Abstract
In the past few years, bone marrow (BM)-derived cells have been used to regenerate damaged cardiovascular tissues post-myocardial infarction. Recent clinical trials have shown controversial results in recovering damaged cardiac tissue. New progress has shown that the underlying mechanisms of cell-based therapy relies more heavily on humoral and paracrine effects rather than on new tissue generation. However, studies have also reported the potential of new endothelial cell generation from BM cells. Thus, efforts have been made to identify cells having higher humoral or therapeutic effects as well as their surface markers. Specifically, BM-derived CD31+ cells were isolated by a surface marker and demonstrated high angio-vasculogenic effects. This article will describe recent advances in the therapeutic use of BM-derived cells and the usefulness of CD31+ cells.
Keywords: angiogenesis, cardiovascular disease, CD31, ischemia, progenitors, stem cells, vasculogenesis
Cardiovascular disease is one of the most serious health challenges worldwide. In the USA, nearly 80 million people are affected by myocardial infarction (MI), stroke and ischemic cardiomyopathy each year [1]. Despite advances in surgical and medical treatment, current therapies are still short of regaining cardiac function. Due to the limited regeneration capacity of the myocardium, severely damaged cardiac muscle following myocardial injury such as MI can lead to adverse ventricular remodeling and heart failure. Until recently, there have been no clinical procedures or medication that has proved to effectively regenerate damaged myocardium and, thus, new approaches have been desired for a long time. The recent identification of adult stem or progenitor cells has raised the possibility of regenerating damaged myocardium, particularly cardiomyocytes and/or vessels. Among those, bone marrow (BM)-derived stem or progenitor cells have been most widely investigated [2–4].
Growing evidence suggests that BM-derived stem cells have a multipotent capacity to differentiate into nonhematopoietic tissues, such as skeletal myoblasts [5,6], endothelium [7], cardiomyocytes [8], gut, lung, skin epithelia [9] and neural cells [10]. This plasticity of BM cells is supported by the detection of donor-derived transplanted BM cells in the heart [11–13] and brain [14]. Based on these promising preclinical data, various BM or peripheral blood (PB)-derived mononuclear cells (MNCs), endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) have been implemented in patients with acute MI or more chronic forms of heart failure. Earlier clinical studies showed improvement of regional or global left ventricular function [15–19]; however, later studies reported marginal or no therapeutic improvement of cardiac function in a similar subset of patients [20–23]. In order to elucidate these conflicting results, more global multicenter trials are needed. More importantly, to enhance the therapeutic effects, there should be ongoing efforts to elucidate the mechanisms of the cell therapy as well as the discovery of new cells with higher therapeutic potential. The following sections will cover the characteristics and the therapeutic potential of the most widely used BM-derived stem or progenitor cells and a recently identified novel angio-vasculogenic cell type, CD31+ cells.
Endothelial progenitor cells
The development of the vascular system is comprised of two processes: vasculogenesis and angiogenesis [24]. Vasculogenesis involves the in situ formation of blood vessels through differentiation into endothelial cells (ECs) from angioblasts or progenitor ECs and gives rise to capillaries [25]; whereas angiogenesis refers to the extension of pre-existing blood vessels by sprouting or intussusceptions of capillaries through proliferation or migration of ECs. Historically, angiogenesis was thought to be responsible for neovascularization in both neonatal and postnatal developmental stages. However, vasculogenesis was believed to occur only during the embryonic developmental period.
In 1997, Asahara et al. reported the existence of circulating progenitor cells or angioblasts isolated from human PB (hPB) that not only displayed EC properties but also showed the potential to differentiate into ECs [26]. These cells were referred to as EPCs. These EPCs were shown to be incorporated into the vasculature in adult animals and induce new vessel formation in ischemic tissues [27]. The transplantation of EPCs into ischemic tissues induced neovascularization and helped regenerate ischemic tissue damage [28]. Although this novel concept of postnatal vasculogenesis has been widely accepted, the precise identification of genuine EPCs has been complicated by the lack of specific markers and phenotype diversity.
Early EPCs
Initially, CD34 or VEGF receptor (VEGFR)-2 was used to isolate circulating EPCs or putative angioblasts from PB [26,29,30]. As there are no known surface markers for specifically isolating circulating EPCs, EPCs were identified by short-term culture of various BM cell fractions in endothelial differentiation media. For example, CD133, which is displayed on immature hematopoietic stem cells (HSCs), was used for culture derivation of EPCs [31]. For therapeutic purposes, short-term culture of MNCs was widely used for deriving EPCs. Whole MNCs were cultured for 4–7 days on vitronectin- or fibronectin-coated dishes and adherent cells were regarded as EPCs (although these cells are not EPCs as a whole but EPC-enriched cells) [26–28,32,33]. Typically these cultured cells displayed endothelial-like characteristics represented by the uptake of acetylated low-density lipoproteins and the binding of lectins and expression of several EC-specific proteins (VEGFR-2, Tie2, vascular endothelial [VE]-cadherin, von Willebrand factor, endothelial nitric oxide synthase and CD146) and showed a low proliferation rate. However, other studies have raised questions regarding the pure endothelial-like features of these EPCs and revealed that they also express monocyte/macrophage markers such as CD45, CD14, CD11b and CD11c [34–37]. More recently these cells were called circulating angiogenic cells [37] as these EPCs rarely give rise to ECs in vivo but contribute to vessel formation mainly through angiogenic effects. Alternative techniques have been used to isolate cells similar to these EPCs, where whole MNCs were seeded on fibronectin-coated plates. After 2 days, only nonadherent cells were collected for removal of mature ECs and macrophages, and subsequently reseeded on fibronectin-coated plates. Colonies were generated after 5–9 days and named colony-forming unit-Hill or colony-forming unit-ECs [38]. Since these cells appear to share similar properties with the aforementioned EPCs, collectively they were called early EPCs.
Late EPCs
Recently, other types of EPCs have been discovered from circulating MNCs, such as outgrowth ECs [39], late EPCs [29] or endothelial colony-forming cells (ECFCs) as these cells appear late (typically more than 2 weeks) in the EPC culture conditions. Although the culture methods are somewhat variable, these cells essentially share common characteristics in cell morphology (round), proliferation rate (rapid) and surface marker expression (EC markers only) [36,40]. For culture derivation of ECFCs, PB or cord blood MNCs were plated on collagen type-1-coated dishes with endothelial growth media-2. After one day, nonadherent cells were depleted and only adherent cells were cultured in endothelial growth media-2. Colonies appeared 5–22 days after plating as monolayers of cobblestone-appearing ECs. ECFCs can form de novo vessels in vitro and have a similar phenotype to cultured ECs [36,40]. Specifically, ECFCs do not express hematopoietic (CD45) and monocytic (CD14) markers but express most EC proteins. However, paracrine effects are limited compared with early EPCs [41]. Their vasculogenic effects were only demonstrated in a Matrigel™ plug assay [41]. Thus, their EC generation capabilities and regenerative effects on vasculature in ischemic animal models need to be addressed. It is possible that these cells are primitive circulating ECs or ECs sloughed off from vessels. The difference between ECFCs and mature ECs also remains to be determined. For clinical applications, the advantages and disadvantages are similar to other cultured cells such as MSCs.
