Abstract
Trastuzumab shows remarkable efficacy in treatment of ErbB2-positive breast cancers when used alone or in combination with other chemotherapeutics. However, acquired resistance develops in most treated patients, necessitating alternate treatment strategies. Increased aerobic glycolysis is a hallmark of cancer and inhibition of glycolysis may offer a promising strategy to preferentially kill cancer cells. In this study, we investigated the antitumor effects of trastuzumab in combination with glycolysis inhibitors in ErbB2-positive breast cancer. We found that trastuzumab inhibits glycolysis via downregulation of heat shock factor 1 (HSF1) and lactate dehydrogenase A (LDH-A) in ErbB2-positive cancer cells, resulting in tumor growth inhibition. Moreover, increased glycolysis via HSF1 and LDH-A contributes to trastuzumab resistance. Importantly, we found that combining trastuzumab with glycolysis inhibition synergistically inhibited trastuzumab-sensitive and -resistant breast cancers in vitro and in vivo, due to more efficient inhibition of glycolysis. Taken together, our findings show how glycolysis inhibition can dramatically enhance the therapeutic efficacy of trastuzumab in ErbB2-positive breast cancers, potentially useful as a strategy to overcome trastuzumab resistance.
Keywords: Warburg effect, glycolysis, HSF1, LDH-A, trastuzumab, ErbB2, resistance
Introduction
Trastuzumab (Herceptin, Genentech Inc.), a humanized ErbB2-targeting monoclonal antibody, was the first successful rational, targeted therapeutic agent approved for clinical use in breast cancer patients (1, 2). Trastuzumab has shown remarkable therapeutic efficacy when used either alone or in combination with other chemotherapeutics to treat ErbB2-positive breast cancer and other types of cancers (1, 3, 4). Several possible mechanisms by which trastuzumab decreases ErbB2 signaling include blockade of ErbB2 receptor dimerization, inhibition of shedding of the extracellular domain, increase of endocytosis, and activation of antibody-dependent cell-mediated cytotoxicity (ADCC) (1). Previous studies showed that trastuzumab inhibits PI3K/Akt signaling by restoring PTEN function (5) and PI3K inhibitors enhanced the antitumor effect of trastuzumab in ErbB2-positive cancer cells (6, 7). Interestingly, in addition to be a major signaling pathway that regulates cell survival, the PI3K/Akt signaling pathway also is a master regulator of cancer cell bioenergetic metabolism (8). However, it is unknown whether targeting cancer cell energy metabolism may serve as a mechanism in trastuzumab-induced tumor inhibition.
Despite its initial efficacy, acquired resistance to trastuzumab develops in a majority of patients who have metastatic breast cancer (5, 9, 10). Possible mechanisms of trastuzumab resistance include: altered interaction between trastuzumab and its target receptor ErbB2, PTEN lost and increased PI3K signaling, p27kip1 downregulation and signaling through other receptors (11). Trastuzumab has emerged as a very important drug for ErbB2-positive breast cancer therapy, and multiple clinical trials are currently being conducted to expand its usage to more breast cancer patients and other types of cancers (1, 12). Thus, understanding the mechanisms of trastuzumab resistance and overcoming its resistance is an urgent task for cancer researchers and clinicians. It has been shown that bioenergetic alteration plays a role in chemoresistance (13), and targeting glycolysis sensitizes cancer cells to chemotherapy (14). However, it is unknown whether the switch of energetic dependency contributes to trastuzumab resistance and whether targeting dysregulated cancer cell metabolism may sensitize cancer cells to trastuzumab.
Warburg, in 1956, observed that most cancer cells take up glucose at higher rates than normal cells but use a smaller fraction of this glucose for oxidative phosphorylation, indicating that cancer cells prefer anaerobic breakdown of glucose for energy rather than mitochondrial oxidative phosphorylation. This phenomenon is called “the Warburg Effect” (15-23). Although the molecular mechanisms underlying the Warburg effect remain unclear, increased glycolysis in cancer cells has been well accepted to be an important process to support malignant phenotypes (18). By taking advantage of the unique metabolic feature of cancer cells, several glycolysis inhibitors have shown to preferentially inhibit cancer cells and spare normal cells (24-26). A synthetic glucose analog, 2-deoxyglucose (2-DG), is one of the glycolysis inhibitors that has been shown to have effects in selectively inhibiting solid tumors and is currently under Phase I/II clinical trials (27-29). Another promising glycolysis inhibitor, oxamate, a specific inhibitor of the lactate dehydrogenase, has also shown promising results and is currently under pre-clinical investigation (14, 30). However, many glycolytic inhibitors have been evaluated after the discovery of the Warburg effect, yet none of them are currently used in the clinic. In addition, studies with xenografts indicated that as a single agent, 2-DG did not significantly enhance tumor cell killing (27). One plausible explanation is that when glycolysis is blocked by glycolytic inhibitors, cancer cells may survive the inhibition through other adaptation mechanisms. Recently, two studies showed that 2-DG may activate prosurvival pathways in cancer cells and treating cancer cells with the combination of 2-DG and an IGF1R inhibitor dramatically reduced cancer cell proliferation and promoted apoptosis (31, 32), indicating that combination 2-DG with other signaling inhibitors in a 2-DG-based therapy may result in enhanced efficacy. Therefore, identifying appropriate anti-cancer reagents to combine with glycolysis inhibitors may hold the key for more efficient cancer cell killing power for glycolysis inhibitors.
Our previous studies showed that the upregulation of lactate dehydrogenase A (LDH-A) by ErbB2 through heat shock factor 1 (HSF1) promotes breast cancer cell glycolysis and growth (33). In this study, we report that trastuzumab inhibits glycolysis via downregulation of HSF1 and LDH-A in ErbB2-positive breast cancer cells, which may provide a new mechanism of trastuzumab-induced tumor inhibition. We also report that increased glycolysis contributes to trastuzumab resistance and that glycolysis inhibitors sensitize ErbB2-positive breast cancer cells to trastuzumab via downregulation of HSF1 and LDH-A. More importantly, glycolysis inhibition resensitizes trastuzumab-resistant cells to trastuzumab both in vitro and in vivo. These novel findings have important implications for the future development of therapeutics for ErbB2-positive and trastuzumab-resistant cancers.
Materials and methods
Cell lines and cell cultures
The breast cancer cells BT474, SKBR3, ZR-7530, and MDA-MB-361 were purchased from ATCC and used within 6 months. BT474, SKBR3 and MDA-MB-361 cells were cultured in DMEM/F-12 (Mediatech Inc.) with 10% FBS. ZR-7530 cells were cultured in RPMI1640 (Mediatech Inc.) with 10% FBS. Trastuzumab-resistant BT474/TR and SKBR3/TRp2 cells, kindly provided by Dr. Rita Nahta, were maintained in DMEM/F-12 (Mediatech Inc.) with 10% FBS and 4 μg/ml trastuzumab. The cells were monthly tested for trastuzumab resistance.
