Abstract
Background
Since the recent introduction of radioimmunotherapy (RIT) using antibodies against cluster of differentiation (CD) 45 for the treatment of lymphoma, the clinical significance of the CD45 antigen has been increasing steadily. Here, we analyzed CD45 expression on lymphocyte subsets using flow cytometry in order to predict the susceptibility of normal lymphocytes to RIT.
Methods
Peripheral blood specimens were collected from 14 healthy individuals aged 25-54 yr. The mean fluorescence intensity (MFI) of the cell surface antigens was measured using a FACSCanto II system (Becton Dickinson Bioscience, USA). MFI values were converted into antibody binding capacity values using a Quantum Simply Cellular microbead kit (Bangs Laboratories, Inc., USA).
Results
Among the lymphocyte subsets, the expression of CD45 was the highest (725,368±42,763) on natural killer T (NKT) cells, 674,030±48,187 on cytotoxic/suppressor T cells, 588,750±48,090 on natural killer (NK) cells, 580,211±29,168 on helper T (Th) cells, and 499,436±21,737 on B cells. The Th cells and NK cells expressed a similar level of CD45 (P=0.502). Forward scatter was the highest in NKT cells (P<0.05), whereas side scatter differed significantly between each of the lymphocyte subsets (P<0.05). CD3 expression was highest in the Th and NKT cells.
Conclusions
NKT cells express the highest levels of CD45 antigen. Therefore, this lymphocyte subset would be most profoundly affected by RIT or pretargeted RIT. The monitoring of this lymphocyte subset during and after RIT should prove helpful.
Keywords: Antibody binding capacity, CD45, Lymphocyte subset
INTRODUCTION
Analysis of lymphocyte subsets via flow cytometry to measure the distribution of helper T (Th) cells, cytotoxic/suppressor T (Ts) cells, B cells, and natural killer (NK) cells is widely employed for the diagnosis and follow-up of several diseases [1]. Recently, quantitative analysis has become possible, as has the measurement of the relative ratios of lymphocyte subsets.
Cluster of differentiation (CD) 45 is a cell surface glycoprotein with a cytoplasmic tyrosine phosphatase domain that catalyzes the removal of phosphates from tyrosine residues in several substrates, and CD45 is believed to function in both the activation and suppression of lymphocytes as well as in T cell maturation [2, 3]. CD45 has previously been shown to play an important role in several diseases. A mutation resulting in the elimination of CD45 expression in white blood cells is associated with the induction of severe combined immunodeficiency [4]. Reduced expression of CD45-protein-tyrosine phosphatase provides protection against anthrax pathogenesis [5]. Recently, radioimmunotherapy (RIT) using anti-CD45 antibodies has become more popular. In an effort to improve the efficacy of this treatment in lymphomas, studies on pretargeted RIT (PRIT) using biotin-labeled anti-CD45 antibodies prior to radiotherapy with avidin-labeled radioisotopes are actively under way [6-9]. Because 85% to 95% of B-cell lymphoma and leukemic cells have relatively high numbers of CD45 (100-300,000 antigenic sites per cell), CD45 can be used to improve RIT targeting of both leukemias and lymphomas [9]. However, normal cells that express more CD45 will be more affected by RIT. Although CD45 is expressed on almost all hematopoietic cells, lymphocytes could be more affected than other cells because lymphocytes express the largest quantities of CD45. The function of each lymphocyte subset is distinct, and it is possible to anticipate which subset would be influenced more profoundly by RIT using an anti-CD45 antibody. However, neither the difference in the expression levels of CD45 antigen on each lymphocyte subset, nor the correlation between CD45 expression and other cellular characteristics, including the levels of additional surface antigens, forward scatter (FSC), and side scatter (SSC) has been adequately studied. In this study, we measured the levels of CD45 expression on lymphocyte subsets using flow cytometry, and analyzed the correlation between the aforementioned factors to predict the susceptibility of normal lymphocytes to RIT. Quantitative analysis of the CD3 antigen on the T cell subset was also analyzed.