Hematopoietic stem cells
Hematopoietic stem cells have the capacity for self-renewal and give rise to every kind of blood cell [42]. HSCs are one of the best characterized stem cells and have been used for the treatment of hematologic disease patients for more than three decades. The transplantation of a single HSC can constitute all hematopoietic cells in an organism and fulfill every stem cell function. Due to their multipotency, reported in earlier studies [43], HSCs were used to regenerate damaged myocardium [8]. These studies have shown that HSCs could generate new cardiomyocytes, ECs and smooth muscle cells and improve post-MI cardiac function. Another versatile capacity of HSCs has been reported with side population cells, which expel Hoechst dye [44]. The differentiation potentials of these cells into cardiomyocytes, ECs and vascular smooth muscle cells were also demonstrated in a MI model [7,8]. However, other studies have argued that HSCs represented by lineage-negative, sca1-positive and c-kit-positive cells do not transdifferentiate into any cardiovascular cells in the infarcted heart after HSC transplantation [45,46]. These studies were performed in murine HSCs and no such experiments were conducted with specific human HSCs other than CD34+ cells and CD133+ cells that are enriched with human HSCs and EPCs. Studies using human CD34+ cells [47–50] or CD133+ cells [51–53] have been reported to improve post-MI cardiac function; however, the therapeutic mechanism turned out to be from nontransdifferentiation effects. As CD34+ cells or CD133+ cells are present in low numbers in circulation, the use of mobilizing cytokines such as granulocyte-colony stimulating factor (G-CSF) for obtaining a large number of cells needed for clinical use incurs high costs and risk to patients.
Mesenchymal stem cells
Mesenchymal stem cells need cell culture for isolation and can be derived from a variety of tissues including BM [54], adipose tissue [55], amniotic fluid [56], cord [57] and umbilical cord blood [58]. They possess self-renewing potential and differentiation capacity to turn into osteoblasts, chondrocytes and adipocytes. Earlier studies have shown that MSCs can be differentiated into cardiomyocytes in vitro [59]. However, differentiation of MSCs into ECs has not been clearly demonstrated. Several groups reported that MSC implantation into infarcted hearts decreased fibrosis, myocardial scarring and improved myocardial regeneration [60–62]. Although earlier studies claimed the mechanism to be myogenesis, follow-up studies have revealed paracrine effects as a main mechanism underlying therapeutic effects [63]. Also, another group demonstrated that in a model of murine hindlimb ischemia, MSC transplantation enhanced tissue repair via secretion of multiple cytokines including VEGF, basic FGF and PlGF rather than incorporation of MSCs into new or remodeling tissues [64,65]. To improve the survival rate of injected MSCs in the infarcted myocardium, genetically modified MSCs (in which heme oxygenase-1 [66] or Akt [67] were overexpressed) were used and were shown to enhance therapeutic efficacy. [62,68]. Clinical trials using MSCs for treating acute MI have shown them to be safe, reduce adverse remodeling and augment myocardial function [16,60,69].
The advantage of MSCs for clinical application lies in the ease of acquisition, isolation and high expansion capability in culture. One interesting characteristic of MSCs is their low immunogenicity, which owes itself to a lack of expression of MHC class II. Studies reported that MSCs have immunomodulatory properties that modulate the function of T cells [70,71]. MSCs are known to induce immune tolerance in allogeneic stem cell transplantations [72,73]. However, the long-term safety of MSC treatment still needs to be monitored due to the use of animal serum, allogeneic cell use, the variable quality resulting from long-term culture expansion and potential tumor formation. Studies have shown that mouse BM-MSCs can transform after long-term culture and induce tumors in mice [74–76].
Therapeutic effects of adult stem/progenitor EPCs, HSCs & MSCs are mainly mediated by nontransdifferentiation effects
Despite the fact that stem or progenitor cells have emerged as a promising therapeutic modality, there have been several controversies regarding therapeutic mechanisms for ischemic diseases. In particular, the plasticity or true tissue generation capacity (transdifferentiation into cardiomyocytes, smooth muscle cells and ECs) of BM-derived cells has been widely debated [45,46,77]. Although therapeutic effects are observed, the occurrence of vasculogenesis and exogenous myogenesis in damaged tissues are very low. Later studies more clearly suggested that the paracrine mechanism could be a major mechanism to mediate therapeutic effects in cardiovascular diseases. BM cells release cytokines such as VEGF, MCP-1, FGF-2, angiopoetin-1 and Wnt [78–80]. MSCs secrete VEGF, FGF-2, PlGF and MCP-1 [65,81] and cultured early EPCs secrete HGF, VEGF and G-CSF [35,74]. One recent study reported an important role of Wnt signaling in mediating angiogenic effects of human fetal CD133+ cells on ischemic wounds [80]. These growth factors promote angiogenesis or cardiomyogenesis, protect against tissue apoptosis or necrosis and induce endogenous resident stem cell migration and proliferation through a paracrine response [63,65,74,80–82]. Additionally, it is now known that these humoral effects are not only attributed to implanted cells, but also to host tissues that had received cell therapy [74,83]. However, the evidence of paracrine effects derived from late EPCs or ECFCs is not clearly reported. Other studies have shown that fusion can be a mechanism for transdifferentiation [84,85]. Although some studies emphasized an important role of fusion for transdifferentiation [86,87], other studies have shown that fusion is only partly or minimally responsible for the phenotypic changes of stem cells [81].