Stable HSF1-overexpressing BT474 cells
BT474 cells were transfected with pcDNA 3.1 (+) or pcDNA 3.1(+)-human wild type HSF1 (WT) using Lipofectamine 2000 (Invitrogen). Twenty-four hours after plasmid transfection, cells were cultured in medium containing 400 μg/ml G418 to screen for stable clones.
Glucose uptake assay
Cells were seeded in 12-well plates at 1 ∼3 × 105 cells/well. Culture media was collected at 48 h and stored at -20°C until assayed. Glucose uptake was measured using an Amplex Red Glucose/Glucose Oxidase Assay Kit (Molecular Probes). Absorbance was measured at 563 nm using a SpectraMax M5 plate reader (Molecular Devices) and the results were normalized to the amount of total protein.
Lactate production assay
Lactate production in the medium was detected by using a Lactate Assay Kit (BioVision). Results were normalized to the amount of total protein.
LDH activity assay
Tumors were extracted for protein, then, their LDH activity was measured using an LDH Assay Kit (BioVision). Results were normalized to the tumor weight.
Western Blot
Western blots were performed as we did previously (34). The following antibodies were used: HSF1 (4356, Cell Signaling Technology), LDH-A (2012, Cell Signaling Technology), HKII (C64G5, Cell Signaling Technology), β-Actin (A2228, Sigma). Immunoreactive bands were visualized by HRP-conjugated secondary antibodies (Bio-Rad) using ECL Western Blotting Substrate (Pierce).
siRNA Experiments
siRNAs for HSF1 and LDHA and the control scrambled siRNA were purchased from Sigma. The downregulation of HSF1 was confirmed with two siRNA sequences to avoid off-target effects. Transfection was performed using the Lipofectamine2000 (Invitrogen).
Twenty-four hours after siRNA transfection, cells were transferred to 24-well plates for glucose uptake and lactate production assay or 96-well plates for drug treatment. Forty-eight hours after transfection, cell lysates were prepared and a Western Blot was performed.
Cell viability assay
Cells (3 × 103∼5 × 103 cells /well) were placed in a 96-well plate. Twenty-four hours later, cells were treated with trastuzumab, 2-DG/OX, or trastuzumab+ 2-DG/OX at the indicated concentration and incubated for the indicated time. Cell viability was determined using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega).
Cell counting
BT474 cells (1 ×105 cells/well) were seeded in 12-well plates. After 24 hrs, cells were treated with or without 100 μg/ml of trastuzumab for 24, 48, 72, and 96 hrs. At different time points, cell viability was measured by Typan Blue staining and direct cell counting using hematocytometer.
Animal experiments
Female athymic nude mice (Harlan Sprague Dawley) were implanted with 0.72 mg of 60 day release 17β-estradiol pellets (Innovative Researchof America). Three days later, BT474 and BT474/TR cells (1×107) in 200μl of PBS and Matrigel (BD Biosciences) mixture (1:1) were injected subcutaneously into a mouse mfp. When the tumor reached >150 mm3, the mice were randomly divided into four groups (8 mice/group) as follows: PBS-treated control; Trastuzumab alone (10 mg/kg, i.p., 2 times/wk × 3 weeks); OX alone (750 mg/kg, i.p., daily for 21 days); and trastuzumab + OX. Mice were weighed weekly and tumor diameters were measured with calipers twice per week for more than 5 weeks. The tumor volumes were calculated with the following formula: volume (mm3) = width × length/2, where W and L are the minor and major diameters (in millimeters), respectively. Unpaired Student's t-tests were used to assess statistical significance.
Results
The combination of trastuzumab and glycolysis inhibitors synergistically inhibit cancer cell growth in vitro
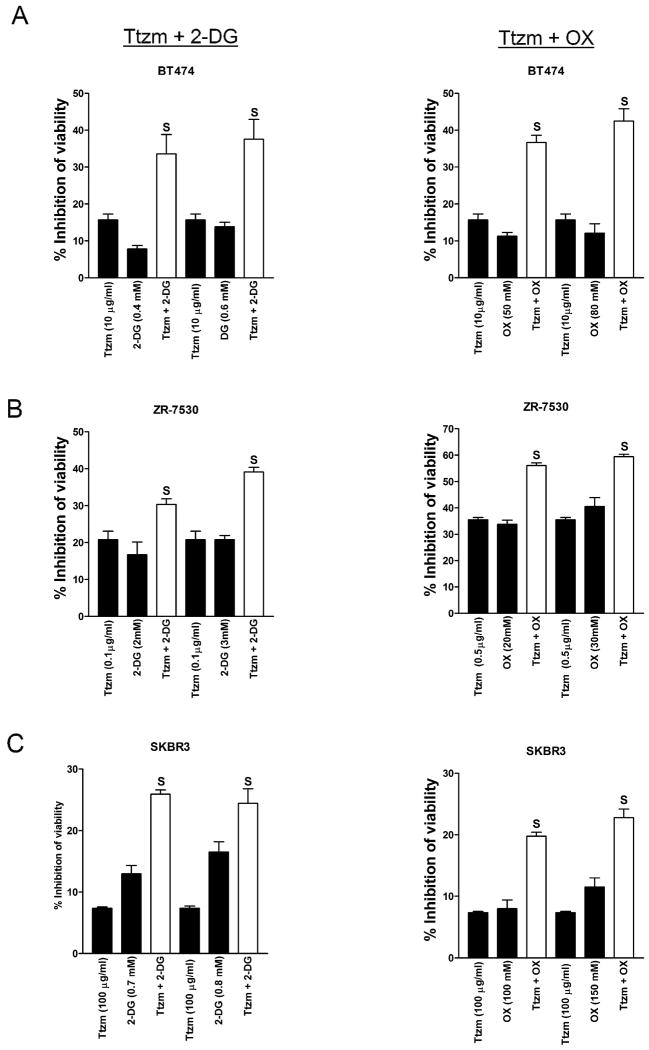
Trastuzumab has been shown to inhibit PI3K/ Akt signaling (5), which is a critical signaling pathway that regulates both cell survival and cancer cell bioenergetic metabolism (8). Our previous studies have shown that overexpression of ErbB2 promotes glycolysis and increases the sensitivity of breast cancer cells to glycolysis inhibitors, such as 2-DG and oxamate (33). Based on these studies, we hypothesized that the combination of trastuzumab with a glycolysis inhibitor may be more efficient than using the agents individually. We first tested the hypothesis in ErbB2-positive BT474 breast cancer cells and found that the combination of trastuzumab and 2-DG (Fig. 1A, left) or oxamate (Fig. 1B, right) dramatically increased the efficacy of trastuzumab to inhibit cell viability compared to use the agents individually. In order to evaluate whether the combinational effect is synergistic or additive, viability data generated from BT474 cells treated with multiple concentrations of trastuzumab were analyzed by the established method of Chou and Talalay using CalcuSyn software (35). The values of the combination index (CI) are shown in Supplementary Table 1 and all of them are less than 1.0, indicating that the combination of trastuzumab and 2-DG/oxamate inhibits cell viability synergistically in BT474 cells. To confirm these results, we used two other ErbB2-positive breast cancer cell lines, ZR-7530 and SKBR3, and the combination also showed synergistic inhibition in both cell lines (Fig. 1 B &C, Table 1). These results demonstrate that targeting glycolysis is a highly efficient strategy to sensitize ErbB2-positive cancer cells to trastuzumab in vitro.