MATERIALS AND METHODS
1. Blood samples
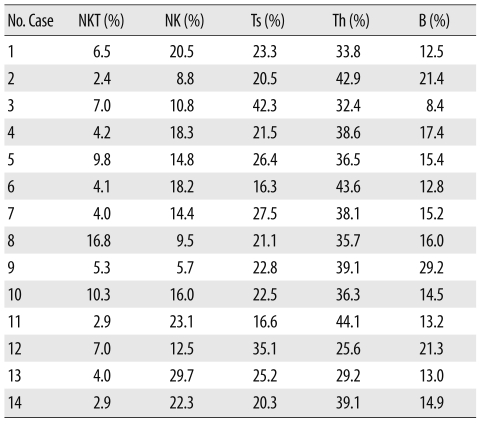
Peripheral blood samples (3 mL) from 14 healthy adults (5 males and 9 females; aged 25 to 54 yr; mean age, 31.4) were collected into dipotassium EDTA blood collection tubes (Becton Dickinson, Franklin Lakes, NJ, USA). The volunteers signed informed consent forms, and the study was approved by the institutional review board. Samples were stored at room temperature and processed within 2 hr after collection. A complete blood cell count and lymphocyte subsets test for all 14 individuals showed normal results (Table 1).
Table 1.
The percentage of each lymphocyte subset in 14 healthy individuals
Abbreviations: NKT, natural killer T cell; NK, natural killer cell; Ts, cytotoxic/suppressor T cell; Th, helper T cell; B, B cell.
2. Comparative quantitative analysis of CD45 expression on the leukocyte subpopulation and each lymphocyte subset
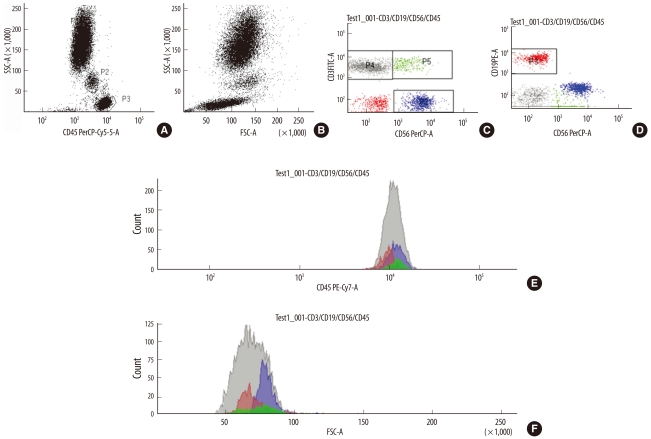
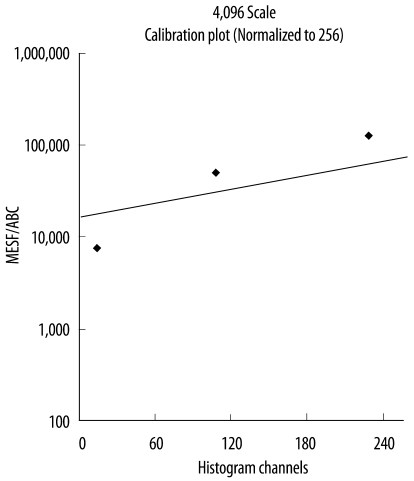
Monoclonal antibodies conjugated with 4 different fluorescent dyes-fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll A protein (PerCP), and phycoerythrin-cyanine 7 (PE-CY7)-were used. In the first tube, 10 µL of monoclonal antibodies against CD4, CD8, CD3, and CD45 that were each labeled with a different fluorescent dye were mixed with 100 µL of blood. In the second tube, 10 µL of monoclonal antibodies against CD3, CD19, CD56, and CD45 that were each labeled with a different fluorescent dye were mixed with 100 µL of blood. The tubes were left in a dark room for 15 min, and then lysed with 2 mL of red blood cell lysing solution (Becton Dickinson) for 15 min. After 2 washes with 3 mL of phosphate-buffered saline (PBS), the samples were resuspended in 1 mL of PBS. Ten thousand events per sample were analyzed and acquired with a flow cytometer (FACSCanto II, Becton Dickinson Bioscience, San Jose, CA, USA). Lymphocytes, granulocytes, and monocytes were gated with CD45 and SSC (Fig. 2A). Lymphocyte subsets were classified as follows: CD3+CD4+ Th cells, CD3+CD8+ Ts cells, CD19+ B cells, CD3-CD56+ NK cells, and CD3+CD56+ natural killer T (NKT) cells. The mean FSC and SSC values were obtained for each cell population. The mean fluorescence intensity (MFI) value for each antigen expressed on each cell population was obtained using BD FACSDiva software (Becton Dickinson Bioscience). The MFI values were converted into antibody-binding capacity (ABC) values using a Quantum Simply Cellular anti-Mouse IgG Kit (Bangs Laboratories, Inc., Fishers, IN, USA). This is a mixture of 5 microbead populations of uniform size, coated with known amounts of goat anti-mouse immunoglobulin G. One drop of each microbead suspension was mixed with 500 µL of PBS. Each of the 7 monoclonal antibodies (anti-CD45-PE-CY7, anti-CD3-FITC, anti-CD3-PerCP, anti-CD19-PE, anti-CD56-PerCP, anti-CD4-PE, and anti-CD8-PerCP) was mixed with 50 µL of microbead mixture in 7 separate tubes and left in the dark for 30 min. The tubes