Abstract
Few brain imaging studies of schizophrenia involve samples with enhanced genetic homogeneity. We compared MRI volumetric data between individuals with 1q21–q23 linked familial schizophrenia associated with NOS1AP and their first and second degree unaffected relatives. We found significant gray matter reductions in the anterior cingulate gyrus in both affected individuals and their unaffected first degree relatives when compared with their unaffected second degree relatives. These results suggest that the changes are primarily due to genetic risk and not illness effects, and may represent an intermediate phenotype.
Keywords: Magnetic resonance imaging, Familial schizophrenia, NOS1AP, Imaging genomics, Anterior cingulate, Voxel-based morphometry
1. Introduction
Structural magnetic resonance imaging (MRI) studies have repeatedly identified regions of gray matter (GM) deficits in schizophrenia compared with unaffected volunteers (Honea et al., 2005). Unaffected relatives of individuals with schizophrenia also have differences in brain structure when compared with unrelated healthy controls, suggesting that some of the volumetric differences seen in schizophrenia may be related to the familial genetic risk of developing the disease (Goghari et al., 2007). However, individuals in the samples studied to date are likely to have various genetic risk factors for schizophrenia, and this etiologic heterogeneity may limit the ability to attribute specific MRI findings to genetic liability. We studied a relatively homogeneous subtype of familial schizophrenia, highly significantly linked to 1q21–q23 (Brzustowicz et al., 2000), comparing affected individuals to their unaffected relatives at two different degrees of relatedness. This allowed us to comment on whether changes may be due to genetic risk as opposed to the illness or its treatment.
2. Methods
2.1. Study sample
We studied 35 subjects (12 males; 23 females) from seven Canadian families of European descent: 11 meeting DSM-IV criteria for narrowly defined schizophrenia (SZ; mean age at onset=23.0 y, SD=5.9 y), and their unaffected first degree (UA1; n=17) and second degree (UA2; n=7) relatives (none of whom met criteria for any schizophrenia spectrum disorder (Wratten et al., 2009)). No participants had a history of serious brain injury or mental retardation. Informed consent was obtained and the study was approved by local Research Ethics Boards. Details of the original study design and recruitment are described elsewhere (Bassett et al., 1993). There were no significant inter-group differences with respect to sex, handedness, or total intracranial volume (TIV). There was a trend towards greater mean full scale IQ with more distant relatedness from an affected individual (SZ: mean=96.0, SD=15.7; UA1: mean=107.2, SD=10.7; UA2: mean=119.3, SD=8.3), with the SZ-UA2 pairwise difference reaching significance (p =0.002) (Husted et al., 2009). The UA2 group was significantly younger (mean=30.6 y, SD=16.0 y) than the well-matched SZ (mean=54.0 y, SD=8.2 y; p<0.001) and UA1 (mean=48.6 y, SD=8.8 y; p=0.002) groups.
2.2. Image acquisition and analysis
A GE Signa 1.5 T MR scanner and coronal three-dimensional inversion prepped RF-spoiled fast gradient recalled echo sequence were used to obtain all images for quantification of cerebrospinal fluid, GM, and white matter (WM) volumes. The 6 min 20 s sequence (TI=300 ms, TR=12 ms, TE=5 ms; flip angle=20°; field of view=20 cm, matrix=256×256) yielded 124 contiguous 1.5 mm-thick sections in the coronal plane. As described elsewhere (Voormolen et al., 2010), we used voxel-based morphometry (VBM) running on Statistical Parametric Mapping 5 (SPM5) with unified segmentation, and VBM5 Toolbox with associated Gaussian Hidden Markov Random Field model, to achieve optimal modulated normalized images for analysis. An overall false discovery rate correction of p<0.05 was used to account for multiple comparisons. Significant voxels and corresponding Talairach coordinates were mapped onto the T1-weighted Montreal Neurological Institute n=305 template, with voxel clusters that survived mapwise correction considered to be significant. We also extracted GM volumes from the modulated VBM images using MARINA to generate anterior cingulate (AC) gyrus masks for region-of-interest (ROI) analyses (Voormolen et al., 2010). Group comparisons for GM measurements derived from VBM and ROI were tested using an analysis of covariance model and IQ, sex, age, and TIV as covariates. Other details of the statistical analyses are as specified elsewhere (Voormolen et al., 2010).
