Abstract
Background
Clostridium difficile infection (CDI) has markedly risen and is associated with hypervirulent ribotype 027 outbreaks in North America and Europe since 2003. The aims of this study were to determine the prevalence of ribotype 027 among C. difficile isolates in Korea, to characterize the ribotype 027 isolates, and to determine the clinical severity of CDI in patients infected with these isolates.
Methods
A total of 1,251 isolates of C. difficile recovered from stool specimens of suspected CDI patients at two tertiary-care hospitals and one commercial laboratory between 2002 and 2009. Genes for toxin A (tcdA), toxin B (tcdB), and binary toxin (cdtA and cdtB) were detected by PCR. Mutation in the tcdC gene was detected by sequencing after PCR amplification. For molecular genotyping, we performed PCR-ribotyping, pulsed-field gel electrophoresis (PFGE), and multilocus variable-number tandem-repeat analysis (MLVA). Minimum inhibitory concentrations of moxifloxacin were determined using Etest strips (AB bioMérieux, Sweden).
Results
We identified 7 isolates as ribotype 027. These isolates had the same tcdC mutation as the epidemic strain, and 6 of them were resistant to moxifloxacin. The isolates were categorized into 3 different PFGE types and 7 different MLVA types. All the 7 cases had occurred sporadically.
Conclusions
C. difficile ribotype 027 is uncommon, but it has emerged in Korea. The spread of this ribotype should be closely monitored in order to avoid an outbreak of CDI in Korea.
Keywords: Clostridium difficile, PCR-ribotype 027, Pulsed-field gel electrophoresis, Multilocus variable-number tandem-repeat analysis
INTRODUCTION
Severe Clostridium difficile infections (CDIs) associated with an emerging epidemic strain, PCR-ribotype 027, have increased in frequency and severity in the U.S., Canada, and the European countries since 2003 [1]. However, reports of ribotype 027 infections in East Asian countries remain rare [2]. One case each was reported from Japan [3] and Korea [4] in 2007 and 2009, respectively. A one-year surveillance study between 2007 and 2008 in Shanghai, China failed to detect any cases, although one case was reported in Hong Kong in 2009 [2]. These results may indicate the onset of spreading ribotype 027 infection in Asian countries.
Ribotype 027 strain had toxin A, toxin B and binary toxin with deletion of toxin regulator gene (tcdC) [1]. According to a previous study, the use of moxifloxacin is associated with ribotype 027 outbreaks, and the isolates obtained in that study were resistant to levofloxacin and moxifloxacin [5]. A Korean isolate was resistant, whereas 1 Japanese isolate was susceptible to moxifloxacin [3, 4]; this indicated a need for further testing of Korean isolates.
The aims of this study were to determine the prevalence of ribotype 027 among the stored C. difficile isolates, to characterize the ribotype 027 isolates, and to evaluate the clinical severity of CDI in patients infected with these strains.
MATERIALS AND METHODS
1. Bacterial isolates and antimicrobial susceptibility testing
A total of 1,251 C. difficile isolates were recovered from stool specimens of suspected CDI patients at two tertiary-care hospitals and one commercial laboratory between 2002 and 2009. The commercial laboratory processed the stool specimens collected from hospitals and clinics without in-house anaerobic microbiological testing facilities. C. difficile selective agar (CDSA; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used to isolate C. difficile under anaerobic incubation. The isolates were identified using conventional tests and the ATB 32A system (bioMerieux, Marcy l'Etoile, France). Minimum inhibitory concentrations (MICs) of moxifloxacin for ribotype 027 isolates were determined using Etest strips (AB bioMérieux, Solna, Sweden).
2. Clinical characteristics of patients
We reviewed the patients' medical records, and according to the criteria proposed by Zar et al. [6], patients with ≥2 points were considered to have severe CDI. One point was given for each of the following criteria: age >60 yr, body temperature >38.3℃, serum albumin <2.5 mg/dL, and peripheral white blood cell count >15,000/µL. Two points were given if there was endoscopic evidence of pseudomembranous colitis (PMC) or if the patient was admitted to the intensive care unit.