were washed twice with PBS, resuspended in 500 µL of PBS, and then analyzed using the same method and instrument settings as those used for analysis of the blood samples. The calibration curve of the MFI for each antibody to the ABC values was set using the MFI values of the 5 microbead populations and the QuickCal program (www.bangslabs.com/products/quickcal). The ABC of each antigen in each cell population was calculated using these calibration curves (Fig. 1).
Fig. 2.
Peripheral blood leukocytes after staining with anti-CD45 showing discrete granulocyte (P1), monocyte (P2), and lymphocyte (P3) populations according to the level of CD45 expression (A) FSC and SSC on the scattergrams (B) The lymphocyte subsets are separated using antibodies to surface antigens. CD3+CD56- T cells (gray) are plotted in the P4 region. CD3+CD56+ NKT cells (green) and CD3-CD56+ NK cells (blue) are plotted in the P5 and P2 regions, respectively (C) CD19+ B cells (red) are plotted in P3 region (D) Evidence for different CD45 expression (E) and FSC levels (F) CD3+CD56+ NKT cells (green) showed the highest CD45 and FSC expression levels. The CD3-CD56+ NK cells (blue) showed high level of FSC also.
Abbreviations: CD, cluster of differentiation; FSC, forward scatter; SSC, side scatter; NKT, natural killer T cell; NK, natural killer cell.
Fig. 1.
Calibration curve of CD45 phycoerythrin-cyanine 7.
Abbreviations: MESF, molecules of equivalent soluble fluorochromes; ABC, antibody-binding capacity.
3. Comparative quantitative analysis of CD3 antigen expression on T lymphocyte subsets
The method used to label the cells and the flow cytometry settings were the same as described above. The calibration curve for the MFI values of the anti-CD3 antibody were set as described above. The ABC values of CD3 on the 3 T lymphocyte subsets (CD3+CD4+, CD3+CD8+, and CD3+CD56+) were calculated using these calibration curves.
4. Statistical analysis
The Kruskal-Wallis test was used to compare the values of CD45 ABC, FSC, and SSC on the cell populations. Two lymphocyte subsets were compared via a Wilcoxon singed rank sum test. The correlation between CD45 ABC, FSC, and SSC in all cell groups was evaluated using Pearson's correlation test. P values of less than 0.05 were considered statistically significant.
RESULTS
1. CD45 expression, FSC, and SSC values in leukocyte subpopulations
The expression of CD45 was the highest on lymphocytes (279,369±101,409), intermediate on monocytes (52,398±15,192), and the lowest on granulocytes (26,890±7,856). The expression levels differed significantly from one another (P=0.001). The FSC was highest in monocytes (134,035±5,877), intermediate in granulocytes (126,370±2,631), and lowest in lymphocytes (82,680±2,430). These values also differed significantly from one another (P=0.001).
The SSC was highest in granulocytes (167,548±15,996), intermediated in monocytes (67,523±3,411), and lowest in lymphocytes (17,777±1,086). Again, these values differed significantly from one another (P=0.001).