Figure 1.
The combination of trastuzumab and glycolysis inhibitors synergistically inhibits cancer cell growth.
A, BT474, B, ZR-7530 and C, SKBR3 cells were seeded in 96-well plates at 5 × 103 cells/well. After 24 hrs, cells were treated with the indicated concentration of trastuzumab (Ttzm), 2-deoxyglucose (2-DG), oxamate (OX), or Ttzm plus 2-DG (left)/OX (right) and incubated for 48 hrs, and cell viability was determined. Data are presented as the percentage of viability inhibition measured in cells not treated with Ttzm and 2-DG/OX. Columns, mean of three independent experiments; bars, SE. S, synergy (CI< 1.0).
Table 1.
Combination index (CI) of Trastuzumab (Ttzm) and 2-deoxy-D-glucose (2-DG) or Oxamate (OX) in breast cancer cells.
| Ttzm (μg/ml) | 2-DG (mM) | CI | |
|---|---|---|---|
| BT474 | 5 | 0.4 | 0.393 |
| 5 | 0.6 | 0.540 | |
| 10 | 0.4 | 0.320 | |
| 10 | 0.6 | 0.430 | |
| ZR-7530 | 0.1 | 2 | 0.303 |
| 0.1 | 3 | 0.391 | |
| 0.5 | 2 | 0.498 | |
| 0.5 | 3 | 0.551 | |
| SKBR3 | 100 | 0.7 | 0.694 |
| 100 | 0.8 | 0.763 | |
| 150 | 0.7 | 0.663 | |
| 150 | 0.8 | 0.582 | |
| BT474/TR | 10 | 0.5 | 0.399 |
| 10 | 1.0 | 0.436 | |
| 100 | 0.5 | 0.212 | |
| 100 | 1.0 | 0.308 | |
| MDA-MB-361 | 10 | 2 | 0.442 |
| 10 | 3 | 0.654 | |
| 100 | 2 | 0.474 | |
| 100 | 3 | 0.538 |
| Ttzm (μg/ml) | OX (mM) | CI | |
|---|---|---|---|
| BT474 | 5 | 50 | 0.001 |
| 5 | 80 | 0.000 | |
| 10 | 50 | 0.000 | |
| 10 | 80 | 3.82e-005 | |
| ZR-7530 | 0.1 | 20 | 0.725 |
| 0.1 | 30 | 0.714 | |
| 0.5 | 20 | 0.591 | |
| 0.5 | 30 | 0.600 | |
| SKBR3 | 20 | 100 | 0.321 |
| 20 | 150 | 0.465 | |
| 100 | 100 | 0.360 | |
| 100 | 150 | 0.471 | |
| BT474/TR | 10 | 50 | 0.509 |
| 10 | 100 | 0.772 | |
| 100 | 50 | 0.496 | |
| 100 | 100 | 0.775 | |
| MDA-MB-361 | 10 | 20 | 0.531 |
| 10 | 30 | 0.695 | |
| 100 | 20 | 0.501 | |
| 100 | 30 | 0.647 |
NOTE: CI<1.0 indicates synergism; CI=1.0 indicates additive effect; CI >1.0 indicates antagonism.
Trastuzumab inhibits glycolysis in human breast cancer cells
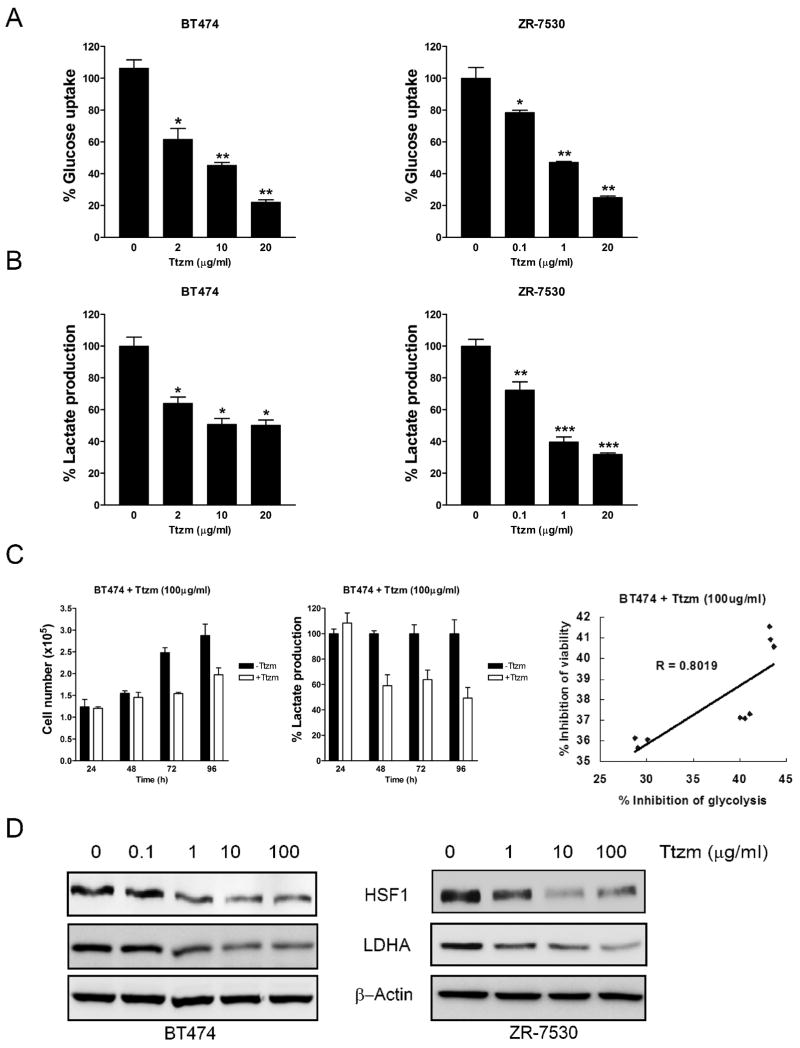
Since trastuzumab has been shown to inhibit metabolism-regulating signaling molecules such as PI3K and mTOR, it is logical to hypothesize that trastuzumab also may inhibit glycolysis. To test this, we treated BT474 and ZR-7530 cells with trastuzumab for 48 hours and measured glucose uptake and lactate production, which are hallmarks of glycolysis. Trastuzumab inhibited glucose uptake (Fig. 2A) and lactate production (Fig. 2B) in a dose-dependent manner in both BT474 and ZR-7530 cells, indicating that trastuzumab effectively inhibits glycolysis in breast cancer cells. To test whether inhibiting glycolysis is simply a consequence of the cell proliferation-inhibiting effect of trastuzumab, we examined which inhibition happened first. We treated BT474 cells with trastuzumab and counted cell number and measured lactate production in the medium at different time points. After 48 hours of trastuzumab treatment, compared to the control group, lactate production decreased by more than 40% (Fig. 2C, middle). However, at this time point, no cell growth inhibition was observed. Cell growth inhibition occurred after 72 h of trastuzumab treatment (Fig. 2C, left). These results showed that the inhibition of glycolysis by trastuzumab precedes cell growth inhibition, indicating that glycolysis inhibition is not simply a consequence of the cell growth inhibition.