Role of CD31 in vascular biology
CD31
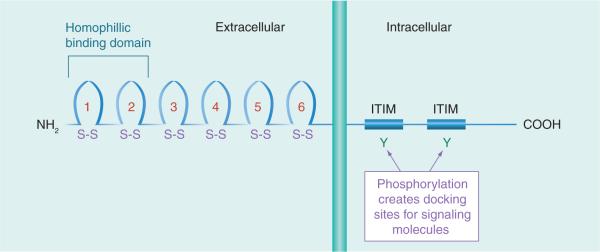
Platelet endothelial cell adhesion molecule (PECAM)-1, also known as CD31, is a 130-kDa molecular weight surface protein that belongs to the immunoglobulin superfamily and consists of six extracellular immunoglobulin folds (Figure 1). In its cytoplasmic domain, there are two immunoreceptor tyrosine inhibitory motifs for interactions with signaling molecules. CD31 is expressed on the cell surface of ECs and hematopoietic cells such as monocytes, platelets, neutrophils, natural killer cells, megakaryocytes and some T cells.
Figure 1. The schematic structure of CD31.
CD31 is a 130-kDa, type I transmembrane glycoprotein, and is a member of the immunoglobulin gene superfamily.
ITIM: Immunoreceptor tyrosine inhibitory motif.
Platelet endothelial cell adhesion molecule-1 mediates homotypic adhesion between adjacent ECs as well as between ECs and leukocytes [88,89]. A role for PECAM-1 in migration through ECs has also been reported for neutrophils and monocytes [90], and subsequently for numerous other cell types including natural killer cells [91], hematopoietic progenitor cells (HPCs) [92] and certain subsets of lymphocytes.
Role of CD31 in cell survival & angiogenesis
Cell–cell and cell–extracellular matrix interactions have been shown to play pivotal roles in coordinating EC proliferation and apoptosis required for proper blood vessel formation and regression. There is growing evidence that CD31 could transduce signals that suppress cell death. It has been proposed that homophilic interactions of CD31 between ECs and monocytes decreases apoptotic EC death [93]. This finding suggests that CD31 homophilic interactions result in the transmission of prosurvival signals. In addition, CD31 engagement has been reported to induce Akt phosphorylation. PECAM-1 itself has also been involved during EC apoptosis by two pathways: a metalloproteinase-dependent cleavage and a caspase-mediated cleavage of the cytoplasmic tail [94].
Given its abundant expression in ECs, CD31 has been shown to be involved in the initial stabilization and formation of cell–cell contacts at lateral junctions of ECs, the maintenance of a vascular permeability barrier, modulation of cell migration, transendothelial migration of monocytes and neutrophils and formation of blood vessels in angiogenesis [88,90,95,96]. Additionally, CD31 was found to form a functional complex with cadherin 5, β-catenin, and F-actin to control EC tube formation [97]. More recent data showed the involvement of CD31 in the adhesion/signaling events necessary for the migration of ECs and subsequent tube formation during angiogenesis, independent of VE-cadherin [98].
Novel role of CD31-expressing MNCs in circulation
Since the major mechanisms underlying therapeutic effects for BM-derived stem or progenitor cells were shown to be humoral effects [63,65,99,100], identifying such cells could lead to the development of next generation cell therapy. Therefore, we explored whether or not we could isolate cells enriched with humoral activities from BM and/or PB. At the same time, we wanted to employ a surface marker to isolate these cells. By avoiding cell culture, direct isolation using a surface marker can circumvent deleterious effects associated with the use of animal serum and extra costs related to cell cultivation. Since CD31 is a well-known EC marker, we hypothesized that CD31+ cells may include cells enriched with vessel-forming activities.
Angiogenic features of CD31+ cells
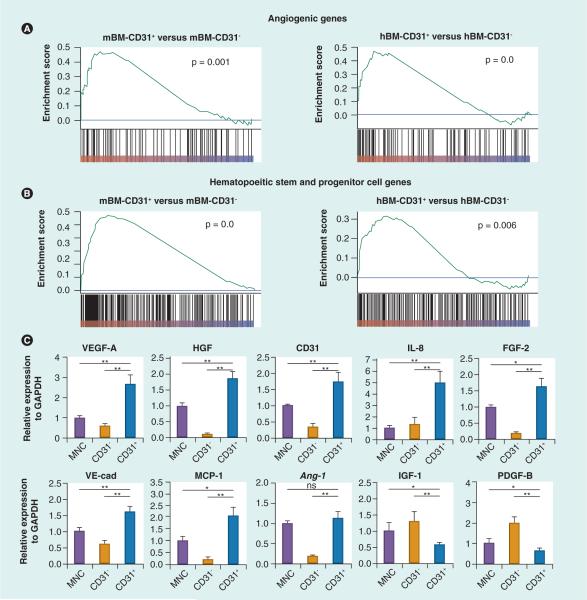
To evaluate the characteristics of CD31+ cells in comparison to CD31− cells, we performed genome-wide gene expression analyses [101,102]; gene set enrichment analysis and hierarchical cluster analysis with mouse BM (mBM), human BM (hBM), and hPB showed that angiogenic genes were globally upregualted in CD31+ cells compared with CD31− cells (Figure 2). The higher expression of angiogenic genes in the CD31+ cells was confirmed by real-time PCR [101,102]. The angiogenic genes were significantly enriched in the CD31+ cells compared with the corresponding CD31− cells. mBM-CD31+ cells expressed very high levels of angiopoietin-1 (Angpt1) and Gata2 compared with the CD31− cells, whereas hBM-CD31+ cells expressed higher levels of heparin-binding EGF-like growth factor (HBEGF) and IL-8 than hBM-CD31− cells. Angpt1 is a well-known angiogenic growth factor [103,104] and Gata2 is a transcription factor, which, when activated, increases angiogenesis [105]. HBEGF is involved in the recruitment of vascular smooth muscle cells [106] and IL-8 is a macrophage-derived mediator of angiogenesis [107]. Neuropilin-1 (NRP1) is the most highly expressed angiogenic gene in hPB-CD31+ cells compared with hPB-CD31− cells. NRP1 is required for vascular development and mediates VEGF-dependent angiogenesis [108].
Figure 2. Gene expression patterns in CD31+ and CD31− cells.
Total RNA was isolated from mBM-CD31+, mBM-CD31−, hBM-CD31+, hBM-CD31−, human peripheral blood (hPB)-CD31+, hPB-CD31− cells, and hPB-MNC and subjected to (A & B) microarray and/or (C) real-time reverse transcriptase PCR. (A & B) Gene set enrichment ana lysis was performed using the microarray data. Gene set enrichment ana lysis shows angiogenic genes (A) and hematopoietic stem and progenitor cell genes (B) are globally upregulated in the CD31+ cell fraction; n = 3. (C) The expression patterns of multiple angiogenic factors in hPB-CD31+ and -CD31− cells. Data are presented as a fold difference compared with the mononuclear cell group; n = 6.
*p < 0.05; **p < 0.01.
GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; hBM: Human bone marrow; mBM: Mouse bone marrow; MCP: Monocyte chemoattractant protein; MNC: Mononuclear cell; ns: Not significant; VE-cad: Vascular endothelial cadherin.
(A & B) Reproduced with permission from [101]. (C) Reproduced with permission from [102].
Vasculogenic effects of CD31
One major focus of our research has been to determine whether any hematopoietic cells contribute to true EC generation and determine the identity of such cells. Studies from other investigators and ours have provided strong evidence that there is still quite a reasonable possibility that certain BM-derived cell populations can give rise to ECs [8,29,109]. However, other studies have strongly argued against these phenomena [46,77,110]. Representative studies that refuted EC generation by hematopoietic cells used MI models [45,46] to prove the vasculogenic effects. However, an MI model allows very minimal engraftment of any cells, not only BM-derived cells. Studies have shown that the engraftment rate of embryonic stem cells differentiated into cardiomyogenic lineages or cardiac stem or progenitor cells are also very minimal in a MI model [111–113]. The constant motion of the heart, ongoing inflammation in acute stages of infarction, and little oxygen and nutrients in the ischemic heart tissue appear to be too harsh for transplanted cells to engraft and survive. Tight junctions between cardiomyocytes could be another hurdle for engraftment of externally injected cells. Furthermore, most studies are more focused on cardiomyocyte transdifferentiation from BM cells rather than ECs. Thus we understood that the counter evidence for transdifferentiation of BM cells into ECs might have been overemphasized. We hypothesized that, if appropriate BM-derived cells are injected into more permissive models, they can give rise to ECs. In fact, recent studies have clearly demonstrated generation of ECs from BM cells in tumor models [114,115]. Together these studies suggest that the generation of ECs from BM or PB cells may not only depend on the cell types but also the experimental models or host environment.
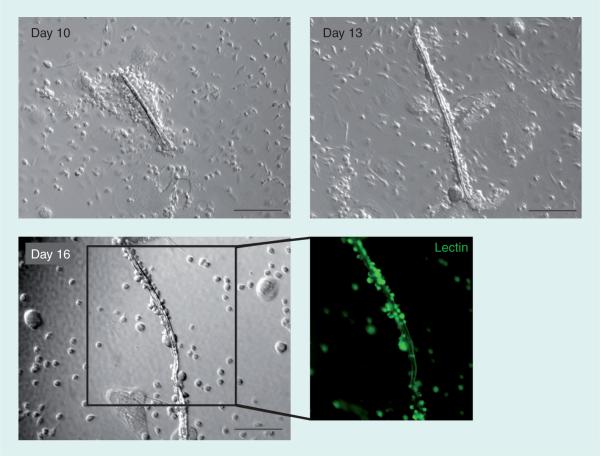
The vasculogenic effects of CD31+ cells have been investigated in our recent studies [101,102]. First, in vitro assays showed that mBM−, hBM− and hPB-CD31+ cells generated a markedly high number of EPCs under culture, compared with the CD31− cells. Results from an in vitro EC differentiation assay using hPB-CD31+ cells showed clear expression of EC-specific markers, such as von Willebrand factor, VEGFR-2, VE-cadherin and CD31, in CD31+ cells. In these culture experiments, we observed intriguing morphological changes of the hPB-CD31+ cells (Figure 3). Under endothelial differentiation conditions, hPB-CD31+ cells formed cellular aggregates on day 7, which subsequently underwent tubular structural changes within the round cell cluster by day 10. Thereafter, these mixed morphologies shifted to complete linear tubular structures that mimicked in vivo vasculogenesis. These tubular structures were positive for lectin and took up acetylated human low-density lipoprotein, indicating EC characteristics.
Figure 3. Human peripheral blood-CD31+ cells formed vascular tube-like structures during ex vivo cultivation.
The CD31+ cells cultured under endothelial growth media-2 started to form tubular structures within the cell cluster at culture day 10. The tube grew into a linear structure stained by lectin (green fluorescence) until day 16, mimicking in vitro vasculogenesis. Magnification 10×, scale bar = 200 μm.
Bona fide evidence of vasculogenesis should be confirmed by in vivo animal models. A mouse model of hindlimb ischemia was used for testing vasculogenic capacity of CD31+ cells [101,102]. As the rate of transdifferentiation of hematopoietic cells into ECs was reported to be 0–50% [35,46,77,109,114,115], a battery of stringent methods and criteria were used to confirm differentiation. Confocal microscopy with 3D reconstruction of multiple images was used as a first step. This technique clearly demonstrated that a fraction of CD31+ cells were colocalized with ECs within the vascular structure even up to 8 weeks after cell injection. To more precisely quantify this transdifferentiation, flow cytometric analysis using enzymatically digested hindlimb tissues was employed. Quantitative flow cytometry showed that up to 4% of the ECs in the ischemic tissues were derived from transplanted mBM- or hPB-CD31+ cells. Fluorescent in situ hybridization of the digested tissues further confirmed vasculogenic capacity of hPB-CD31+ cells [102]. No previous studies adopted all of these technologies to prove transdifferentiation of hematopoietic cells. These data clearly indicate that functional ECs can be derived from directly injected CD31+ cells in the ischemic tissue.
Higher adhesion capacity of CD31+ cells
Studies have shown that engraftment of transplanted neonatal cardiomyocytes is less than 25% within 24 h of MI in animal models [116] and 5% after 1 h postinjection of CD34+ cells in human patients with MI [117]. Our study also demonstrated that most of the cultured early EPCs injected directly into the myocardium post-MI, disappeared within a week [100]. To enhance therapeutic effects of cell therapy, stable engraftment and survival of transplanted cells appears to be critical. One recent study suggested an association between durable engraftment of the transplanted EPCs and maintenance of functional improvement in experimental diabetic neuropathy [118]. When cultured, early EPCs were intramuscularly transplanted along the nerve, they engrafted into the diabetic nerve along the vasa nervorum for more than 12 weeks and improved neural function for more than 8 weeks [118].