3. PCR assay for toxin genes
C. difficile toxin genes were detected by performing PCR as described in previous studies [7, 8]. Primer sequences used in this study are listed in Table 1. C. difficile VPI 10463 (A+ B+, CDT-), 3608/03 (A-B-, CDT-), SE844 (A+B+, CDT+), and 1470 (A-B+, CDT-) were used as controls for the PCR assays. The obtained amplicons were commercially sequenced (Macrogen, Seoul, Korea). The deduced amino acid sequences were compared to those of the strain VPI 10463.
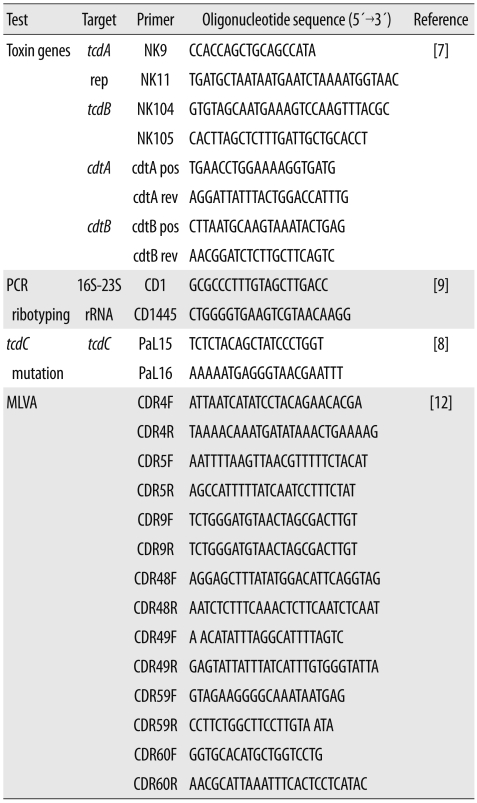
Table 1.
Primers used in this study
Abbreviations: MLVA, multilocus variable-number tandem-repeat analysis; CDR, Clostridium difficile repeat.
4. PCR-ribotyping
PCR-ribotyping was performed according to a previously described method [9] with minor modification. We added 10 µL of crude nucleic acid as template to a 50-µL PCR mix-ture containing 10 mM Tris-HCl (pH 8.3) and 50 mM KCl (GeneAmp 1×PCR Buffer II; Applied Biosystems, Foster City, CA, USA), 4.0 mM MgCl2, 0.4 mM dNTP, 1.5 U Taq DNA polymerase (AmpliTaq Gold, Applied Biosystems), and 0.4 µM primers (CD1 and CD1445).
5. Pulsed-field gel electrophoresis and multilocus variable-number tandem-repeat analysis typing
Pulsed-field gel electrophoresis (PFGE) analysis was performed by modified method of Alonso et al. [10]. In brief, we prepared plugs of chromosomal DNA from fresh colonies cultured for 24 hr and treated them with high concentration of proteinase K (75 U/mL). Thiourea was added to the gel and running buffer used for PFGE [11].
Multilocus variable-number tandem-repeat analysis (ML-VA) was performed by PCR amplification and sequence analysis of 7 selected C. difficile repeat (CDR) loci; CDR4, CDR5, CDR9, CDR48, CDR49, CDR59, and CDR60. The primer sets used are listed in Table 1 [12]. The copy numbers of each of the 7 CDR loci were concatenated to generate an MLVA type for each isolate.
RESULTS
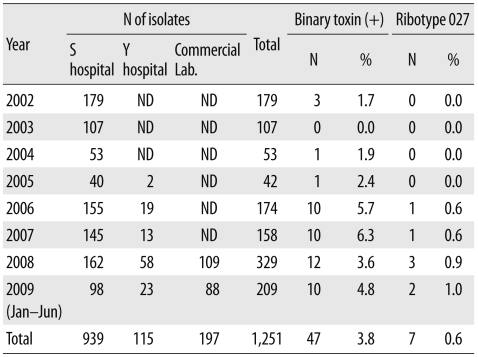
Of the 1,251 C. difficile isolates, 47 (3.8%) were PCR positive for the toxin A, toxin B, and binary toxin genes, and 7 (0.6%) were identified as ribotype 027 by PCR-ribotyping. The first isolate to be identified as ribotype 027 was from a sample obtained in 2006, and the prevalence of ribotype 027 was 0.6% in 2006 and 2007, 0.9% in 2008, and 1.0% in 2009 (Table 2).