Figure 2.
Trastuzumab inhibits glycolysis in human breast cancer cells.
A, The human breast cancer cells BT474 (left) and ZR-7530 (right) were seeded in 12-well plates at 3 × 105 cells/well. After 24 hrs, cells were treated with the indicated concentrations of Ttzm for 48 hrs, and glucose uptake was measured. Data are presented as the percentage of glucose uptake measured in cells not treated with Ttzm. B, BT474 (left) and ZR-7530 (right) cells were treated as described in panel (A) and lactate production was measured. Data are presented as the percentage of lactate production measured in cells not treated with Ttzm. C, BT474 cells were seeded in 12-well plates at 1 × 105 cells/well. After 24 hrs, cells were treated with 100 μg/ml of Ttzm for 24, 48, 72 and 96 hrs. At different time points, cell viability was measured by direct cell counting (left) and lactate production in the medium was measured (middle). Data are presented as the percentage of lactate production measured in cells not treated with Ttzm. Columns, mean of three independent experiments; bars, SE. *, P<0.05, **, P<0.01, ***, P<0.001. Correlation scatter plot of inhibition of glycolysis (lactate production) and inhibition of viability in BT474 cells (R=0.8019, right). D, BT474 (left) and ZR-7530 (right) cells were seeded in 6-well plates at 3 × 105 cells/well. After 24 hrs, cells were treated with the indicated concentrations of Ttzm for 48 hrs. The cell lysates were prepared and Western blot analyses were carried out with antibodies against HSF1, LDH-A and β-Actin.
To study the relationship between the inhibitory effects on glycolysis and cell viability by trastuzumab, we collected data from three independent experiments and analyzed the correlation. As shown in the correlation scatter graph, the inhibition of glycolysis (lactate production) correlates well with the inhibition of cell viability by trastuzumab (R=0.8019, Fig. 2C, right).
Our previous studies showed that the ErbB2-HSF1-LDHA pathway has a major role in regulating glucose metabolism in breast cancer cells (33). To test whether trastuzumab inhibits glycolysis through this pathway, we treated BT474 and ZR-7530 cells with increasing concentrations of trastuzumab for 48 hrs and found that trastuzumab decreased protein expression of HSF1 and LDH in both cell lines (Fig. 2D). We also treated SKBR3 cells with increasing concentrations of trastuzumab for 48 hrs and found similar results (Supplementary Fig. S1), suggesting that trastuzumab inhibits glycolysis via downregulation of HSF1 and LDHA in breast cancer cells.
HSF1 contributes to the resistance of breast cancer cells to trastuzumab
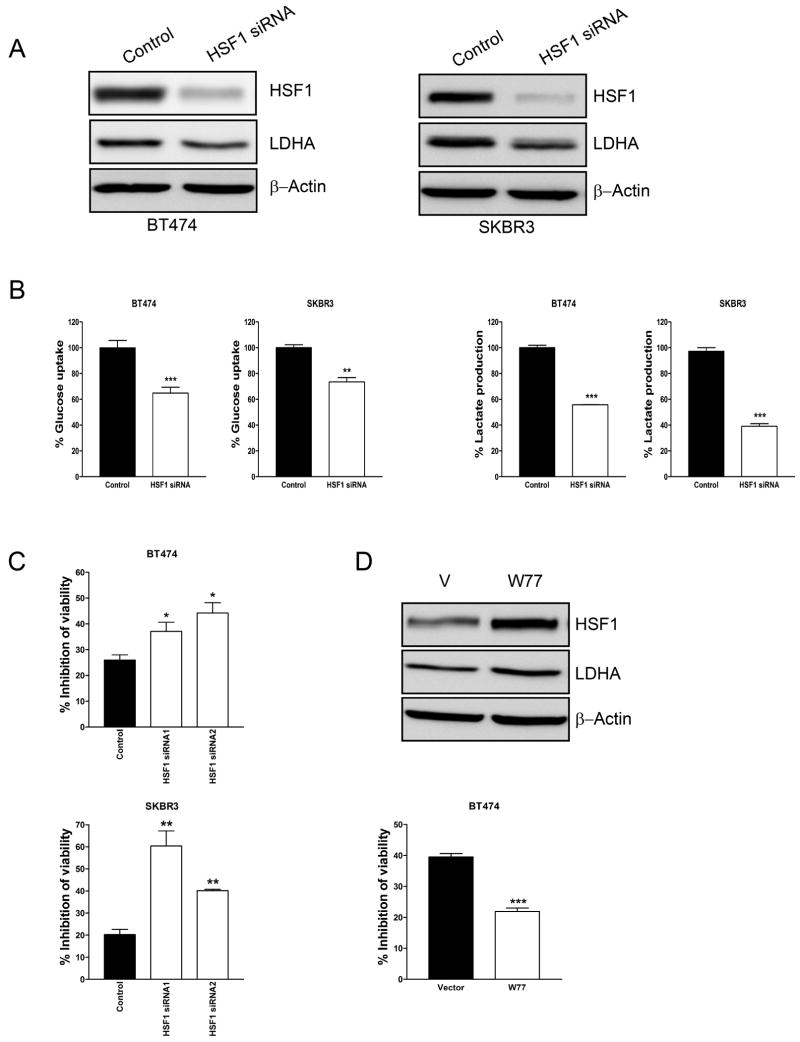
Previously we reported that HSF1 transcriptionally activates LDH-A and promotes glycolysis in ErbB2-positive breast cancer cells and in HSF1 knockout mouse embryonic fibroblasts (33). Here we further confirmed these results in ErbB2-positive BT474 and SKBR3 cells. When HSF1 was downregulated by siRNA, the protein levels of LDH-A also decreased in both BT474 and SKBR3 cells (Fig. 3A). Furthermore, HSF1 siRNA significantly reduced glucose uptake (Fig. 3B, left) and lactate production (Fig. 3B, right). Together with the results reveal that trastuzumab inhibits glycolysis and HSF1 expression in breast cancer cells (Fig. 2), these results further indicate that downregulation of HSF1 contributes to glycolysis inhibition by trastuzumab. Next, we examined whether HSF1 plays a role in the resistance of breast cancer cells to trastuzumab. BT474 and SKBR3 cells were treated with two different HSF1 siRNAs to downregulate HSF1 and then treated with trastuzumab. Downregulation of HSF1 significantly increased the sensitivity of both BT474 (Fig. 3C, upper) and SKBR3 cells (Fig. 3C, lower) to trastuzumab. Moreover, the resistance of cancer cells to trastuzumab is dependent on the protein levels of HSF1 (data not shown). We also used LDH-A siRNA to downregulate LDH-A in BT474 cells and treated with trastuzumab. Downregulation of LDH-A robustically increased the sensitivity of BT474 cells to trastuzumab (Supplementary. Fig. S2). To further confirm these results, we constructed stable HSF1-overexpressing BT474 cells and found that overexpression of HSF1 upregulated LDH-A protein levels (Fig. 3D, upper) and increased the resistance of cancer cells to trastuzumab (Fig. 3D, lower). These results indicate that downregulation of HSF1 contributes to the glycolysis inhibition by trastuzumab and that the HSF1/LDH-A axis plays an important role in the resistance of cancer cells to trastuzumab.