Cells that successfully adhere to the extra-cellular matrix have a higher chance of survival by avoiding anoikis (apoptosis caused by lack of adhesion to extracellular matrix). This adhesion capacity may play an important role in cell survival, particularly in the ischemic environment. CD31 was originally cloned as an adhesion molecule between cells [89,119], and it has been shown to mediate cell-cell adhesion mainly through hemophilic interactions between CD31-expressing cells [120]. Genome-wide gene expression data showed a high expression of genes related to adhesion, transmembrane structure, chemokine production and reception, and extracellular matrix in hPB-CD31+ cells [102]. Cell adhesion assays further demonstrated that CD31+ cells have a higher adhesion capacity to various extracellular matrix proteins such as collagen, laminin, vitronectin, and fibronectins than CD31− cells [101,102]. In fact, the confocal microscopic data and FACS analysis for digested tissues verified higher engraftment of mBM-CD31+ cells compared with mBM-CD31− cells in cell transplantation studies with a hindlimb ischemia model. CXCR4/ SDF-1 is well known to mediate cell engraftment and migration. However, the expression of CXCR4 was not different between CD31+ cells and CD31− cells, suggesting that the higher engraftment of CD31+ cells does not result from the difference in expression levels of CXCR4. The higher engraftment of CD31+ cells is likely to augment the angiogenic and vasculogenic ability of mBM- and hPB-CD31+ cells [101,102]. CD31 is the first marker used to isolate a BM cell subpopulation that has higher adhesion and engraftment potential.
Enrichment of hematopoietic stem & progenitor cells in CD31+ cells
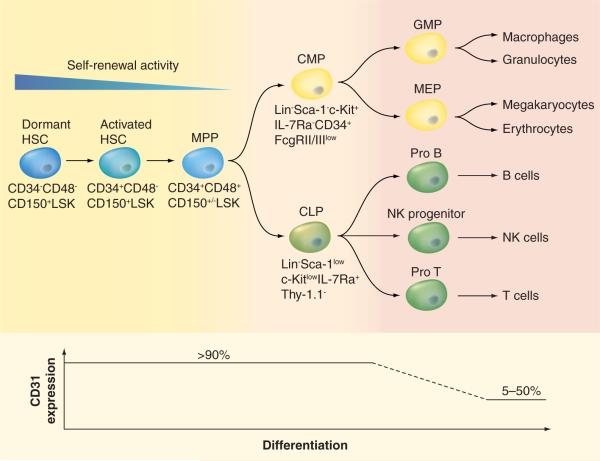
Gene expression studies have shown that the levels of hematopoietic stem and progenitor cell genes were higher in mBM-CD31+ cells and hBM-CD31+ cells than mBM-CD31− cells and hBM-CD31− cells, respectively (Figure 2) [101], supporting the notion that hematopoietic stem and progenitor cells are enriched in the CD31+ cells. FACS analysis confirmed that more than 90% of HSCs, multipotent progenitor cells, common lymphoid progenitor cells, and common myeloid progenitor cells in mBM express CD31 [101]. In vitro colony-forming assays and in vivo BM cell transplantation experiments further support that hematopoietic stem and progenitor cells are almost exclusively included in mBMCD31+ cells [101]. Similarly, in hBM, CD31 was expressed in 99.8% of CD34+CD133+ and 89% of CD34+CD133−, indicating that HSCs and most HPCs express CD31 [101]. In vitro hematopoietic colony-forming assays revealed that clonogenic HPCs are enriched in hPBCD31+ cells [102]. Together, regardless of whether human or mouse, HSCs and HPCs are almost exclusively present in CD31+ cell fractions.
CD34+ cells are effective for improving ischemic cardiovascular diseases in animal models [121] and human patients [49,122] with no documented adverse effects. Given that HSCs/ EPCs that are heavily enriched in the CD34+ cell fraction are almost exclusively present in the CD31+ population, and that HSCs/EPCs have been reported to be vital for therapeutic neovascularization in ischemic cardiovascular diseases, it is important to address the role of HSC/EPC versus non-HSC/EPC populations among CD31+ cells in ischemic cardiovascular repair. One important thing to note is that this non-HSC/EPC population constitutes the majority of the CD31+ cells. When this population (hBM-CD34−CD31+ cells) was compared with hBM-CD34+ cells, therapeutic effects between these two groups were similar in improving mouse limb ischemia, suggesting that non-HSC/EPC populations among the CD31+ cell fraction also play an important role in therapeutic neovascularization.
Other potentially beneficial effects of CD31+ cells
Given that mBM-, hPB- and hBM-CD31+ cells include immune cells such as B, T and myeloid cells (Figure 4), it is possible that the CD31+ cells have an immunomodulatory function to reduce tissue damage and accelerate tissue regeneration. Inflammation is a double-edged sword that can destroy the tissue or can contribute to the regeneration of tissue. Since the transplantation of CD31+ cells resulted in the regeneration of ischemic tissue, it is possible that immune cells that contribute to tissue regeneration [123,124] may be enriched in CD31+ cells and that tissue-destroying immune cells are relatively depleted in the CD31+ cell fraction. However, further investigation is required to determine whether the CD31+ cells have favorable immunomodulatory functions and/or are enriched with tissue-regenerating immune cells.
Figure 4. Expression of CD31 decreases as the hematopoietic stem and progenitor cells undergo differentiation.
CLP: Common lymphoid progenitor; CMP: Common myeloid progenitor; GMP: Granulocyte–monocyte progenitor; HSC: Hematopoietic stem cell; LSK: Lin−Sca-1+c-Kit+; MEP: Megakaryocyte–erythroid progenitor; MPP: Multipotential progenitor; NK: Natural killer.
Clinical application of CD31+ cells
One major advantage of CD31+ cells over CD34+ or CD133+ cells is their prevalence in circulating blood. Approximately 30–35% of total MNCs of hPB are CD31+. For instance, if 60 million CD31+ cells are needed for transplantation, only 100 ml of blood is needed [122,125]. Thus, there is no need to use mobilizing agents, such as G-CSF, for collecting CD31+ cells [122]. This leads to the reduction in cost of cell therapy, simplification of treatment procedures and removal of potential adverse effects associated with mobilizing agents.
Another advantage of CD31+ cells over the cultured EPCs or MSCs is the avoidance of cell culture. The major limitation of using cultured cells for clinical use is whether or not cells can be expanded in large scale in a safe manner. Most EPC or MSC expansion protocols utilize media containing fetal bovine serum. Since fetal bovine serum contains growth factors, attachment factors and vital nutrients, it has been one of the most widely used ingredients for cell culture. However, the use of xenogeneic serum can pose a risk such as disease transmission, harmful immunizing effects or adverse effects caused by unknown factors. Potential concerns include viral, prion and zoonose contamination. In fact, antifetal bovine serum antibodies and inflammatory reactions have been detected and those could have immunological adverse events and be a factor influencing the therapeutic results [126].