2. CD45 expression, FSC, and SSC values on lymphocyte subsets
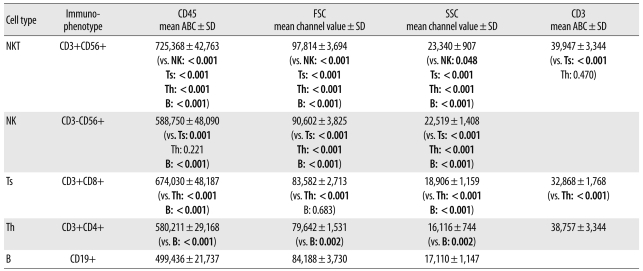
The expression of CD45 and the FSC and SSC values for each lymphocyte subset are shown in Table 2 and Fig. 2. The pattern of CD45 expression on each lymphocyte subset was same in all 14 subjects. CD45 expression on the lymphocyte subsets from the highest to the lowest was 725, 368±42,763 on NKT cells, 674,030±48,187 on Ts cells, 588,750±48,090 on NK cells, 580,211±29,168 on Th cells, and 499,436±21,737 on B cells. The Th cells and NK cells expressed similar levels of CD45 (P=0.502).
Table 2.
Expression level of CD45 and forward and side scatter values for each lymphocyte subset. Two lymphocyte subsets were compared via Wilcoxon singned rank sum test (P values)
Bold type: P value <0.05.
Abbreviations: CD, cluster of differentiation; ABC, antibody-binding capacity; NKT, natural killer T cell; NK, natural killer cell; Ts, cytotoxic/suppressor T cell; Th, helper T cell; FSC, forward scatter; SSC, side scatter; B, B cell.
The FSC was the highest in NKT cells, the next highest in NK cells, and the lowest in Th cells. The FSC in Ts cells and B cells was similar (P=0.589) (Fig. 2).
The SSC was the highest in NKT cells, and the next highest in NK cells, followed by Ts cells, B cells, and Th cells indescending order. The SSC values differed significantly between each of the lymphocyte subsets (P<0.05).
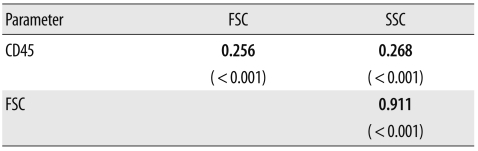
The CD45 expression level increased in proportion to the FSC (r=0.256, P=0.001) and the SSC (r=0.268, P=0.001). The FSC value was strongly associated with SSC (r=0.911, P=0.000) (Table 3).
Table 3.
Correlation among CD45, FSC, and SSC (P value) in lymphocytes evaluated via Pearson's correlation test
Bold type: P value <0.05.
Abbreviations: CD, cluster of differentiation; FSC, forward scatter; SSC, side scatter.
3. Expression of CD3 on CD3+ lymphocyte subsets
CD3 expression was the highest in Th cells (38,757±3,344) and NKT cells (39,947±5,143) compared with Ts cells (32,868±1,768, P=0.001). We noted no significant difference between the Th and NKT cells (P=0.470) (Table 2).
DISCUSSION
Using CD45, SSC, and FSC, the leukocytes were well separated and could be gated to analyze the expression of other antigens. The FSC and SSC values were higher in monocytes and granulocytes than in lymphocytes. The lymphocytes are classified into small lymphocytes and large lymphocytes according to morphology, and when flattened on glass slides, their sizes were 6-9 µm and 9-15 µm in diameter, respectively [10, 11]. The medium or large-sized lymphocytes are known to be a mixed population consisting of NK cells and Ts cells. Although many NK cells show typical morphology of large granular lymphocytes, a significant proportion of NK cells are indistinguishable from other small lymphocytes, and may even be agranular [11]. In this study, the FSC was the highest in the NKT cells. The FSC in NK cells was second highest, and that in Th cells was the lowest. The FSC value in Ts cells and B cells was similar (P=0.589).
The size of the B lymphocytes increased when G0 phase transitioned to G1 phase [12, 13]; however, no difference in cell size was observed between young and senescent T cells [14]. In this study, the circulating lymphocytes showed cha-racteristic sizes according to subset. SSC was also the highest in the NKT cells, and SSC was proportional to FSC in each lymphocyte subset. SSC shows the degree of cell complexity, and the granules in the large granular lymphocytes may have contributed to these high SSC values.