Table 2.
Prevalence of binary toxin-producing Clostridium difficile and PCR-ribotype 027
Abbreviation: ND, not done.
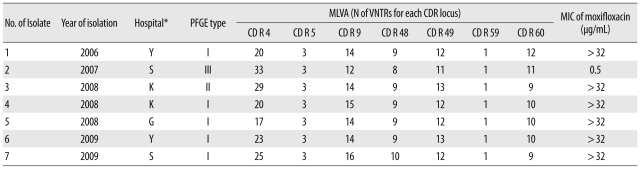
All the ribotype 027 isolates had an 18-bp deletion from position 330 to 347 and a 1-bp deletion at position 117 in the tcdC gene sequence. We observed identical PFGE patterns for 5 isolates (isolates 1, 4, 5, 6, and 7). However, the patterns for isolates 2 and 3 differed from those of the other isolates in 3 and 2 bands, respectively (Table 3, Fig. 1).
Table 3.
Molecular and phenotypic characteristics of the Clostridium difficile PCR-ribotype 027
*Two hospitals, hospitals K and G, sent the samples to a commercial laboratory, and the samples were redirected to the S hospital in Seoul where the test was performed.
Abbreviations: PFGE, pulsed-field gel electrophoresis; MLVA, multilocus variable-number tandem-repeat analysis; VNTR, variable number tandem repeat; CDR, C. difficile repeat; MIC, minimum inhibitory concentration.
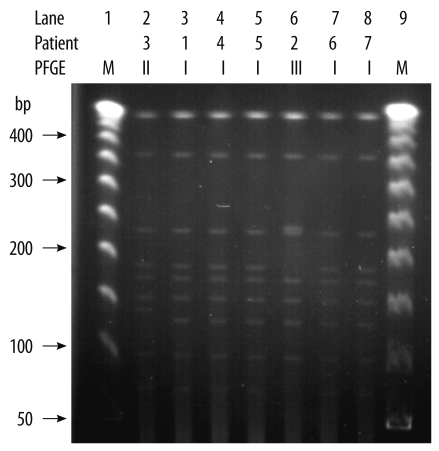
Fig. 1.
Pulsed-field gel electrophoresis (PFGE) patterns of SmaI-digested genomic DNA obtained from the ribotype 027 isolates. Lanes 1 and 9 were loaded with a 50-kb ladder (molecular marker). The PFGE patterns for isolates 2 and 3 differed in 5 bands. The remaining 5 isolates had identical patterns, and their pattern differed from that of isolates 2 and 3 in 3 and 2 bands, respectively.
All the 7 isolates had different MLVA types. However, all the isolates had the same number of variable number tandem repeats (VNTRs) at the CDR5 and CDR59 loci (Table 3).
The MIC of moxifloxacin was >32 µg/mL for 6 of the 7 isolates and 0.5 µg/mL for the remaining 1 isolate (isolate 2).
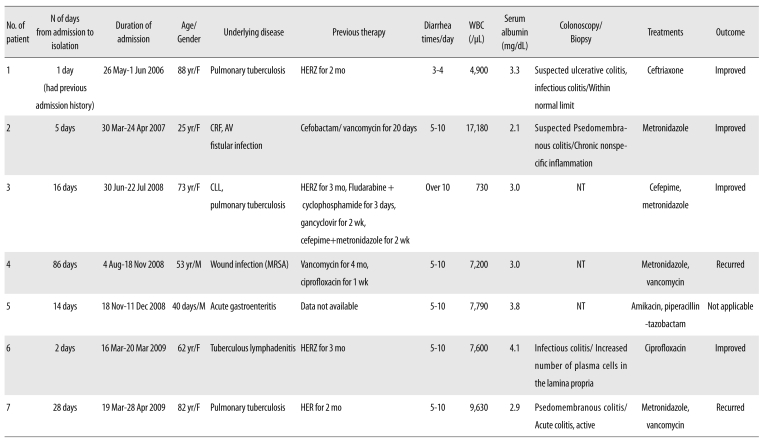
Brief histories of patients infected with ribotype 027 are summarized in Table 4. The ages of the 7 patients (2 men and 5 women) were in the range of 25 to 88 yr, except a clinically-irrelevant 40-day-old infant with acute gastroenteritis.
