Fig. 4A and B). The drug combination was very effective in inhibiting cell proliferation and does not have any toxic effects (Fig. 4C).
Fig. 4.
Effect of nilotinib and selumetinib (AZD6244) combination therapy on schwannoma proliferation and viability. (A and B) Combination of low concentrations of nilotinib and the MEK1/2 inhibitor selumetinib (AZD6244) is more potent towards basal and PDGF-DD–mediated proliferation of schwannoma cells than each drug alone. The cells were cultured for 72 hours in serum-free medium containing PDGF-DD (100 ng/mL), nilotinib (0.25 µM), and selumetinib (AZD6244) (0.5 µM). (C) Cell viability is not affected by combined nilotinib (0.25 µM) and selumetinib (AZD6244; 0.5 µM) treatment. Cell proliferation was determined by BrdU incorporation and 4′,6-diamidino-2-phenylindole (1 µg/mL), and cell viability was determined by propidium iodide (PI) at 2.5 µg/mL. Data are mean±SEM.
Discussion
With use of our in vitro model, we previously identified PDGFR-β as a promising therapeutic target for schwannoma treatment.5 Because of the benign character of these slow-growing tumors, long-term therapy is expected; therefore, adverse effects are an important issue. In this study, we investigated 2 drugs that target PDGFR—imatinib and nilotinib—and compared their efficiency with the previously tested drug sorafenib.5 Imatinib and nilotinib have been successfully used to treat CML and GIST, displaying manageable side effects.9,11 Imatinib has also been tested in the immortalized human HEI-193 schwannoma cell line, inhibiting cell proliferation.18 These data, although very important, are not fully reflective of the disease, because they are based on only 1 cell line and not on primary human cells coming from multiple patients. In addition, in contrast to human primary schwannoma cells,5 HEI-193 cells are immortalized and are not merlin-null cells, because they express the active merlin 3 isoform, which is sufficient to suppress cell proliferation.19 Therefore, our studies employed merlin-deficient human primary schwannoma cells and provide an important complement to previous work on imatinib in cell lines, to evaluate drugs for their potential in clinical trials involving patients with NF2 and sporadic schwannoma. In addition, we evaluated nilotinib, which was developed as a successor of imatinib.
We showed that imatinib and nilotinib effectively inhibit PDGF-DD–mediated PDGFR-β, ERK1/2, and AKT activation and phosphorylation. Basal activity of those targets was also inhibited by both drugs, except for ERK1/2, which was inhibited only by nilotinib. Interestingly, ERK1/2 is much less sensitive to those inhibitors, probably in association with compensatory mechanisms triggered by strong AKT activation. Surprisingly, only a lower concentration of nilotinib (1 μM) inhibited the basal ERK1/2 activity; at a higher concentration (10 μM), it lost its inhibitory effect. The same phenomenon was observed in the inhibition of basal PDGFR-β and AKT activity: there complete loss of efficiency was observed, in contrast to the experience with PDGF-DD–mediated activity, which was significantly inhibited. The decreased sensitivity to 10 μM of nilotinib could be due to the inhibition of a negative feedback loop. The decreased sensitivity to the inhibitory effects of imatinib and nilotinib toward inhibition of basal versus PDGF-DD–stimulated PDGFR-β, AKT, and ERK1/2 could be explained by the actions of other factors released from schwannoma cells in an autocrine manner.20 Nilotinib is more effective than imatinib, displaying a maximal inhibitory effect at a concentration 10-fold lower than that of imatinib and one-half that of its steady-state trough plasma concentration (1.95 μM) observed in patients with CML who are treated with the standard dose.21 Imatinib displays its maximal inhibitory effect on PDGF-DD–mediated PDGFR-β and AKT activity at a concentration of 10 μM, which is much higher than plasma concentration (1.98 μM).
