Abstract
Microangiopathic hemolytic anemia (MAHA) occurs occasionally as a paraneoplastic syndrome in some solid tumors, but MAHA accompanied by signet ring cell carcinoma of an unknown origin is very rare. In this study, we present the case of an 80-yr-old man who was admitted to the hospital because of a 1-month history of lower back pain and dyspnea. He was diagnosed with MAHA on the basis of the laboratory findings that revealed anemia with schistocytes, decreased haptoglobin levels, and a negative direct Coombs' test. Bone marrow examination, which was performed because of the progression of anemia, revealed bone marrow metastases of signet ring cell carcinoma with extensive bone marrow necrosis. However, the primary origin of this signet ring cell carcinoma was not found. When the cause of progressive MAHA is unknown, the possibility of cancer-associated MAHA must be excluded by performing additional tumor workup, including the detection of tumor markers, gastric and colorectal endoscopic examinations, bone marrow examinations, and positron emission tomography-computed tomography or bone scans.
Keywords: Bone marrow metastasis, Microangiopathic hemolytic anemia, Signet ring cell carcinoma
INTRODUCTION
In 1962, Brain et al. [1] coined the term microangiopathic hemolytic anemia (MAHA) for characterizing hemolytic anemia with fragmented red blood cells in the peripheral blood smears. Subsequently, many studies have elucidated the pathogenesis and common causes of MAHA [2-7]. One of the common causes of MAHA is cancer, particularly gastric, breast, and lung cancers [2-7]. Thus far, only a few cases of MAHA associated with signet ring cell carcinoma (SRCC) of unknown origin have been reported [8, 9].
In this report, we describe an 80-yr-old patient who presented with MAHA due to metastatic SRCC of unknown origin.
CASE REPORT
An 80-yr-old man was admitted to the hospital for poor oral intake, back pain, dyspnea, and a 1-month history of jaundice. The patient was diagnosed with MAHA on the basis of the laboratory findings, which showed anemia with schistocytes and polychromasia, nucleated red blood cells on the blood smear (Fig. 1), decreased haptoglobin levels (1.2 mg/dL), a negative direct Coombs' test, and elevated serum levels of total bilirubin (7.38 mg/dL), lactate dehydrogenase (LDH) (1,849 IU/L), and alkaline phosphatase (ALP) (610 IU/L). Among the tumor markers that were assessed, carcinoembryonic antigen level was elevated (785.0 ng/mL). In order to rule out the possibility of solid tumor origin, endoscopic examinations of the upper and lower gastrointestinal tract and abdominal ultrasonography were performed, with unremarkable findings. Thoracic, abdominal, and pelvic computed tomography also showed unremarkable findings. However, multiple bone metastases in both the pelvic bones, humeri, and femora and the sternum were detected on positron emission tomography-computed tomography (PET-CT). Because of the progression of MAHA, thrombocytopenia, and hyperbilirubinemia, bone marrow examination was performed. In the bone marrow aspirates, clusters of abnormal, large non-hematopoietic cells were observed (Fig. 1). The bone marrow biopsy sections showed infiltration of signet ring-shaped atypical large cells with extensive bone marrow necrosis (BMN) (Fig. 2). The signet ring cells were positive for CK20 stain and negative for CK7 stain, and the cytoplasm of these cells was positive for Alcian blue stain (Fig. 3). Finally, we diagnosed the patient with SRCC of unknown origin because the primary site of SRCC (a subtype of adenocarcinoma) could not be confirmed even after a thorough examination, which included endoscopic examination of the lower and upper gastrointestinal tract and ultrasonography of the abdomen.
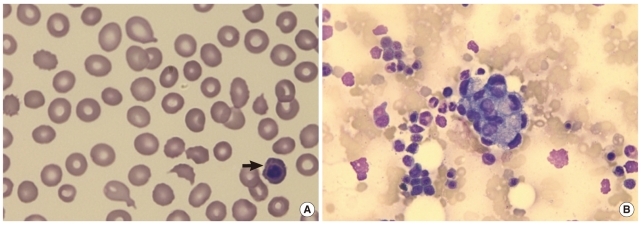
Fig. 1.
(A) Schistocytes, polychromasia, and a nucleated red blood cell (arrow) (peripheral blood smear, hematoxylin and eosin stain, ×1,000); (B) non-hematopoietic, large cell clusters are visible (bone marrow aspirate, Wright & Giemsa stain, ×400).