3. Results and discussion
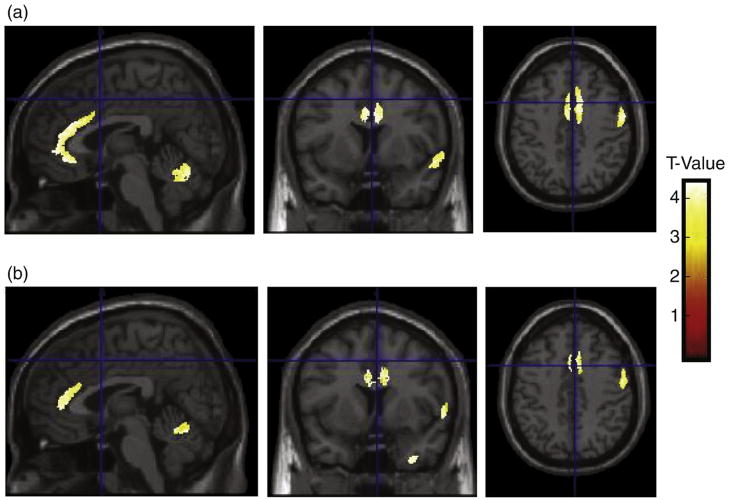
The VBM finding of largest cluster size and greatest significance was located in the right AC gyrus (Talairach coordinates {10, 18, 21}; 9714 voxels; p=0.026). Pairwise comparisons demonstrated significantly lower GM volume in the right AC gyrus in the schizophrenia group compared with their unaffected second degree relatives (Talairach coordinates {10, 17, 20}; 39,548 voxels; p=0.001; Fig. 1(a)). There were similar right AC gyrus differences between the groups of unaffected relatives with a smaller cluster size (Talairach coordinates {10, 21, 20}; 6894 voxels; p=0.004; Fig. 1(b)). Significant GM signals of more limited cluster size were also observed in the right superior temporal gyrus and right posterior cerebellum (Fig. 1). There were no significant GM differences between the SZ and UA1 groups, nor any significant WM differences between the three groups. Interestingly, we found no significant effect of the functional risk allele rs12742393 in the candidate gene NOS1AP on brain structure in the individuals studied, despite the fact the families show significant linkage disequilibrium with this variant to schizophrenia (Wratten et al., 2009). This suggests other variants may be more relevant to the expression of this phenotype.
Fig. 1.
VBM group differences in regional GM in1q21–q23 linked familial schizophrenia. Separate T contrasts were used to test for differences. Group data are plotted on sections of a normalized template brain scan. (a) SZ and UA2 groups show significant GM differences of smaller cluster size in the right AC gyrus (see text), right superior temporal gyrus (Talairach coordinates {44, −27, 5}; p=0.006), and right posterior cerebellum (Talairach coordinates {56, −55, −22}; p=0.018). (b) UA1 and UA2 groups show comparable significant GM differences of smaller cluster size in the right AC gyrus (see text), right superior temporal gyrus (Talairach coordinates {41, −29, 5}; p=0.031), and right posterior cerebellum (Talairach coordinates {55, −73, −29}; p=0.022).
ROI analyses confirmed the VBM volumetric results and showed GM AC volume deficits bilaterally (Table 1). There were non-significantly lower bilateral GM AC volumes in the SZ group compared with the UA1 group. Notably, replication of these ROI cingulate findings using a Siemens 1.5 T MR scanner, similar scan sequence, and identical ROI methods in seven other 1q21–q23 linked families (SZ: n=15; UA1: n=16; UA2: n=5) provided further support (data not shown).
Table 1.
Gray matter anterior cingulate (AC) ROI findings.
| Volume (cm3) | Comparison group
|
p value of analysis*
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SZ (n=11)
|
UA1 (n=17)
|
UA2 (n=7)
|
Three-way comparison** |
Two-way comparison*** |
||||||
| Mean | SD | Mean | SD | Mean | SD | SZ vs. UA1 | SZ vs. UA2 | UA1 vs. UA2 | ||
| Left AC gyrus | 20.02 | 1.76 | 21.49 | 1.84 | 25.31 | 1.53 | <0.0001 | 0.1839 | 0.0013 | 0.0032 |
| Right AC gyrus | 19.91 | 1.75 | 21.48 | 1.77 | 25.05 | 1.52 | <0.0001 | 0.1337 | 0.0014 | 0.0046 |
Results significant at p<0.05 are in bold.