Other potential benefits over uncultured BM-derived MNCs or unfractionated cells is the removal of unnecessary cells that may induce adverse effects such as calcification [127] or aggravation of ischemia [128]. We did not observe such adverse effects when we tested the CD31+ cells in a mouse model of hindlimb ischemia [101,102]. Recent studies suggested that BM-MNCs [129] or G-CSF-mobilized PB-MNCs [130] are effective in the treatment of patients with critical limb ischemia. When we compared hBM-MNCs with hBM-CD31+ cells in a mouse model of hindlimb ischemia, the therapeutic efficacy of CD31+ cells was superior to that of BM-MNCs. Thus, CD31+ cell selection by removing nonangiogenic and highly inflammatory cells included in the CD31− cell fraction appear to have higher therapeutic effects and less potential toxicity. If a xeno-derived antibody against CD31 is used, patients might develop an allergenic reaction caused by the xeno-derived protein. However, given that many xeno-derived antibodies have been safely used in human patients, this risk would be minimal.
Future perspective
To identify more angiogenic and/or vasculogenic cells, further experimental investigation is required. Since CD31+ cells are a heterogeneous cell population that include T and B lymphocytes and myelomonocytic cells, more angiogenic or vasculogenic cells can be found by using additional markers. We recently demonstrated that vasculogenic cells are more abundant in CD14−CD31+ mBM cell populations than in the rest of the population of CD31+ mBM cells [101]. The addition of other markers will help narrow down the identification of more angiogenic and vasculogenic cells. Mechanistically, we also need to determine the role of CD31 itself in the improvement of ischemia. CD31 knockout mice display a grossly normal phenotype. However, the response of CD31 knockout mice to pathologic conditions including ischemia or inflammation has not been tested. Moreover, considering the possibility of compensation for CD31 knockout effects during development, conditional deletion of CD31 genes may lead to the identification of novel roles of CD31 in regeneration or repair of ischemic cardiovascular diseases.
As the therapeutic effects of CD31+ cells have been clearly demonstrated in experimental hindlimb ischemia, clinical trials with CD31+ cells should be the next step. The first target could be patients with critical limb ischemia or intractable wounds. This cell therapy can be expanded to the treatment of other ischemic cardiovascular diseases including MI, stroke and diabetic neuro pathy. Although there are increasing reports that the efficacy of myocardial repair or regeneration with BM-derived cells may be minimal or modest, studies with peripheral vascular obstructive disease are very limited and most studies used unselected BM or PB-MNCs [131]. However, a recent presentation showed that CD34+ cells are effective in the treatment of critical limb ischemia patients [132]. As such, CD31+ cells will be a very promising option for critical limb ischemia patients. Another good reason that CD31+ cells will be effective for treating at least vascular ischemia, is that peripheral tissues have more room for cell engraftment and present a less hostile environment for cell survival. A recent diabetic neuropathy study also indicated that the host environment is a more important factor in determining engraftment and survival [118,133]. Although CD31+ cells have robust angiogenic and vasculogenic capability, it cannot be ruled out that CD31− cells can play a significant role in neovascularization. MSCs and pericytes are derived from the CD31− cell fraction [101,134] and these mural cells appear to play an important role in late-stage vessel formation, contributing to vascular maturation [135].
A decade of experience with BM cell therapy for cardiovascular diseases has yielded new insight into the mechanisms underlying this process. Important discoveries include the major role of the humoral and paracrine mechanisms in cell therapy, the importance of the host environment in cell engraftment and therapeutic effects, and the advantages of selected cells. Based on these new observations and discoveries, it is time to develop next generation adult cell therapy. Together, cell therapy with CD31+ cells for pulmonary venoocclusive disease can be a reasonable option to meet this end. Further scientific investigation will require the discovery of more therapeutically effective cell types, surface markers for genuine EPCs and appropriate target diseases for cell therapy.
Executive summary.
-
■
Various subsets of bone marrow (BM)-derived cells were identified, characterized and attempted cardiovascular regeneration or repair.
-
■
Early endothelial progenitor cells (EPCs) or circulating angiogenic cells are isolated in short-term ex vivo culture, and share characteristics of monocytes and endothelial cells (ECs). Early EPCs have strong humoral effects, including angiogenic effects, and are effective for repairing ischemic cardiovascular diseases in animal models.
-
■
Late EPCs are isolated by relatively long-term ex vivo culture and share features of mature ECs. The paracrine and therapeutic effects remain to be determined.
-
■
Human CD34+ cells are an EPC-enriched population and are effective for the treatment of ischemic cardiovascular diseases. The cell isolation process needs BM cell mobilizing reagents and apheresis, and the treatment is costly. Their transdifferentiation capacity to cardiomyocytes and ECs in vivo is questionable.
-
■
Mesenchymal stem cells are derived in ex vivo culture and their transdifferentiation capacity to cardiomyocytes or ECs in vivo is none too minimal. MSCs have paracrine and immunomodulatory function.
-
■
The major mechanisms underlying the therapeutic effects of BM-derived stem or progenitor cells on cardiovascular diseases are humoral or paracrine effects.
-
■
CD31 (Platelet endothelial cell adhesion molecule-1) is a cell adhesion molecule expressed in endothelial cells, platelets and various hematopoietic cells, and has been known as a representative marker for ECs. Recent series of papers have revealed that CD31 can be used as a marker for a BM-derived mononuclear cell fraction that is highly enriched with angiogenic, vasculogenic and hematopoietic stem or progenitor cells.
-
■
Hematopoietic stem and progenitor cells are exclusively included in the BM- or peripheral blood (PB)-derived CD31+ cell population.
-
■
BM- and PB-derived CD31+ cells have robust angiogenic capacity and include genuine vasculogenic cells or true EPCs.
-
■
BM- and PB-derived CD31+ cells possess high adhesion capability allowing longer engraftment and retention in ischemic cardiovascular tissues.
-
■
BM- and PB-derived CD31+ cells are effective for inducing neovascularization and recovering ischemic tissue injuries.
-
■
BM- and PB-derived CD31+ cells have advantages over other BM-derived stem or progenitor cells in therapeutic use as the CD31+ cells are more abundant than CD34+ or CD133+ cells, and do not require BM mobilizing reagents and ex vivo culture.
Acknowledgement
The authors would like to thank Kenneth Kelly for critical reading of the manuscript.