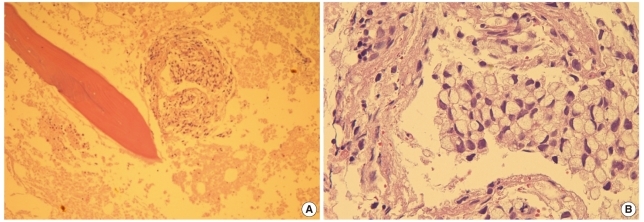
Fig. 2.
(A) Trephine biopsy section reveals clusters of malignant cells with extensive necrosis (center, bone marrow [BM] biopsy section, hematoxylin and eosin stain [H&E], ×100). (B) Tumor cells show eccentrically located nuclei and abundant mucinous cytoplasm, consistent with signet ring cell carcinoma (BM biopsy section, H&E, ×400).
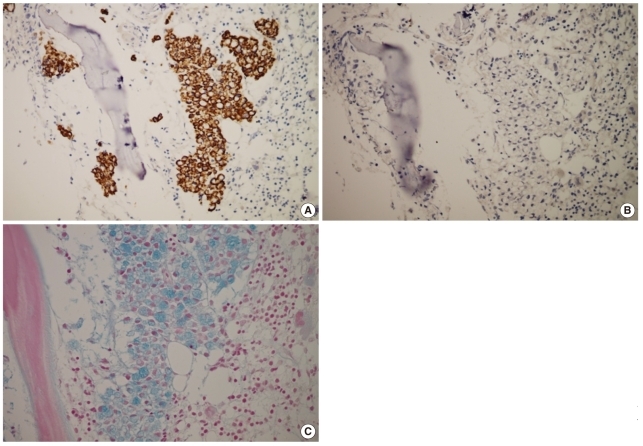
Fig. 3.
Results of immunohistochemical analysis and Alcian blue staining. Tumor cells are positive for CK20 (A) and negative for CK7 stains (B). (C) Alcian blue stain reveals cytoplasmic mucin positivity in the tumor cells.
The patient received red blood cell and platelet transfusion because of the low platelet count (7.8×109/L) and low hemoglobin level (9.1 g/dL). However, the hemoglobin level and platelet count did not increase even after transfusion. Persistently high serum levels of bilirubin, LDH, and ALP were observed. The patient refused to undergo further treatment and was discharged 20 days after admission. He died 3 weeks after discharge from the hospital.
DISCUSSION
MAHA can occur as a paraneoplastic syndrome in cancer patients, and it may present as the first manifestation of a malignant tumor. The most common tumors associated with MAHA are gastric, breast, and lung cancers and malignancies of unknown origin [2-7]. A Korean study reported that 14 (25.5%) out of 55 MAHA patients had gastric cancer [10]. In our patient, MAHA was the initial finding of SRCC of unknown origin.
Cancer-associated MAHA (CA-MAHA) is a rare and fatal complication of malignant tumors. Among patients with bone marrow metastases from cancer, MAHA patients have a worse prognosis than patients without MAHA [11]. Most CA-MAHA patients die within a few weeks after the diagnosis [12], and the most common cause of death is infection [10]. Our patient died 4 weeks after he was diagnosed with CA-MAHA. On average, the median time interval between the initial diagnosis of MAHA and the diagnosis of the underlying malignancy is 6 days (range, 2-14 days), and bone marrow examinations and bone scans are usually performed to detect CA-MAHA [12]. Our patient was diagnosed with CA-MAHA on the basis of the findings of bone marrow examination and PET-CT conducted 7 days after the diagnosis of MAHA. Many studies have recommended a thorough tumor workup, including bone marrow examination, in cases of progressive MAHA of unknown origin [2, 8, 9, 11, 12].
Characteristic laboratory findings of CA-MAHA are anemia with schistocytes, thrombocytopenia, leukoerythroblastic anemia, decreased haptoglobin levels, and elevated serum levels of ALP, LDH, and bilirubin [2, 10, 12]. MAHA is also seen in many different diseases such as idiopathic thrombotic thrombocytopenic purpura (TTP), disseminated intravascular coagulation (DIC), hemolytic uremic syndrome (HUS), and vasculitis. Patients with these diseases have similar laboratory findings and clinical manifestations; therefore, the detection of the underlying diseases is important for appropriate and timely treatment [12]. Bone pain and respiratory symptoms are observed more frequently in CA-MAHA than in non-CA-MAHA [12, 13]. Our patient experienced bone pain and dyspnea.