Table 4.
Clinical features of patients infected with the Clostridium difficile PCR-ribotype 027
Abbreviations: WBC, white blood cell; HERZ, isoniazid, ethambutol, rifampin, pyrazinamide; mo, month; CRF, chronic renal failure; AV, arteriovenous; NT, not tested; MRSA, methicillin-resistant Staphylococcus aureus; HER, isoniazid, ethambutol, rifampin.
All patients had been hospitalized for some underlying diseases. Four patients had received anti-tuberculous medication 2-4 weeks before hospitalization. None of the patients had travelled overseas. Symptoms and their severity varied among the patients, ranging from mild colitis to severe PMC. Except for patient 2, none of the patients showed high fever (>38.0℃), increased leukocyte count (>15,000/µL), increased serum creatinine (>2.0 mg/dL), or decreased serum albumin (<2.5 mg/dL). Patient 2 showed high leukocyte count and serum creatinine level due to underlying chronic renal failure and an arteriovenous fistula infection.
Two (patient nos. 2 and 7) cases were diagnosed with suspected PMC and PMC by colonoscopic biopsy and considered probably severe (patient no. 2) or severe (patient no. 7) according to the criteria proposed by Zar FA et al. [6].
Metronidazole was administered to 4 patients (patient nos. 2, 3, 4, and 7) to treat CDI. Because of poor response, for patients 4 and 7, the regimen was changed to vancomycin, but the infection recurred. Two patients (patient nos. 1 and 6) recovered without CDI treatment.
DISCUSSION
Although the first case of ribotype 027 infection in Korea was reported in 2009 [4], we found that ribotype 027 was present in Korea as early as 2006. Since 2006, the prevalence of ribotype 027 isolates has been 0.8% (7/870). Except for patient no. 6, all the patients had most likely acquired the infection in a hospital. We recovered these isolates from 7 patients who had visited 4 different hospitals located in Seoul and Gyeonggi province; this indicated an early stage of dissemination to Korean hospitals. Two of these hospitals sent the samples to a commercial laboratory for culturing C. difficile, and the samples were redirected to the S hospital in Seoul where the tests were performed. The presence of ribotype 027 in an infant was clinically irrelevant; however, this finding suggested that the isolate was easily transmissible.
Severe diseases are more likely to be caused by ribotype 027 strains than by non-027 strains, because the ribotype 027 strains produces excessive amounts of toxins A and B as a result of tcdC deletion [5]. There is no consensus about the definition of severe CDI. However, as per the criteria proposed by Zar et al. [6], 2 of the 7 cases in this study, like the first case reported in Korea [4], were either probably severe (case no. 2) or severe (case no. 7). In North America, patients infected with the ribotype 027 isolates had severe symptoms more frequently; however, a study in England showed no such evidence [13].
Mortality due to ribotype 027 infection increases with the patient's age. In a Canadian study, 44.1% of the patients were older than 65 yr, and 8.1% of them died within 30 days after diagnosis [14]. In our study, 3 out of 7 patients (42.8%) were older than 65 yr, and none of them died from CDI.
Anti-tuberculous agents are rarely associated with CDI. Among these agents, only rifampin is considered a cause of CDIs because rifampin has an antibiotic effect on a wide range of bacteria, whereas isoniazid and ethambutol have little or no activity against anaerobes [15]. In our study, 4 patients had received rifampin.
In our study, 6 of the 7 isolates were resistant to moxifloxacin; this was also true for the recent epidemic-causing PCR-ribotype 027 strains isolated in other countries. In contrast, the PCR-ribotype 027 strains isolated prior to 2001 were su-sceptible to moxifloxacin [5].