Figure 3.
Inhibition of HSF1 sensitizes breast cancer cells to trastuzumab.
A, BT474 (left) and SKBR3 (right) cells were transfected with scramble siRNA (Control) or HSF1 siRNA. 48 hrs after siRNA transfection, cell lysates were prepared and immunoblot analyses were carried out with antibodies against HSF1, LDH-A and β-Actin. B, 24 hrs after siRNA transfection, cells were transferred to 24-well plates for glucose uptake (left) and lactate production (right) assays. Data are shown as a percentage relative to control-transfected cells. C, BT474 (upper) and SKBR3 (lower) cells were transfected with scramble siRNA (Control) or two HSF1 siRNAs (HSF1 siRNA1 or HSF1 siRNA2). 24 hrs after siRNA transfection, cells were transferred to 96-well plates. The next day, cells were treated with 100 μg/ml of Ttzm for 72 hrs, and cell viability was determined. Data are presented as the percentage of viability inhibition measured in cells not treated with Ttzm. D, Western blot with anti-HSF1 and anti-LDHA antibody of total cell extracts from stable HSF1-overexpressing BT474 cell lines W77 (V: vector). β-actin was used a loading control (upper). HSF1-overexpressing cells (W77) were seeded in 96-well plates at 5 × 103 cells/well. The next day, cells were treated with 100 μg/ml trastuzumab for 72 hrs and cell viability was detected. Data are presented as the percentage of viability inhibition measured in cells treated without Ttzm. Columns, mean of three independent experiments; bars, SE. *, P<0.05, **, P<0.01, ***, P<0.001.
The combination of trastuzumab and glycolysis inhibitors better inhibits glycolysis in cancer cells
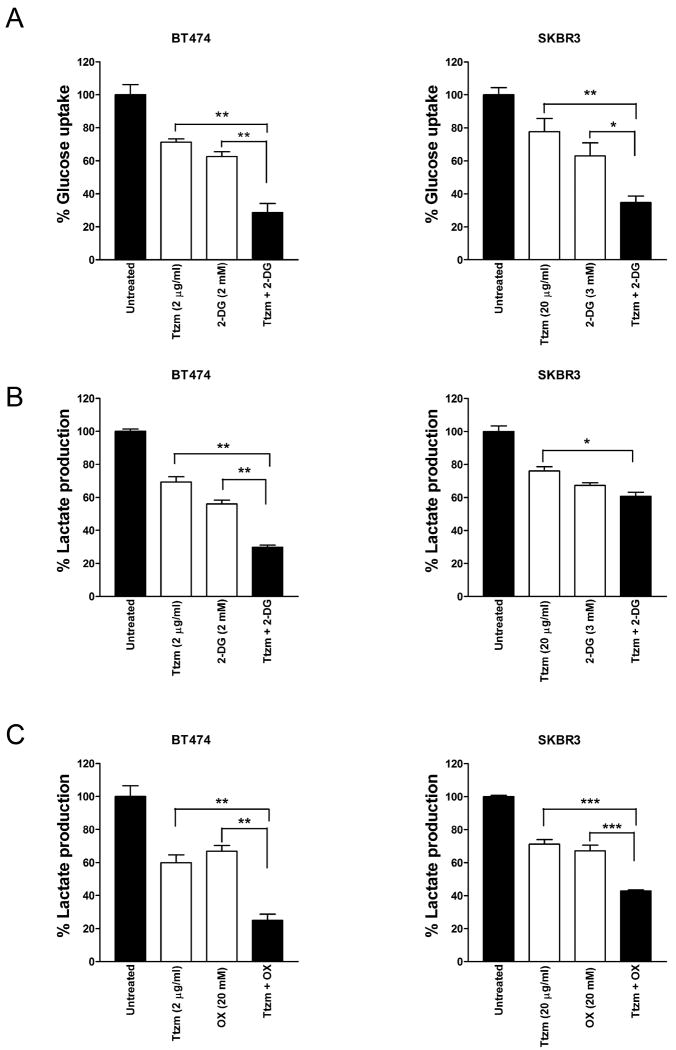
Since trastuzumab inhibits glycolysis via downregulation of HSF1 and LDH-A in breast cancer cells (Fig. 2 and Fig. 3), we reasoned that the combination of trastuzumab and 2-DG or oxamate may inhibit glycolysis more effectively than either agent alone. When we treated BT474 and SKBR3 cells with trastuzumab or 2-DG alone, or trastuzumab plus 2-DG, the combination of trastuzumab and 2-DG showed a much stronger inhibitory effect on both glucose uptake (Fig. 4A) and lactate production (Fig. 4B) compared to either trastuzumab or 2-DG alone. Similarly, trastuzumab plus oxamate inhibited lactate production much more effectively than either agent alone in both BT474 and SKBR3 cells (Fig. 4C). These results indicate that the combination of trastuzumab and glycolysis inhibition better blocks glycolysis in ErbB2-positive breast cancer cells.
Figure 4.
The combination of trastuzumab and glycolysis inhibitors better inhibit glycolysis in cancer cells.
A, BT474 (left) and SKBR3 (right) cells were seeded in 12-well plates at 3 × 105 or 1 × 105 cells/well, respectively. After 24 hrs, cells were treated with the indicated concentration of Ttzm, 2-DG, or Ttzm plus 2-DG and incubated for 48 hours, and glucose uptake in the medium was measured. Data are presented as the percentage of glucose uptake measured in cells not treated with Ttzm and 2-DG. B, BT474 cells (left) and SKBR3 (right) were treated as described under Panel (a) and lactate production in the medium was measured. Data are presented as the percentage of lactate production measured in cells not treated with Ttzm and 2-DG. C, BT474 (left) and SKBR3 (right) cells were seeded in 12-well plates at 3 × 105 or 1 × 105 cells/well, respectively. After 24 hrs, cells were treated with the indicated concentration of Ttzm, OX, or Ttzm plus OX and incubated for 48 hours, and lactate production in the medium was measured. Data are presented as the percentage of lactate production measured in cells not treated with Ttzm and OX. Columns, mean of three independent experiments; bars, SE. *, P<0.05, **, P<0.01, ***, P<0.001.