Although several studies have suggested that fibrinoid necrosis of the bone marrow and tumor cell emboli of the arteries, arterioles, and capillaries are the causes of CA-MAHA, the pathogenesis of CA-MAHA remains unclear [14, 15]. Tumor-derived factors, procoagulants, and some anti-cancer drugs are also considered causative agents of CA-MAHA [16]. Some studies have reported that a decrease in the levels of the von Willebrand factor (vWF)-cleaving protease, ADAMTS13 plays a role in CA-MAHA [12]. We were unable to evaluate the presence of tumor emboli of the arteries, ADAMTS13, or tumor necrosis factor alpha (TNF-α) in this case because the patient was lost to follow-up after discharge.
SRCC is a subtype of mucin-producing adenocarcinomas. SRCC can arise from virtually all organs, but the most common sites of occurrence are the stomach, breast, and colon [17, 18]. Mucin-producing adenocarcinomas and SRCC show clinicopathological differences [19-23]. Gastrointestinal tract SRCC is more common in the young and in males and has a worse prognosis than mucin-producing adenocarcinomas. SRCC is frequently complicated by metastases to the regional lymph nodes, peritoneal surfaces, ovaries, lungs, and bone marrow. The immunohistochemical characteristics of SRCC can be used to differentiate the primary site of SRCC. Usually, the CK7 negative staining and CK20 positive staining patterns of the tumor cells observed in this case are more frequently found in colon SRCC patients than in gastric SRCC patients [24].
A bone marrow biopsy revealed extensive BMN in our patient. BMN is a rare and fatal condition that is defined as the necrosis of myeloid tissue and medullary stroma with preservation of the bone [25]. The prevalence of BMN differs according to the population studied and the grading scales used, but the prevalence of extensive BMN is usually below 0.5% in unselected populations [26, 27]. The prevalence of BMN in CA-MAHA is unknown, but approximately 50% of the BMN cases are accompanied by MAHA [17, 18]. Only a few cases of BMN with CA-MAHA have been reported [3]. The most common underlying diseases in patients with BMN are malignancies, particularly acute leukemia and gastric cancer [26-28].
There is no definitive treatment of choice for CA-MAHA. The low platelet count and hemoglobin level make red blood cell and platelet transfusion obligatory. Chemotherapy is the most reliable treatment of choice for the underlying cancer [3, 9, 11, 28].
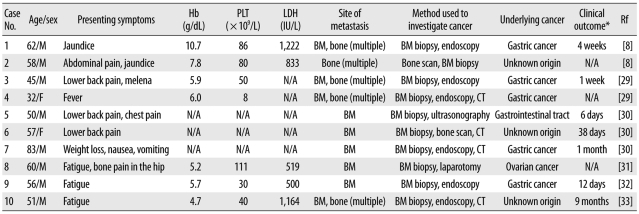
We reviewed 10 previously reported cases of metastatic SRCC that showed MAHA as an initial clinical finding (Table 1) [29-33]. The most common origins of SRCC are the stomach and unknown origins. All the patients showed bone marrow and/or bone metastasis, and therefore, bone marrow examination played an important role in the diagnosis. The time interval between the diagnosis of CA-MAHA and death was less than 6 weeks. The presence of BMN was not assessed in all the patients.
Table 1.
Clinical features of 10 cases of metastatic signet ring cell carcinoma with microangiopathic hemolytic anemia as an initial clinical finding
*Time interval between the diagnosis of underlying cancer and death.
Abbreviations: PLT, platelet; LDH, lactate dehydrogenase; Rf, reference; N/A, not available; BM, bone marrow; CT, computed tomography.