This work was supported in part by NIH grants, RO1HL084471, R21HL097353, RC1 GM092035 and HHSN268201000043C (Program of Excellence in Nanotechnology Award), PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, and Stem Cell Research Center of the 21st Century Frontier Research Program grant SC4300, funded by the Ministry of Science and Technology, Republic of Korea.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J. Clin. Invest. 2005;115(3):572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laflamme MA, Murry CE. Regenerating the heart. Nat. Biotechnol. 2005;23(7):845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 4.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ. Res. 2005;96(2):151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 6.Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 7.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 9.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 10.Brazelton TR, Rossi FM, Keshet GI, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 11.Minami E, Laflamme MA, Saffitz JE, et al. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112(19):2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 12.Laflamme MA, Myerson D, Saffitz JE, et al. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ. Res. 2002;90(6):634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 13.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N. Engl. J. Med. 2002;346(1):5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 14.van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 16.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assmus B, Rolf A, Erbs S, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ. Heart Fail. 2010;3(1):89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 18.Tatsumi T, Ashihara E, Yasui T, et al. Intracoronary transplantation of non-expanded peripheral blood-derived mononuclear cells promotes improvement of cardiac function in patients with acute myocardial infarction. Circ. J. 2007;71(8):1199–1207. doi: 10.1253/circj.71.1199. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen JA, Ylitalo K, Hedberg P, et al. Determinants of functional recovery after myocardial infarction of patients treated with bone marrow-derived stem cells after thrombolytic therapy. Heart. 2010;96(5):362–367. doi: 10.1136/hrt.2009.171694. [DOI] [PubMed] [Google Scholar]
- 20.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 21.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 22.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 23.Penicka M, Horak J, Kobylka P, et al. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction: a prematurely terminated randomized study. J. Am. Coll. Cardiol. 2007;49(24):2373–2374. doi: 10.1016/j.jacc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert SF. Developmental Biology. 5th Edition Sinauer Associates; Sunderland, MA, USA: 1997. [Google Scholar]
- 25.Risau W, Flamme I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]; ■■ First paper to report the existence of endothelial progenitor cells in adult peripheral blood.
- 27.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 28.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl Acad. Sci. USA. 2000;97(7):3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92(2):362–367. [PubMed] [Google Scholar]
- 30.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 31.Fernandez Pujol B, Lucibello FC, Gehling UM, et al. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65(5):287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 32.Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ. Res. 2000;87(6):434–439. doi: 10.1161/01.res.87.6.434. [DOI] [PubMed] [Google Scholar]
- 33.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J. Clin. Invest. 2001;108(3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmeisser A, Garlichs CD, Zhang H, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc. Res. 2001;49(3):671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 35.Rehman J, Li J, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 36.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106(5):1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 37.Gulati R, Jevremovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ. Res. 2003;93(11):1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 38.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]; ■■ Describes the identity of late endothelial progenitor cells and the diversity of endothelial progenitor cells.
- 41.Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 42.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 43.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 44.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 46.Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Zhang S, Rabinovich B, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ. Res. 2010;106(12):1904–1911. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh ET, Zhang S, Wu HD, et al. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108(17):2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 49.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a Phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115(25):3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 50.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114(20):2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]; ■■ Provides the first evidence that human peripheral blood-derived CD34+ cells are effective for myocardial ischemia.
- 51.Flores-Ramirez R, Uribe-Longoria A, Rangel-Fuentes MM, et al. Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc. Revasc. Med. 2010;11(2):72–78. doi: 10.1016/j.carrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Schots R, De Keulenaer G, Schoors D, et al. Evidence that intracoronary-injected CD133+ peripheral blood progenitor cells home to the myocardium in chronic postinfarction heart failure. Exp. Hematol. 2007;35(12):1884–1890. doi: 10.1016/j.exphem.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Leor J, Guetta E, Feinberg MS, et al. Human umbilical cord blood-derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006;24(3):772–780. doi: 10.1634/stemcells.2005-0212. [DOI] [PubMed] [Google Scholar]
- 54.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 55.Madonna R, Geng YJ, De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler. Thromb. Vasc. Biol. 2009;29(11):1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 56.Antonucci I, Stuppia L, Kaneko Y, et al. Amniotic fluid as rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2010 doi: 10.3727/096368910X539074. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciavarella S, Dammacco F, De Matteo M, et al. Umbilical cord mesenchymal stem cells: role of regulatory genes in their differentiation to osteoblasts. Stem Cells Dev. 2009;18(8):1211–1220. doi: 10.1089/scd.2008.0340. [DOI] [PubMed] [Google Scholar]
- 59.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl Acad. Sci. USA. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaquet K, Krause KT, Denschel J, et al. Reduction of myocardial scar size after implantation of mesenchymal stem cells in rats: what is the mechanism? Stem Cells Dev. 2005;14(3):299–309. doi: 10.1089/scd.2005.14.299. [DOI] [PubMed] [Google Scholar]
- 62.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 63.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 64.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates that therapeutic effects of mesenchymal stem cells in ischemic heart disease are mainly mediated by paracrine effects.
- 65.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 66.Melo LG, Agrawal R, Zhang L, et al. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105(5):602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, McCullough KD, Franke TF, et al. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 2000;275(19):14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 68.Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 2005;46(7):1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 69.Boyle AJ, McNiece IK, Hare JM. Mesenchymal stem cell therapy for cardiac repair. Methods Mol. Biol. 2010;660:65–84. doi: 10.1007/978-1-60761-705-1_5. [DOI] [PubMed] [Google Scholar]
- 70.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 71.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 72.Majumdar MK, Keane-Moore M, Buyaner D, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 2003;10(2):228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 73.Barry FP, Murphy JM, English K, et al. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14(3):252–265. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 74.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25(2):371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 75.Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24(4):1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 76.Zhou YF, Bosch-Marce M, Okuyama H, et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66(22):10849–10854. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- 77.Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ. Res. 2004;94(2):230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 78.Fuchs U, Zittermann A, Suhr O, et al. Heart transplantation in a 68-year-old patient with senile systemic amyloidosis. Am. J. Transplant. 2005;5(5):1159–1162. doi: 10.1111/j.1600-6143.2005.00805.x. [DOI] [PubMed] [Google Scholar]