The combination of trastuzumab and glycolytic inhibitors effectively inhibits the growth of trastuzumab-resistant cells in vitro
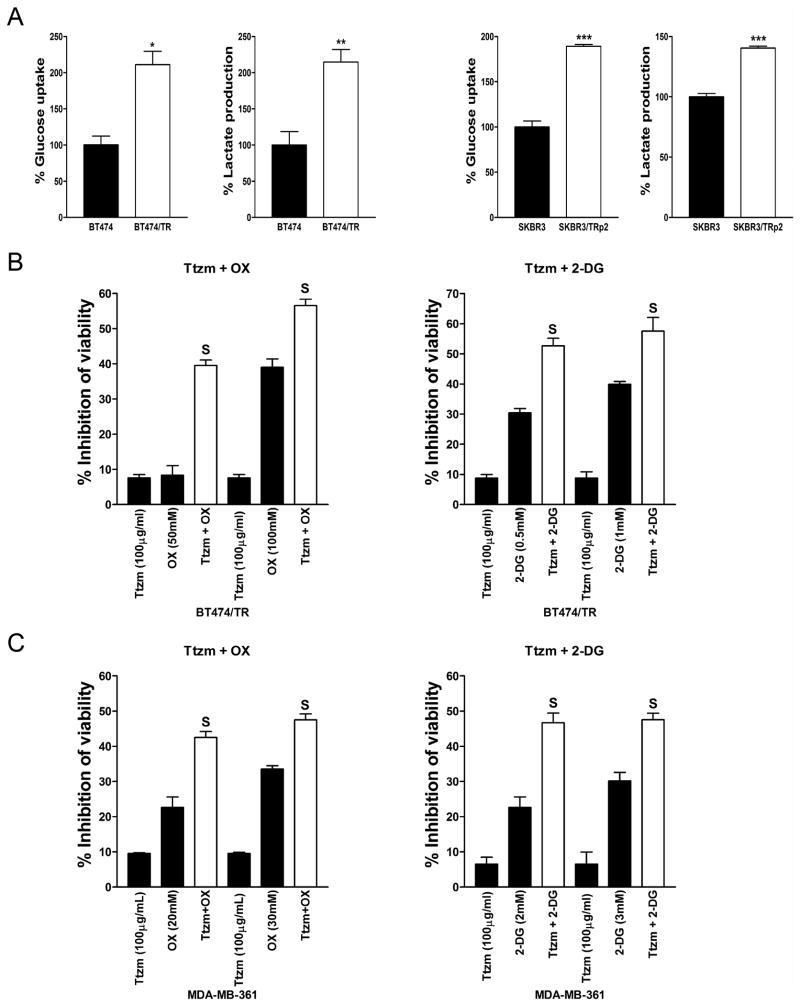
The treatment of ErbB2-positive human breast cancer BT474 and SKBR3 cells with gradually increasing concentrations of trastuzumab led to an acquired resistance to trastuzumab (36). To examine whether trastuzumab-resistant cells have altered glycolysis, we compared the parental BT474 cells with their trastuzumab-resistant counterparts for glucose uptake and lactate production. Indeed, compared to the parental cells, trastuzumab-resistant BT474/TR cells have increased glucose uptake and lactate production (Fig. 5A, left), indicating that trastuzumab-resistant cells have increased glycolysis. To confirm these results, we performed similar experiments in parental SKBR3 and trastuzumab-resistant SKBR3/TRp2 cells and observed similar results (Fig. 5A, right). We also compared HSF1 and LDH-A expression levels in these cells and found that trastuzumab-resistant SKBR3/TRp2 and BT474/TR cells have significantly higher HSF1 and LDH-A levels (Supplementary Fig. S3), suggesting that trastuzumab-resistant cells have increased glycolysis resulting from upregulation of HSF1 and LDH-A. Since trastuzumab-resistant cells have increased glycolysis, we explored whether a glycolysis inhibitor could sensitize trastuzumab-resistant cells to trastuzumab. We found that the combination of trastuzumab and 2-DG/oxamate synergistically inhibits the growth of BT474/TR cells (Fig. 5B and Table 1). We also confirmed these results in another trastuzumab-resistant cell line MDA-MB-361 (Fig. 5C and Table 1). These data indicate that increased glycolysis contributes to trastuzumab resistance and the combination of trastuzumab and 2-DG or oxamate effectively inhibits trastuzumab-resistant cancer cells in vitro.
Figure 5.
The combination of trastuzumab and a glycolysis inhibitor effectively inhibits trastuzumab-resistant breast cancer cells.
A, BT474, trastuzumab-resistant BT474/TR (left), SKBR3 and trastuzumab-resistant SKBR3/TRp2 cells (right) were seeded in 12-well plates at 2 × 105 cells/well. After 48 hrs, the medium was collected and glucose uptake and lactate production were measured. Data are shown as a percentage relative to BT474 (left) or SKBR3 (right). B, BT474/TR cells were seeded in 96-well plates at 5 × 103 cells/well. After 24 hr, cells were treated with the indicated concentration of Ttzm, 2-DG/OX, or Ttzm plus 2-DG/OX and incubated for 48 hrs, and cell viability was determined. Data are presented as the percentage of viability inhibition measured in cells not treated with Ttzm and 2-DG/OX. C, MDA-MB-361 cells were seeded and treated as described in B. Columns, mean of three independent experiments; bars, SE. S, synergy.