In conclusion, MAHA can be seen in different diseases; it is observed as a paraneoplastic syndrome in cancers, particularly gastric cancers. CA-MAHA is usually accompanied by multiple bone or bone marrow metastases. Therefore, bone marrow examination can be used as a primary diagnostic tool to investigate the underlying cancer. The prognosis of patients with CA-MAHA is generally poor, and adequate chemotherapy is the only reliable treatment. Because of the high prevalence of gastric cancers in the Korean population, additional tumor workup, including the detection of tumor markers, gastric and colorectal endoscopic examination, bone marrow examination, and PET-CT or bone scan are recommended in cases of progressive MAHA of unknown origin.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Brain MC, Dacie JV, Hourihane DO. Microangiopathic haemolytic anaemia: the possible role of vascular lesions in pathogenesis. Br J Haematol. 1962;8:358–374. doi: 10.1111/j.1365-2141.1962.tb06541.x. [DOI] [PubMed] [Google Scholar]
- 2.Elliott MA, Letendre L, Gastineau DA, Winters JL, Pruthi RK, Heit JA. Cancer-associated microangiopathic hemolytic anemia with thrombocytopenia: an important diagnostic consideration. Eur J Haematol. 2010;85:43–50. doi: 10.1111/j.1600-0609.2010.01448.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee JL, Lee JH, Kim MK, Cho HS, Bae YK, Cho KH, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34:476–480. doi: 10.1093/jjco/hyh082. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ, Choi HM, Jung BG, Lee CK, Chung SY. Two cases of microangiopathic hemolytic anemia in patients with gastric cancer. Korean J Hematol. 1990;25:575–579. [Google Scholar]
- 5.Suh HC, Min YH, Kang WC, Lee S, Chong SY, Hahn JS, et al. A case of bone marrow metastatic malignant melanoma presenting microangiopathic hemolytic anemia as initial clinical findings. Korean J Hematol. 1996;31:457–464. [Google Scholar]
- 6.Youk DW, Choi MK, Kim KH, Kim HK, Park CW, Kim BK, et al. Microangiopathic homolytic anemia in patients with gastrointestinal malignancy. Korean J Hematol. 1983;18:207–214. [Google Scholar]
- 7.Lee MA, Shim SI. A review of ten cases of microangiopathic hemolytic anemia. Korean J Clin Pathol. 1994;14:43–48. [Google Scholar]
- 8.Arkenau HT, Müssig O, Buhr T, Jend HH, Porschen R. Microangiopathic hemolytic anemia (MAHA) as paraneoplastic syndrome in metastasized signet ring cell carcinomas: case reports and review of the literature. Z Gastroenterol. 2005;43:719–722. doi: 10.1055/s-2005-858259. [DOI] [PubMed] [Google Scholar]
- 9.Abdel Samie A, Sandritter B, Theilmann L. Severe microangiopathic hemolytic anemia as first manifestation of a CUP syndrome. Rapid hematologic remission under polychemotherapy. Med Klin (Munich) 2004;99:148–153. doi: 10.1007/s00063-004-1023-8. [DOI] [PubMed] [Google Scholar]
- 10.Hahn JS, Lee DH, Lee SJ, Min YH, Ko YW. A clinical study on microangiopathic hemolytic anemia. Korean J Hematol. 1991;26:263–279. [Google Scholar]
- 11.Etoh T, Baba H, Taketomi A, Nakashima H, Kohnoe S, Seo Y, et al. Diffuse bone metastasis with hematologic disorders from gastric cancer: clinicopathological features and prognosis. Oncol Rep. 1999;6:601–605. doi: 10.3892/or.6.3.601. [DOI] [PubMed] [Google Scholar]
- 12.Francis KK, Kalyanam N, Terrell DR, Vesely SK, George JN. Disseminated malignancy misdiagnosed as thrombotic thrombocytopenic purpura: A report of 10 patients and a systematic review of published cases. Oncologist. 2007;12:11–19. doi: 10.1634/theoncologist.12-1-11. [DOI] [PubMed] [Google Scholar]
- 13.Oberic L, Buffet M, Schwarzinger M, Veyradier A, Clabault K, Malot S, et al. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009;14:769–779. doi: 10.1634/theoncologist.2009-0067. [DOI] [PubMed] [Google Scholar]