The lymphocytes are characterized by the highest CD45 expression and low SSC. In this study, the level of CD45 expression was significantly higher in the lymphocytes than in the monocytes and granulocytes. Because most B-cell lymphoma and leukemic cells have relatively high numbers of CD45 (100-300,000 antigenic sites per leukemic cell), it has been suggested that CD45 could be used to improve RIT targeting of both leukemias and lymphomas [6, 8, 9, 15]. The CD45 antigen has been shown to be superior to CD20 for targeting in RIT [6, 8]. Cells harboring more CD45 antigen would be effectively eradicated during RIT using an antibody against CD45. Because there is only a weak correlation between CD45, ABC, and FSC (r<0.3), the difference in CD45 expression among the lymphocyte subsets is not attributable to cell size, but rather the density of CD45 on the cell surface. Carulli et al. [16] previously employed the CD45 index [MFI of pathologic B lymphocytes/MFI of T lymphocytes (internal control)] in the differential diagnosis of various mature B cell lymphomas based on the lack of a difference in CD45 expression between normal B and T cells. In this study, CD45 expression varied among normal lymphocyte subsets and even within T cell subsets. Because there is interlaboratory variation in MFI values, the MFI cannot be used to compare the amount of surface antigens [17]. However, the antibody binding capacity of an antigen could be employed for interlaboratory comparison of the quantitative analysis of leukocyte membrane antigen expression via flow cytometry [18]. This study is, to the best of our knowledge, the first report on the ABC of CD45 antigen on different lymphocyte subsets. We determined that NKT cells expressed the highest levels of CD45 antigen; therefore, this lymphocyte subset would be most profoundly affected by RIT or PRIT. Monitoring this lymphocyte subset during and after RIT may prove helpful.
The CD3 antigen is a component of the T cell receptor (TCR) complex and is involved in the transduction of stimulatory signals after antigen recognition [2]. It is expressed at high levels in mature T cells and is downregulated after antigen recognition and activation [19]. Th cells have been reported to express more CD3 antigen than Ts cells despite their smaller cell size [20-22]. In this study, the highest CD3 expression was observed in Th cells and NKT cells. We noted no significant difference between the Th and NKT cells. Unlike antigens that stimulate only specific T cells, anti-CD3 antibody functions as a polyclonal activator of T cells. In addition, antibodies against CD3 proteins frequen-tly independently stimulate T cell functional responses, which are identical to antigen-induced responses [2].
In summary, NKT cells express the highest levels of CD45 antigen. Therefore, this lymphocyte subset would be most affected by RIT or PRIT. The monitoring of this lymphocyte subset during and after RIT is expected to prove helpful.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kee SJ The Korean society for laboratory medicine, editor. Laboratory medicine. 4th ed. Seoul: E public; 2009. Laboratory evaluation of the cellular immune system; pp. 601–610. [Google Scholar]
- 2.Abbas AK, Lichtman AH, et al., editors. Cellular and molecular immunology. 6th updated ed. Philadelphia: Saunders; 2010. pp. 137–152. [Google Scholar]
- 3.Falahati R, Leitenberg D. Selective regulation of TCR signaling pathways by the CD45 protein tyrosine phosphatase during thymocyte development. J Immunol. 2008;181:6082–6091. doi: 10.4049/jimmunol.181.9.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder ME. Investigation of signal transduction defects. In: Detrick B, Hamilton RG, et al., editors. Manual of molecular and clinical laboratory immunology. 7th ed. Washington, D.C: ASM Press; 2006. pp. 901–905. [Google Scholar]