The combination of trastuzumab and oxamate synergistically inhibits tumor growth in vivo
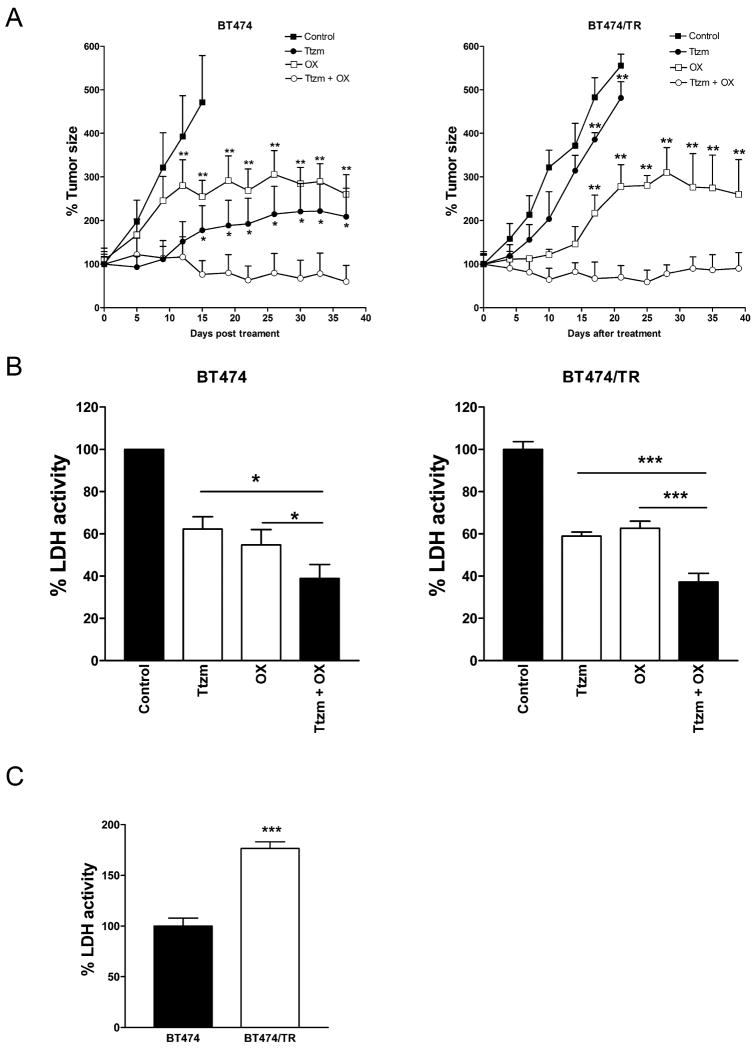
Our aforementioned results showed that the combination of trastuzumab and a glycolysis inhibitor synergistically inhibited the growth of both trastuzumab-sensitive and -resistant cells in vitro (Fig. 1 and Fig. 5). To confirm these results in vivo, we tested the combination treatment of trastuzumab and oxamate in a xenograft nude mice model. BT474 (Fig. 6A, left) and its trastuzumab-resistant variant BT474/TR cells (Fig. 6A, right) were inoculated into the mammary fat pads of 6-week-old nude mice. After tumors formed, we treated tumor xenografts with trastuzumab (10 mg/kg, i.p., 2 times/wk × 3 weeks), oxamate (750 mg/kg, i.p., daily for 21 days), or a combination of the agents. As we expected, compared to the control group, trastuzumab inhibits BT474-derived tumor growth (Fig. 6A, left). However, BT474/TR-derived tumors were resistant to trastuzumab treatment (Fig. 6A, right). The oxamate treatment inhibits both BT474 and BT474/TR-derived tumor growth. Remarkably, the combination of trastuzumab and oxamate was much more efficient in inhibiting the growth of both BT474 and BT474/TR tumors than either of the single agents and retained the inhibitory effect well beyond the time of cessation of treatment at day 22 (Fig. 6A).
Figure 6.
The combination of trastuzumab and oxamate effectively inhibits tumor growth in vivo.
A, Pre-established BT474 (left) or BT474/TR (right) tumor xenografts were treated with Control (PBS), Ttzm (10mg/kg, i.p., 2×/wk × 3 wks), OX (750mg/kg, i.p., daily × 21 days), or a combination of the agents. Data are presented as the relative tumor size. Statistical analysis of the differences of average tumor sizes in the single treatment group and the combination group was performed using the unpaired Student's t test. *, P<0.05, **, P<0.01. B, Tumors derived from BT474 cells (left) or BT474/TR cells (right) were lysed for LDH activity assay. Data are presented as the percentage of LDH activity measured in the control group. C, LDH activity in tumors derived from BT474 or BT474/TR cells. Data are shown as a percentage relative to BT474 cells-formed tumors. Columns, mean of three independent tumors; bars, SE. *, P<0.05, ***, P<0.001.
To test whether the combination of trastuzumab and oxamate efficiently inhibits LDH activity in the xenograft tumors, we evaluated LDH's activity in the control, trastuzumab, oxamate, or trastuzumab plus oxamate treated BT474 (Fig. 6B, left) or BT474/TR (Fig. 6B, right) tumors. We found that the combination treatment was more efficient in the inhibition of LDH activity compared to either of the single agents in both types of tumors, suggesting that glycolysis inhibition contributes to tumor growth inhibition in vivo.
To test whether increased LDH activity contributes to trastuzumab resistance in vivo, we compared LDH activity between BT474 and BT474/TR tumors. Compared to BT474 tumors, BT474/TR tumors have significantly greater LDH activity (Fig. 6C), which is consistent with the data obtained in vitro (Fig. 5A). These results further support that increased glycolysis contributes to trastuzumab resistance.
Discussion
Thus far, the mechanisms by which trastuzumab inhibits ErbB2-positive breast cancer development are still not fully defined. Previous studies showed that trastuzumab inhibits PI3K/Akt signaling (5), which is not only a regulator of cell survival, but also a regulator of cellular metabolism. However, it is unknown whether altered glycolysis may serve as a mechanism in trastuzumab-mediated cancer inhibition. In this study, we use multiple ErbB2-positive cell lines (BT474, SKBR3, MDA-MB-361, and ZR-7530) to address the role of trastuzumab in glycolysis inhibition in breast cancer cells. We found that trastuzumab decreased glucose and lactate production in these cells, indicating that trastuzumab indeed inhibits glycolysis in these cells. The inhibition of glycolysis by trastuzumab precedes cell growth inhibition, indicating that glycolysis inhibition is not simply a consequence of cell growth inhibition by trastuzumab. Since dysregulated glycolysis contributes to cancer cell's malignant behavior, these results suggest that inhibition of glycolysis may serve as a mechanism for the tumor inhibitory effect of trastuzumab.
It has been reported that bioenergetic alteration plays a role in chemoresistance (13), and we have reported that targeting glycolysis can sensitize cancer cells to chemotherapy (14). However, it is unknown whether the switch of energetic dependency contributes to trastuzumab resistance. To examine whether trastuzumab-resistant cells have altered glycolysis, we compared glucose uptake and lactate production between parental BT474 or SKBR3 cells and their trastuzumab-resistant counterparts (BT474/TR or SKBR3/TRp2 cells). Compared to parental cells, trastuzumab-resistant cells show increased glycolysis, as indicated by higher glucose uptake and lactate production. In vivo studies also showed that BT474/TR-derived tumors have a higher LDH activity than BT474-derived tumors. These data suggested that increased glycolysis contributes to trastuzumab resistance. This may serve as a novel mechanism of trastuzumab resistance.
Our previous study showed that the ErbB2-HSF1-LDHA pathway plays a major role in regulating glycolysis in breast cancer cells (33). Here, for the first time, we show that HSF1 is upregulated in trastuzumab-resistant cells and that the downregulation of HSF1 or LDHA increases the sensitivity of breast cancer cells to trastuzumab. Moreover, overexpression of HSF1 decreased the sensitivity to trastuzumab. These novel findings indicated that HSF1/LDH-A enhanced glycolysis contributes to the resistance of breast cancer cells to trastuzumab.