- 14.Hilgard P, Gordon-Smith EC. Microangiopathic haemolytic anaemia and experimental tumour-cell emboli. Br J Haematol. 1974;26:651–659. doi: 10.1111/j.1365-2141.1974.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 15.Murgo AJ. Thrombotic microangiopathy in the cancer patient including those induced by chemotherapeutic agents. Semin Hematol. 1987;24:161–177. [PubMed] [Google Scholar]
- 16.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer. 1982;50:775–781. doi: 10.1002/1097-0142(19820815)50:4<775::aid-cncr2820500425>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Connelly JH, Robey-Cafferty SS, el-Naggar AK, Cleary KR. Exophytic signet-ring cell carcinoma of the colorectum. Arch Pathol Lab Med. 1991;115:134–136. [PubMed] [Google Scholar]
- 19.Kim KS, Kim YD, Han KH, Lee SH, Kim JH, Choi HY, et al. Endoscopic findings and clinicopathological characteristics of signet ring cell carcinoma of the stomach. Korean J Med. 2007;73:596–602. [Google Scholar]
- 20.Saito S, Iwaki H. Mucin-producing carcinoma of the prostate: review of 88 cases. Urology. 1999;54:141–144. doi: 10.1016/s0090-4295(98)00595-0. [DOI] [PubMed] [Google Scholar]
- 21.Sung CO, Seo JW, Kim KM, Do IG, Kim SW, Park CK. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol. 2008;21:1533–1541. doi: 10.1038/modpathol.2008.170. [DOI] [PubMed] [Google Scholar]
- 22.Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121–130. doi: 10.1620/tjem.186.121. [DOI] [PubMed] [Google Scholar]
- 23.Akamatsu S, Takahashi A, Ito M, Ogura K. Primary signet-ring cell carcinoma of the urinary bladder. Urology. 2010;75:615–618. doi: 10.1016/j.urology.2009.06.065. [DOI] [PubMed] [Google Scholar]
- 24.Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer. 2002;38:758–763. doi: 10.1016/s0959-8049(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 25.Nies BA, Kundel DW, Thomas LB, Freireich EJ. Leukopenia, bone pain, and bone necrosis in patients with acute leukemia: a clinicopathologic complex. Ann Intern Med. 1965;62:698–705. doi: 10.7326/0003-4819-62-4-698. [DOI] [PubMed] [Google Scholar]
- 26.Janssens AM, Offner FC, Van Hove WZ. Bone marrow necrosis. Cancer. 2000;88:1769–1780. [PubMed] [Google Scholar]
- 27.Hahn JS, Lee HL, Lee SJ, Min YH, Ko YW, Yang WI. A clinical study on bone marrow necrosis. Korean J Hematol. 1991;26:135–149. [Google Scholar]
- 28.Jeong GY, Yoon HR, Kim SH. A case of bone marrow necrosis preceeding acute monoblastic leukemia. Korean J Clin Pathol. 1999;19:172–176. [Google Scholar]
- 29.Kaidar-Person O, Nasrallah H, Haim N, Dann EJ, Bar-Sela G. Disseminated carcinoma diagnosed by bone marrow biopsy in patients with microangiopathic hemolytic anemia and thrombocytopenia: a report of two cases with gastric cancer and a review of the literature. J Gastrointest Cancer. 2010 doi: 10.1007/s12029-010-9204-6. in press. [DOI] [PubMed] [Google Scholar]
- 30.Ozkalemkas F, Ali R, Ozkocaman V, Ozcelik T, Ozan U, Ozturk H, et al. The bone marrow aspirate and biopsy in the diagnosis of unsuspected nonhematologic malignancy: a clinical study of 19 cases. BMC Cancer. 2005;5:144. doi: 10.1186/1471-2407-5-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadikoylu G, Barutca S, Tataroglu C, Kafkas S, Erpek H, Meydan N, et al. Thrombotic thrombocytopenic purpura as the first manifestation of metastatic adenocarcinoma in a young woman. Transfus Apher Sci. 2010;42:39–42. doi: 10.1016/j.transci.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya Y, Ishibashi H, Ohtsuka T, Nagasawa K, Niho Y, Ide K. Mucin-producing signet ring cell carcinoma of stomach accompanied by microangiopathic hemolytic anemia and disseminated intravascular coagulation: a case report. Fukuoka Igaku Zasshi. 1989;80:477–481. [PubMed] [Google Scholar]
- 33.Abdel Samie A, Sandritter B, Theilmann L. Severe microangiopathic hemolytic anemia as first manifestation of a CUP syndrome. Rapid hematologic remission under polychemotherapy. Med Klin (Munich) 2004;99:148–153. doi: 10.1007/s00063-004-1023-8. [DOI] [PubMed] [Google Scholar]