We subsequently tested the inhibitory potential of imatinib and nilotinib on schwannoma proliferation. When used for 3 days in our human in vitro model, imatinib and nilotinib both significantly inhibited PDGF-DD–mediated proliferation to the basal levels at concentrations slightly higher (in the case of imatinib) or 4 times lower (in the case of nilotinib) than their steady state trough of plasma concentrations at the standard therapeutic dose.21 In accordance with the observation made regarding inhibition of signaling pathways, nilotinib displayed 6-fold greater potency than that of imatinib. Basal proliferation is also inhibited by both drugs, with 10-fold higher efficiency of nilotinib (0.5 μM) versus imatinib (5 μM). The discrepancy between total cell number determined by DAPI staining and the number of dividing cells determined by BrdU incorporation occurred because cell proliferation of primary schwannoma cells cannot be synchronized, meaning that different cell populations are at different stages of the cell cycle at the time of fixation and staining. Moreover, schwannoma cells have a long doubling time, making BrdU the more sensitive procedure. The difference in imatinib and nilotinib potency is likely due to the higher cell-membrane permeability of nilotinib, which has a more lypophilic character than imatinib and lack of reliance of active transport mechanisms to enter cells.22
Thus, in schwannomas, PDGFR-β overexpression and activation and ERK1/2 and AKT activation seem to sensitize schwannoma cells to imatinib and nilotinib. In addition, the overexpression of c-KIT in human schwannoma tissue may add to the tumor sensitivity to those drugs.18 Of interest, AKT inhibition is strong. From our previous study, we know that schwannoma cells display stronger basal AKT activity than do Schwann cells.5 Our data differ from the results obtained by Matei et al,23 who showed that, in ovarian cancer cell lines, high AKT activity induces partial resistance to PDGFR inhibition by imatinib in vitro. This discrepancy is likely due to the different biological backgrounds of schwannomas and ovarian cancers and possibly due to the malignant character of the latter.
We did not test either imatinib or nilotinib on PDGF-DD–mediated Schwann cell proliferation as in our in vitro model PDGF-DD is not mitogenic for those cells as shown previously.5,6 Moreover, as opposed to schwannoma cells, mature Schwann cells do not proliferate in vivo unless the nerve is injured.24 No toxicity was observed in our human in vitro model, as shown by cell viability tests using PI and DAPI, in either Schwann or schwannoma cells in response to imatinib or nilotinib treatment. Moreover, human toxicity data for both imatinib and nilotinib are available.
Because our aim was to define the most effective drug, we compared nilotinib with sorafenib.5 Both sorafenib and nilotinib strongly inhibited basal and PDGF-DD–stimulated ERK1/2 and AKT activity and proliferation of schwannoma cells, displaying similar inhibitory efficiencies. Nilotinib reaches its maximal effects on proliferation at a concentration ∼4 times lower than plasma trough levels, and sorafenib reaches this state at a concentration ∼9 times lower than plasma trough levels.5,25 Thus, sorafenib seemed slightly more effective in our human in vitro model but causes more severe adverse effects than does nilotinib. The reason why sorafenib is more effective than nilotinib is probably because sorafenib, in addition to PDGFR, also inhibits downstream of the Raf/MEK1/2/ERK1/2 pathway, which is activated not only by PDGF-DD but also by other growth factors released from schwannoma. The Raf/MEK1/2/ERK1/2 pathway was previously shown to play a crucial role in schwannoma development, and its inhibition is required to decrease schwannoma pathological proliferation.5,6 c-KIT seemed to play a lesser role in schwannoma pathobiology than did the Raf/MEK1/2/ERK1/2 pathway and PDGFR, and even though both sorafenib and nilotinib inhibited c-KIT, sorafenib is still more effective, suggesting the importance of Raf. To prove that the MEK1/2/ERK1/2 pathway should be inhibited in addition to PDGFR, we combined low concentrations of nilotinib (0.25 μM) with the MEK1/2 inhibitor selumetinib (AZD6244; 0.5 μM) and investigated whether the combined drugs would display a stronger inhibitory effect than would either drug alone. Indeed, we showed that a low concentration of selumetinib (AZD6244) introduced together with a low concentration of nilotinib resulted in a significant increase in the efficiency of nilotinib toward inhibition of schwannoma growth. Thus, these data agree with our hypothesis that inhibition of the MEK1/2/ERK1/2 pathway is synergistic with inhibition of PDGFR in reducing schwannoma proliferation. One could argue that the combination of receptor and MEK1/2 inhibitors is necessary to diminish the positive feedback caused by the MEK1/2 inhibitor itself, increasing receptor phosphorylation/activity.26 However, in our experience, selumetinib (AZD6244) used alone does not lose its inhibitory effect on schwannoma proliferation (Ammoun et al6 and current data), thus excluding the development of any positive feedback toward PDGFR. The drug combination was not toxic in our model, thus decreasing—but not completely eliminating—the possibility that patients would develop adverse effects. Therefore, the combination of nilotinib with selumetinib (AZD6244) could be an alternative option to nilotinib monotherapy to be tested in future clinical trials of merlin-deficient tumors. The drugs—individually and in combination—were not toxic in our model, thus limiting but not excluding the possibility of that side effects could develop after long-term use in patients. Interestingly, in combination, both drugs were effective at rather low concentrations in our human in vitro model. Tolerability in humans can only be judged after long-term use by patients and not from preclinical data. For the drugs individually, these data already exist and indicate good tolerability. However, any tolerability issues might be ameliorated through modification of dosing schedules, such that the maximum concentrations of the 2 drugs do not coincide.