- 5.Panchal RG, Ulrich RL, Bradfute SB, Lane D, Ruthel G, Kenny TA, et al. Reduced expression of CD45 protein-tyrosine phosphatase provides protection against anthrax pathogenesis. J Biol Chem. 2009;284:12874–12885. doi: 10.1074/jbc.M809633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green DJ, Pagel JM, Pantelias A, Hedin N, Lin Y, Wilbur DS, et al. Pretargeted radioimmunotherapy for B-cell lymphomas. Clin Cancer Res. 2007;13:5598s–5603s. doi: 10.1158/1078-0432.CCR-07-1223. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Pagel JM, Axworthy D, Pantelias A, Hedin N, Press OW. A genetically engineered anti-CD45 single-chain antibody-streptavidin fusion protein for pretargeted radioimmunotherapy of hematologic malignancies. Cancer Res. 2006;66:3884–3892. doi: 10.1158/0008-5472.CAN-05-3443. [DOI] [PubMed] [Google Scholar]
- 8.Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340–2348. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 9.Green DJ, Pagel JM, Nemecek ER, Lin Y, Kenoyer A, Pantelias A, et al. Pretargeting CD45 enhances the selective delivery of radiation to hematolymphoid tissues in nonhuman primates. Blood. 2009;114:1226–1235. doi: 10.1182/blood-2009-03-210344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broome HE. Morphology of lymphocytes and plasma cells. In: Lichtman M, editor. Williams hematology. 7th ed. New York: McGraw-Hill; 2006. pp. 1023–1030. [Google Scholar]
- 11.Trinchieri G, Lanier LL. Functions of natural killer cells. In: Lichtman M, editor. Williams hematology. 7th ed. New York: McGraw-Hill; 2006. pp. 1077–1082. [Google Scholar]
- 12.Thompson CB, Scher I, Schaefer ME, Lindsten T, Finkelman FD, Mond JJ. Size-dependent B lymphocyte subpopulations: relationship of cell volume to surface phenotype, cell cycle, proliferative response, and requirements for antibody production to TNP-Ficoll and TNP-BA. J Immunol. 1984;133:2333–2342. [PubMed] [Google Scholar]
- 13.Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci USA. 1999;96:13180–13185. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perillo NL, Naeim F, Walford RL, Effros RB. In vitro cellular aging in T-lymphocyte cultures: analysis of DNA content and cell size. Exp Cell Res. 1993;207:131–135. doi: 10.1006/excr.1993.1171. [DOI] [PubMed] [Google Scholar]
- 15.Press OW, Farr AG, Borroz KI, Anderson SK, Martin PJ. Endocytosis and degradation of monoclonal antibodies targeting human B-cell malignancies. Cancer Res. 1989;49:4906–4912. [PubMed] [Google Scholar]
- 16.Carulli G, Cannizzo E, Zucca A, Buda G, Orciuolo E, Marini A, et al. CD45 expression in low-grade B-cell non-Hodgkin's lymphomas. Leuk Res. 2008;32:263–267. doi: 10.1016/j.leukres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Ratei R, Karawajew L, Lacombe F, Jagoda K, Del Poeta G, Kraan J, et al. Normal lymphocytes from leukemic samples as an internal quality control for fluorescence intensity in immunophenotyping of acute leukemias. Cytometry B Clin Cytom. 2006;70:1–9. doi: 10.1002/cyto.b.20075. [DOI] [PubMed] [Google Scholar]
- 18.Bikoue A, George F, Poncelet P, Mutin M, Janossy G, Sampol J. Quantitative analysis of leukocyte membrane antigen expression: normal adult values. Cytometry. 1996;26:137–147. doi: 10.1002/(SICI)1097-0320(19960615)26:2<137::AID-CYTO7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 19.Beverley P. The importance of T3 in the activation of T lymphocytes. Nature. 1983;304:398–399. doi: 10.1038/304398b0. [DOI] [PubMed] [Google Scholar]
- 20.Lenkei R, Andersson B. Determination of the antibody binding capacity of lymphocyte membrane antigens by flow cytometry in 58 blood donors. J Immunol Methods. 1995;183:267–277. doi: 10.1016/0022-1759(95)00064-h. [DOI] [PubMed] [Google Scholar]
- 21.Ginaldi L, Farahat N, Matutes E, De Martinis M, Morilla R, Catovsky D. Differential expression of T cell antigens in normal peripheral blood lymphocytes: a quantitative analysis by flow cytometry. J Clin Pathol. 1996;49:539–544. doi: 10.1136/jcp.49.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam D, Lindberg AA, Christensson B. Peripheral blood cell preparation influences the level of expression of leukocyte cell surface markers as assessed with quantitative multicolor flow cytometry. Cytometry. 1995;22:128–134. doi: 10.1002/cyto.990220208. [DOI] [PubMed] [Google Scholar]