Analysis of covariance model with sex, IQ, age, and TIV as covariates.
Post-hoc Tukey–Kramer test.
Small sample size limited the scope of detectable results. However, the main finding was highly significant, attesting to the large effect size of the GM deficits in the AC observed in this sample with enhanced genetic homogeneity. The results were robust to a sensitivity analysis, sequentially removing one subject at a time. The use of unaffected second degree relatives was a novel means of controlling for other familial factors that may have decreased noise and increased the observable signal from the main schizophrenia disease-related genetic factors. The resulting trade-off with respect to the significantly younger age of this group is a methodological concern and prevents us from drawing wide-ranging conclusions on the basis of this initial investigation. Aging, however, may not significantly affect GM volumes in the AC cortex (Jernigan et al., 1991), the site of our main finding.
The significant differences between UA1 and UA2 groups using both VBM and ROI methods enables attribution of this finding to genetic liability and suggests that GM AC deficits may represent an intermediate phenotype in this form of familial schizophrenia. Many studies indicate dysfunction of the AC cortex in schizophrenia, including mild expression under the diagnostic threshold (Filbey et al., 2008; Fornito et al., 2009). Our results support the hypothesis that the cingulate gyrus is one of the cortical regions most affected by unexpressed genetic liability to schizophrenia (Goghari et al., 2007).
Acknowledgments
The authors thank the families for their longstanding participation in the study, and staff and students at our Clinical Genetics Research Program who assisted in data collection. Special thanks to Gladys Wong for helping with manuscript formatting and submission, and to Sean Bekeschus for helping with figure design.
Role of funding source
Author Costain is supported by a Canadian Institutes of Health Research (CIHR) MD/PhD Studentship and a McLaughlin Centre for Molecular Medicine award. This research was supported by a CIHR grant (MOP-53216), a National Institute of Mental Health grant (R01 MH64220), a National Alliance for Research on Schizophrenia and Depression/Staglin Family Music Festival Schizophrenia Research Award, and a Canada Research Chair in Schizophrenia Genetics (Author Bassett). These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
Authors Bassett and Chow designed the study and wrote the protocol. Authors Crawley and Mikulis assisted with image collection and analysis. Author Brzustowicz performed the molecular analysis. Author Ho undertook the statistical analysis. Author Costain wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
None of the authors have financial interests that might present a conflict of interest.
References
- Bassett AS, Collins EJ, Nuttall SE, Honer WG. Positive and negative symptoms in families with schizophrenia. Schizophr Res. 1993;11:9–19. doi: 10.1016/0920-9964(93)90033-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzustowicz LM, Hodgkinson KA, Chow EWC, Honer WG, Bassett AS. Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21–q22. Science. 2000;288:678–682. doi: 10.1126/science.288.5466.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Russell T, Morris RG, Murray RM, McDonald C. Functional magnetic resonance imaging (fMRI) of attention processes in presumed obligate carriers of schizophrenia: preliminary findings. Ann Gen Psychiatry. 2008;7:18. doi: 10.1186/1744-859X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17:415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Husted J, Lim S, Chow EWC, Greenwood C, Bassett AS. Heritability of neurocognitive traits in familial schizophrenia. Am J Med Genet B. 2009;150B:845–853. doi: 10.1002/ajmg.b.30907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Voormolen EHJ, Wei C, Chow EWC, Bassett AS, Mikulis D, Crawley AP. Voxel-based morphometry and automated lobar region of interest volumetry: the trade-off between spatial scale and statistical correction. Neuroimage. 2010;49:587–596. doi: 10.1016/j.neuroimage.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wratten N, Memoli H, Huang Y, Dulencin A, Matteson P, Cornacchia M, Azaro M, Messenger J, Hayter J, Bassett A, Buyske S, Millonig J, Vieland V, Brzustowicz L. Identification of a schizophrenia-associated functional noncoding variant in NOS1AP. Am J Psychiatry. 2009;166:434–441. doi: 10.1176/appi.ajp.2008.08081266. [DOI] [PMC free article] [PubMed] [Google Scholar]