In summary, we demonstrated that both imatinib and nilotinib could be used for treatment of schwannoma and other NF2-related tumors. Nilotinib, however, seems to be more efficient than imatinib, exerting its maximal effect on schwannoma growth at concentrations 6 to 10 times lower than that of imatinib and 4 times lower than its plasma concentration. We suggest that nilotinib is presumably clinically more relevant than imatinib, because it can be used at lower doses, limiting advese effects. Moreover, the bioavailability of nilotinib would be increased because of its lipophilicity, facilitating the drugs' penetration into the tumor.5 Taking into account that schwannomas are located outside of the blood-brain barrier makes systemic therapies with small-molecular inhibitors more effective. In addition, we showed that the MEK1/2 inhibitor selumetinib (AZD6244) increases the inhibitory effect of nilotinib, potentiating the clinical relevance of this therapy.
Conflict of interest statement. None declared.
Funding
This study was funded by Peninsula College of Medicine & Dentistry and Novartis.
References
- 1.Hanemann CO. Magic but treatable? Tumours due to loss of merlin. Brain. 2008;131:606–615. doi: 10.1093/brain/awm249. doi:10.1093/brain/awm249. [DOI] [PubMed] [Google Scholar]
- 2.Hanemann CO, Evans DG. News on the genetics, epidemiology, medical care and translational research of Schwannomas. J Neurol. 2006;253:1533–1541. doi: 10.1007/s00415-006-0347-0. doi:10.1007/s00415-006-0347-0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum C, Kluwe L, Mautner VF, et al. Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis. 1998;5:55–64. doi: 10.1006/nbdi.1998.0179. doi:10.1006/nbdi.1998.0179. [DOI] [PubMed] [Google Scholar]
- 4.Schulze KM, Hanemann CO, Muller HW, et al. Transduction of wild-type merlin into human schwannoma cells decreases schwannoma cell growth and induces apoptosis. Hum Mol Genet. 2002;11:69–76. doi: 10.1093/hmg/11.1.69. doi:10.1093/hmg/11.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Ammoun S, Flaiz C, Ristic N, et al. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. doi:10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 6.Ammoun S, Ristic N, Matthies C, et al. Targeting ERK1/2 activation and proliferation in human primary schwannoma cells with MEK1/2 inhibitor AZD6244. Neurobiol Dis. 2010;37:141–146. doi: 10.1016/j.nbd.2009.09.017. doi:10.1016/j.nbd.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Ammoun S, Cunliffe CH, Allen JC, et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 2010;12:834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraenzer JT, Pan H, Minimo L, Jr, et al. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–1500. [PubMed] [Google Scholar]
- 9.Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2010;184:3–20. doi: 10.1007/978-3-642-01222-8_1. doi:10.1007/978-3-642-01222-8_1. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. doi:10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 11.Deremer DL, Ustun C, Natarajan K. Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Clin Ther. 2008;30:1956–1975. doi: 10.1016/j.clinthera.2008.11.014. doi:10.1016/j.clinthera.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Utermark T, Kaempchen K, Antoniadis G, et al. Reduced apoptosis rates in human schwannomas. Brain Pathol. 2005;15:17–22. doi: 10.1111/j.1750-3639.2005.tb00095.x. doi:10.1111/j.1750-3639.2005.