PFGE patterns of 5 isolates (1, 4, 5, 6, and 7) were identical. Compared to the pattern of these 5 isolates, the pattern for isolates 2 and 3 differed in 3 and 2 bands, respectively (Fig. 1). All the isolates were categorized as different MLVA types. The VNTR loci used to discriminate these isolates were CDR4, CDR9, CD48, CDR49, and CDR60. MLVA typing indicated that these isolates were not clones. All the 7 cases had occurred sporadically.
For differentiating the isolates, MLVA was more useful than PFGE.
C. difficile ribotype 027 is uncommon, but it has emerged in Korea. The spread of this ribotype should be closely monitored in order to avoid an outbreak of CDI in Korea.
Acknowledgement
This study was supported by a faculty research grant from Yonsei University College of Medicine (grant 6-2008-0269). We thank Thomas V. Reily (University of Western Australia, Perth, WA, Australia) for providing reference strains and Myungsook Kim (Yonsei University Health System, Seoul, Korea), Kwangwoo Kim (Yonsei University Health System, Seoul, Korea), and Gwanghee Byun (Kyunghee University, Yongin, Korea) for laboratory assistance.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 2.Cheng VC, Yam WC, Chan JF, To KK, Ho PL, Yuen KY. Clostridium difficile ribotype 027 arrives in Hong Kong. Int J Antimicrob Agents. 2009;34:492–493. doi: 10.1016/j.ijantimicag.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Ito Y, van den Berg RJ, Kuijper EJ, Arakawa Y. First isolation of Clostridium difficile 027 in Japan. Euro Surveill. 2007;12:E070111.3. doi: 10.2807/esw.12.02.03110-en. [DOI] [PubMed] [Google Scholar]
- 4.Tae CH, Jung SA, Song HJ, Kim SE, Choi HJ, Lee M, et al. The first case of antibiotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J Korean Med Sci. 2009;24:520–524. doi: 10.3346/jkms.2009.24.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 6.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 7.Terhes G, Urbán E, Sóki J, Hamid KA, Nagy E. Community-acqu-ired Clostridium difficile diarrhea caused by binary toxin, toxin A, and toxin B gene-positive isolates in Hungary. J Clin Microbiol. 2004;42:4316–4318. doi: 10.1128/JCM.42.9.4316-4318.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spigaglia P, Mastrantonio P. Comparative analysis of Clostridium difficile clinical isolates belonging to different genetic lineages and time periods. J Med Microbiol. 2004;53:1129–1136. doi: 10.1099/jmm.0.45682-0. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill GL, Ogunsola FT, Brazier JS, Duerden BI. Modification of a PCR ribotyping method for application as a routine typing scheme for Clostridium difficile. Anaerobe. 1996;2:205–209. [Google Scholar]
- 10.Alonso R, Martín A, Peláez T, Marín M, Rodríguez-Creixéms M, Bouza E. An improved protocol for pulsed-field gel electrophoresis typing of Clostridium difficile. J Med Microbiol. 2005;54:155–157. doi: 10.1099/jmm.0.45808-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Kim H, Seo Y, Yong D, Jeong SH, Chong Y, et al. Molecular characterization of toxin A-negative, toxin B-positive variant stra-ins of Clostridium difficile isolated in Korea. Diagn Microbiol Infect Dis. 2010;67:198–201. doi: 10.1016/j.diagmicrobio.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Marsh JW, O'Leary MM, Shutt KA, Pasculle AW, Johnson S, Gerding DN, et al. Multilocus variable-number tandem-repeat analysis for investigation of Clostridium difficile transmission in Hospitals. J Clin Microbiol. 2006;44:2558–2566. doi: 10.1128/JCM.02364-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan OW, Rodrigues B, Elston T, Verlander NQ, Brown DF, Brazier J, et al. Clinical severity of Clostridium difficile PCR ribotype 027: a case-case study. PLoS One. 2008;3:e1812. doi: 10.1371/journal.pone.0001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung SW, Jeon SW, Do BH, Kim SG, Ha SS, Cho CM, et al. Clinical aspects of rifampicin-associated pseudomembranous colitis. J Clin Gastroenterol. 2007;41:38–40. doi: 10.1097/MCG.0b013e31802dfaf7. [DOI] [PubMed] [Google Scholar]