- 79.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 80.Barcelos LS, Duplaa C, Krankel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ. Res. 2009;104(9):1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoon YS, Wecker A, Heyd L, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J. Clin. Invest. 2005;115(2):326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell Cardiol. 2005;39(5):733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 83.Tateno K, Minamino T, Toko H, et al. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ. Res. 2006;98(9):1194–1202. doi: 10.1161/01.RES.0000219901.13974.15. [DOI] [PubMed] [Google Scholar]
- 84.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416(6880):542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 85.Ying QL, Nichols J, Evans EP, et al. Changing potency by spontaneous fusion. Nature. 2002;416(6880):545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 86.Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 2004;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 87.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 88.Albelda SM, Muller WA, Buck CA, et al. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell–cell adhesion molecule. J. Cell Biol. 1991;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie Y, Muller WA. Molecular cloning and adhesive properties of murine platelet/endothelial cell adhesion molecule 1. Proc. Natl Acad. Sci. USA. 1993;90(12):5569–5573. doi: 10.1073/pnas.90.12.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muller WA, Weigl SA, Deng X, et al. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berman ME, Xie Y, Muller WA. Roles of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and β 2 integrin activation. J. Immunol. 1996;156(4):1515–1524. [PubMed] [Google Scholar]
- 92.Voermans C, Rood PM, Hordijk PL, et al. Adhesion molecules involved in transendothelial migration of human hematopoietic progenitor cells. Stem Cells. 2000;18(6):435–443. doi: 10.1634/stemcells.18-6-435. [DOI] [PubMed] [Google Scholar]
- 93.Noble KE, Wickremasinghe RG, DeCornet C, et al. Monocytes stimulate expression of the Bcl-2 family member, A1, in endothelial cells and confer protection against apoptosis. J. Immunol. 1999;162(3):1376–1383. [PubMed] [Google Scholar]
- 94.Ilan N, Mohsenin A, Cheung L, et al. PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. FASEB J. 2001;5(2):362–372. doi: 10.1096/fj.00-0372com. [DOI] [PubMed] [Google Scholar]
- 95.Newman PJ, Berndt MC, Gorski J, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 96.DeLisser HM, Christofidou-Solomidou M, Strieter RM, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. 1997;151(3):671–677. [PMC free article] [PubMed] [Google Scholar]
- 97.Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J. Immunol. 1997;158(7):3408–3416. [PubMed] [Google Scholar]
- 98.Cao G, O'Brien CD, Zhou Z, et al. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am. J. Physiol. Cell Physiol. 2002;282(5):C1181–C1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- 99.Uemura R, Xu M, Ahmad N, et al. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 100.Cho HJ, Lee N, Lee JY, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J. Exp. Med. 2007;204(13):3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim H, Cho HJ, Kim SW, et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ. Res. 2010;107(5):602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ First paper showing that CD31 is a marker to identify highly angiogenic and vasculogenic cells in mouse and human bone marrow hematopoietic cells.
- 102.Kim SW, Kim H, Cho HJ, et al. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J. Am. Coll. Cardiol. 2010;56(7):593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ First paper showing that human peripheral blood-derived CD31+ cells have superior therapeutic effects over CD31− cells owing to their strong angiogenic and vasculogenic properties.
- 103.Cho CH, Kammerer RA, Lee HJ, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc. Natl Acad. Sci. USA. 2004;101(15):5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jones N, Iljin K, Dumont DJ, et al. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2001;2(4):257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- 105.Mammoto A, Connor KM, Mammoto T, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457(7233):1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iivanainen E, Nelimarkka L, Elenius V, et al. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 2003;17(12):1609–1621. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 107.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 108.Lee P, Goishi K, Davidson AJ, et al. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc. Natl Acad. Sci. USA. 2002;99(16):10470–10475. doi: 10.1073/pnas.162366299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7(11):1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 110.O'Neill TJ, 4th, Wamhoff BR, Owens GK, et al. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ. Res. 2005;97(10):1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 111.Smits AM, van Laake LW, den Ouden K, et al. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc. Res. 2009;83(3):527–535. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 112.Tang XL, Rokosh DG, Guo Y, et al. Cardiac progenitor cells and bone marrow-derived very small embryonic-like stem cells for cardiac repair after myocardial infarction. Circ. J. 2010;74(3):390–404. doi: 10.1253/circj.cj-09-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 114.Nolan DJ, Ciarrocchi A, Mellick AS, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21(12):1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao D, Nolan DJ, Mellick AS, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 116.Muller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell Cardiol. 2002;34(2):107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 117.Musialek P, Tekieli L, Kostkiewicz M, et al. Randomized transcoronary delivery of CD34+ cells with perfusion versus stop-flow method in patients with recent myocardial infarction: early cardiac retention of (99m)Tc-labeled cells activity. J. Nucl. Cardiol. 2010;18(1):104–116. doi: 10.1007/s12350-010-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeong JO, Kim MO, Kim H, et al. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009;119(5):699–708. doi: 10.1161/CIRCULATIONAHA.108.789297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zocchi MR, Poggi A. Lymphocyteendothelial cell adhesion molecules at the primary tumor site in human lung and renal cell carcinomas. J. Natl Cancer Inst. 1993;85(3):246–247. doi: 10.1093/jnci/85.3.246. [DOI] [PubMed] [Google Scholar]
- 120.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2007;27(2):2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 121.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107(3):461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 122.Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34+ cells in patients with critical limb ischemia: a Phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27(11):2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 123.Rafei M, Hsieh J, Zehntner S, et al. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat. Med. 2009;15(9):1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- 124.Stagg J, Galipeau J. Immune plasticity of bone marrow-derived mesenchymal stromal cells. Handb. Exp. Pharmacol. 2007;(180):45–66. doi: 10.1007/978-3-540-68976-8_3. [DOI] [PubMed] [Google Scholar]
- 125.Kang HJ, Kim HS, Zhang SY, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363(9411):751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 126.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc. Natl Acad. Sci. USA. 2002;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoon YS, Park JS, Tkebuchava T, et al. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109(25):3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 128.Miyamoto K, Nishigami K, Nagaya N, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114(24):2679–2684. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 129.Iso Y, Soda T, Sato T, et al. Impact of implanted bone marrow progenitor cell composition on limb salvage after cell implantation in patients with critical limb ischemia. Atherosclerosis. 2010;209(1):167–172. doi: 10.1016/j.atherosclerosis.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 130.Horie T, Onodera R, Akamastu M, et al. Long-term clinical outcomes for patients with lower limb ischemia implanted with G-CSF-mobilized autologous peripheral blood mononuclear cells. Atherosclerosis. 2010;208(2):461–466. doi: 10.1016/j.atherosclerosis.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 131.Al Mheid I, Quyyumi AA. Cell therapy in peripheral arterial disease. Angiology. 2008;59(6):705–716. doi: 10.1177/0003319708321584. [DOI] [PubMed] [Google Scholar]
- 132.Losordo D, Kibbe M, Mendelsohn F, et al. Randomized, double-blind, placebo controlled trial of autologous CD34+ cell therapy for critical limb ischemia: 1 year results. Circulation. 2010;122:A16920. [Google Scholar]
- 133.Kim H, Park JS, Choi YJ, et al. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells. 2009;27(7):1686–1696. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 135.Campagnolo P, Cesselli D, Al Haj Zen A, et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121(15):1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]