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaempchen K, Mielke K, Utermark T, et al. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12:1211–1221. doi: 10.1093/hmg/ddg146. doi:10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- 14.Hanemann CO, Bartelt-Kirbach B, Diebold R, et al. Differential gene expression between human schwannoma and control Schwann cells. Neuropathol Appl Neurobiol. 2006;32:605–614. doi: 10.1111/j.1365-2990.2006.00769.x. doi:10.1111/j.1365-2990.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 15.Pirraco A, Coelho P, Rocha A, et al. Imatinib targets PDGF signaling in melanoma and host smooth muscle neighboring cells. J Cell Biochem. 2010;111:433–441. doi: 10.1002/jcb.22725. [DOI] [PubMed] [Google Scholar]
- 16.Biswas SK, Zhao Y, Sandirasegarane L. Imatinib induces apoptosis by inhibiting. Mol Vis. 2009;15:1599–1610. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Adrian FJ, Jahnke W, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463:501–506. doi: 10.1038/nature08675. doi:10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee J, Kamnasaran D, Balasubramaniam A, et al. Human schwannomas express activated platelet-derived growth factor receptors and c-kit and are growth inhibited by Gleevec (Imatinib Mesylate) Cancer Res. 2009;69:5099–5107. doi: 10.1158/0008-5472.CAN-08-4475. doi:10.1158/0008-5472.CAN-08-4475. [DOI] [PubMed] [Google Scholar]
- 19.Lepont P, Stickney JT, Foster LA, et al. Point mutation in the NF2 gene of HEI-193 human schwannoma cells results in the expression of a merlin isoform with attenuated growth suppressive activity. Mutat Res. 2008;637:142–151. doi: 10.1016/j.mrfmmm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright JA, Huang A. Growth factors in mechanisms of malignancy: roles for TGF-beta and FGF. Histol Histopathol. 1996;11:521–536. [PubMed] [Google Scholar]
- 21.Tanaka C, Yin OQ, Sethuraman V, et al. Clinical pharmacokinetics of the BCR-ABL tyrosine kinase inhibitor nilotinib. Clin Pharmacol Ther. 2010;87:197–203. doi: 10.1038/clpt.2009.208. doi:10.1038/clpt.2009.208. [DOI] [PubMed] [Google Scholar]
- 22.Davies A, Jordanides NE, Giannoudis A, et al. Nilotinib concentration in cell lines and primary CD34(+) chronic myeloid leukemia cells is not mediated by active uptake or efflux by major drug transporters. Leukemia. 2009;23:1999–2006. doi: 10.1038/leu.2009.166. doi:10.1038/leu.2009.166. [DOI] [PubMed] [Google Scholar]
- 23.Matei D, Chang DD, Jeng MH. Imatinib mesylate (Gleevec) inhibits ovarian cancer cell growth through a mechanism dependent on platelet-derived growth factor receptor alpha and Akt inactivation. Clin Cancer Res. 2004;10:681–690. doi: 10.1158/1078-0432.ccr-0754-03. doi:10.1158/1078-0432.CCR-0754-03. [DOI] [PubMed] [Google Scholar]
- 24.Atanasoski S, Boentert M, De VL, et al. Postnatal Schwann cell proliferation but not myelination is strictly and uniquely dependent on cyclin-dependent kinase 4 (cdk4) Mol Cell Neurosci. 2008;37:519–527. doi: 10.1016/j.mcn.2007.11.005. doi:10.1016/j.mcn.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. doi:10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 26.Kang ES, Oh MA, Lee SA, et al. EGFR phosphorylation-dependent formation of cell-cell contacts by Ras/Erks cascade inhibition. Biochim Biophys Acta. 2007;1773:833–843. doi: 10.1016/j.bbamcr.2007.02.003. doi:10.1016/j.bbamcr.2007.02.003. [DOI] [PubMed] [Google Scholar]