The increased dependency of cancer cells upon the glycolytic pathway is well accepted as an important process that supports malignant phenotypes (18). Inhibition of glycolysis as a strategy to preferentially kill cancer cells and the development of glycolytic inhibitors as anticancer agents has become a rapidly growing area in cancer research. Several glycolytic inhibitors with promising anticancer activity are currently at various stages of pre-clinical and clinical development (15, 24, 25). 2-deoxyglucose (2-DG) has been shown to have effects in selectively inhibiting solid tumors and is currently under Phase I/II clinical trials (27-29). Another promising glycolysis inhibitor, oxamate, a specific inhibitor of the lactate dehydrogenase, also has shown promising results and is currently under pre-clinical investigation (30). However, 2-DG alone did not show a significant effect upon tumor growth in vivo (27). Recently, it has been reported that 2-DG may activate prosurvival signaling pathways in cancer cells (31, 32), suggesting that the combination of 2-DG with other signaling inhibitors in a 2-DG-based therapy may result in enhanced therapeutic efficacy. Here we show that the combination of 2-DG with a PI3K/Akt inhibiting agent, such as trastuzumab, synergistically inhibited tumor growth in vitro. These results are consistent with a recent study showing that treating cancer cells with the combination of 2-DG and an IGF1R inhibitor dramatically reduced cancer cell proliferation and promoted significant apoptosis (31, 32).
For the first time, we report that the combination of trastuzumab with a glycolysis inhibitors, 2-DG or oxamate, synergistically inhibits breast cancer cell growth. More importantly, this combinational therapy effectively inhibits the growth of trastuzumab-resistant cancer cells in vitro and in vivo. Interestingly, glycolysis inhibition by trastuzumab precedes cell growth inhibition and the combination treatment is more efficient at inhibiting glycolysis both in vitro and in vivo compared to either of the single agents alone. These novel findings indicate that glycolysis inhibition contributes to the synergistic anti-tumor effect of combinational therapy, and targeting glycolysis could be an effective strategy for overcoming trastuzumab resistance in cancer therapy.
In summary, we report that trastuzumab inhibits glycolysis via downregulation of HSF1 and LDH-A in ErbB2-positive breast cancer cells, which may serve as a novel mechanism for trastuzumab induced tumor growth inhibition. We also demonstrate that the combination of trastuzumab and glycolysis inhibition synergistically inhibits growth of both trastuzumab-sensitive and -resistant breast cancer cells in vitro and in vivo, which correlates to a more efficient inhibition of glycolysis. Moreover, we are the first to report that the increased glycolysis mediated by HSF1 and LDH-A contributes to trastuzumab resistance. These findings revealed that the switch of energetic dependency to glycolysis contributes to trastuzumab resistance, and glycolysis inhibition is a promising therapeutic strategy to overcome trastuzumab resistance. This is important for the future design of combined therapeutics targeting both ErbB2 and glycolysis to treat ErbB2-positive and trastuzumab-resistant breast cancers.
Supplementary Material
Acknowledgments
We thank Dr. Michele Schuler and the USA Animal Research Facility veterinary staff for technical support, and Ms. Amy Brown for editorial assistance.
Grant Support: We are grateful to the support from The Vincent F. Kilborn, Jr. Cancer Research Foundation (M. Tan), NIH Grant RO1CA149646 (M. Tan) and Radiumhospitalets Legater Award Project 334003 (M. Tan and O. Fodstad).
References
- 1.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051–8. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–8. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–8. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 8.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 9.Lan KH, Lu CH, Yu D. Mechanisms of trastuzumab resistance and their clinical implications. Ann N Y Acad Sci. 2005;1059:70–5. doi: 10.1196/annals.1339.026. [DOI] [PubMed] [Google Scholar]
- 10.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 11.Bedard PL, de Azambuja E, Cardoso F. Beyond trastuzumab: overcoming resistance to targeted HER-2 therapy in breast cancer. Curr Cancer Drug Targets. 2009;9:148–62. doi: 10.2174/156800909787581024. [DOI] [PubMed] [Google Scholar]
- 12.Tan M, Yu D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance. Adv Exp Med Biol. 2007;608:119–29. doi: 10.1007/978-0-387-74039-3_9. [DOI] [PubMed] [Google Scholar]
- 13.Evans A, Bates V, Troy H, Hewitt S, Holbeck S, Chung YL, et al. Glut-1 as a therapeutic target: increased chemoresistance and HIF-1-independent link with cell turnover is revealed through COMPARE analysis and metabolomic studies. Cancer Chemother Pharmacol. 2008;61:377–93. doi: 10.1007/s00280-007-0480-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33. doi: 10.1186/1476-4598-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–74. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 16.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Gatenby RA, Gillies RJ. Glycolysis in cancer: a potential target for therapy. Int J Biochem Cell Biol. 2007;39:1358–66. doi: 10.1016/j.biocel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49 2:24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 19.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–7. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–30. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 24.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 25.Scatena R, Bottoni P, Pontoglio A, Mastrototaro L, Giardina B. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin Investig Drugs. 2008;17:1533–45. doi: 10.1517/13543784.17.10.1533. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Liu H, Riker AI, Fodstad O, Ledoux SP, Wilson GL, et al. Emerging metabolic targets in cancer therapy. Front Biosci. 2011;16:1844–60. doi: 10.2741/3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, et al. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–4. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Zhu H, Liu DX, Niu TK, Ren X, Patel R, et al. Silencing of elongation factor-2 kinase potentiates the effect of 2-deoxy-D-glucose against human glioma cells through blunting of autophagy. Cancer Res. 2009;69:2453–60. doi: 10.1158/0008-5472.CAN-08-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XD, Deslandes E, Villedieu M, Poulain L, Duval M, Gauduchon P, et al. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res. 2006;26:3561–6. [PubMed] [Google Scholar]
- 30.Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci U S A. 2005;102:5992–7. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008;7:809–17. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 32.Zhong D, Xiong L, Liu T, Liu X, Liu X, Chen J, et al. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J Biol Chem. 2009;284:23225–33. doi: 10.1074/jbc.M109.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao YH, Zhou M, Liu H, Ding Y, Khong HT, Yu D, et al. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- 34.Tan M, Jing T, Lan KH, Neal CL, Li P, Lee S, et al. Phosphorylation on tyrosine-15 of p34(Cdc2) by ErbB2 inhibits p34(Cdc2) activation and is involved in resistance to taxol-induced apoptosis. Mol Cell. 2002;9:993–1004. doi: 10.1016/s1097-2765(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 35.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 36.Rowe DL, Ozbay T, Bender LM, Nahta R. Nordihydroguaiaretic acid, a cytotoxic insulin-like growth factor-I receptor/HER2 inhibitor in trastuzumab-resistant breast cancer. Mol Cancer Ther. 2008;7:1900–8. doi: 10.1158/1535-7163.MCT-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.