Abstract
The present study traced the emergence of the neural circuits for reading in five-year-old children of diverse pre-literacy ability. In the fall and winter of kindergarten, children performed a one-back task with letter versus false font stimuli during fMRI scanning. At the start of kindergarten, children with on-track pre-literacy skills (OT) recruited bilateral temporo-parietal regions for the letter > false font comparison. In contrast, children at-risk for reading difficulty (AR) showed no differential activation in this region. Following 3 months of kindergarten and, for AR children, supplemental reading instruction, OT children showed left-lateralized activation in the temporo-parietal region, whereas AR children showed bilateral activation and recruitment of frontal regions including the anterior cingulate cortex. These data suggest that typical reading development is associated with initial recruitment and subsequent disengagement of right hemisphere homologous regions while atypical reading development may be associated with compensatory recruitment of frontal regions.
Over the past 20 years, a number of neuroimaging studies have examined the nature, development, dysfunction, and remediation of cortical circuits for reading. Studies of proficient, adult readers have identified three left-hemisphere regions that comprise a putative reading network. These include dorsal and ventral posterior regions and one anterior region (Pugh et al., 2000, 2001; Schlaggar and McCandliss, 2007). The posterior dorsal region, located at and around the temporo-parietal junction, including the posterior part of the superior temporal gyrus, supramarginal gyrus, and angular gyrus, has been hypothesized to be recruited for phonological processing (Church et al., 2008; Temple, 2002) and the conversion of orthographical (visual) information to phonological (auditory) form (Pugh et al., 2000, 2001; Shaywitz et al., 1998), which involves integration of multi-modal information (Booth et al., 2002; van Atteveldt et al., 2004). The posterior ventral region, localized in the inferior temporal gyrus and known as the visual word form area, has been postulated to support visual word recognition based on orthographical regularities of a given language (Cohen et al., 2000; McCandliss et al., 2003). Finally, the anterior region, centered in and around the inferior frontal gyrus (Pugh et al., 2000, 2001), has been hypothesized to support articulatory recoding, that is, the conversion of phonological information to motoric information of articulatory organs, during reading (Pugh et al., 2000, 2001).
Studies of adults with dyslexia indicate patterns of atypical activation in the posterior dorsal and anterior regions of the reading network. For example, when performing phonological processing tasks with visually presented words or letters, adults with dyslexia show reduced activation relative to normal readers in the left posterior dorsal region (Brunswick et al., 1999; Paulesu et al., 1996; Rumsey et al., 1992; Rumsey et al., 1997; Shaywitz et al., 1998). In contrast, recruitment in the homologous region in the right-hemisphere has been reported in some studies of adults with dyslexia (Pugh et al., 2000; Rumsey et al., 1999). Furthermore, it has been shown that adults with dyslexia who received a phonologically based intervention increased the recruitment of right as well as left posterior dorsal regions in a phonological manipulation task (Eden et al., 2004). With respect to the anterior region, abnormal activations, both over-(Brunswick et al., 1999; Paulesu et al., 1996; Shaywitz et al., 1998) and underactivation (Paulesu et al., 1996), have been reported in adults with dyslexia performing these and similar tasks. Recruitment of the right posterior dorsal region and overactivation of anterior region has been interpreted as reflecting the use of compensatory strategies for phonological processing tasks (Brunswick et al., 1999; Eden et al., 2004).
More recently, research has examined the reading network in beginning readers six years of age and older. In typically developing children, the posterior dorsal region is recruited in letter rhyming (Temple et al., 2001) and implicit word reading (Turkeltaub et al., 2003) tasks, while underactivation of this area is reported in children with developmental dyslexia (Hoeft et al., 2006; Shaywitz et al., 2002; Temple et al., 2003). Using magnetoencephalography (MEG), similar findings are reported in a study with 6–7 year olds with or without a risk for developing reading problems (Simos et al., 2002). Furthermore, several functional magnetic resonance imaging (fMRI) and MEG studies report increased activation in the posterior dorsal region in children with or at risk for dyslexia after successful reading intervention (Shaywitz et al., 2004; Simos et al., 2007; Simos et al., 2005; Temple et al., 2003). A finding from a cross-sectional developmental study of 6 to 22 year olds (Turkeltaub et al., 2003) suggests that the posterior dorsal region comes to be recruited earlier than other regions in the reading network. Results in the same study also suggest that the development of reading ability involves progressive disengagement of the right-hemisphere homologues of the reading network.
These previous studies provide evidence on the development of reading circuits in children who have already had at least a year of formal schooling and in many cases several years. However, to date no study has examined children under the age of six years. Yet, this is the age when many children are first exposed to print. Thus, examining the neural circuits for reading in children at the age of first school entry provides an opportunity to examine the emergence of reading circuits in pre-reading children. Furthermore, given that children’s pre-literacy skills at school entry can predict their reading performance many years later, longitudinal studies of children at this age provides the opportunity to examine possible differences in the neural trajectory across the first months of reading instruction in children either on-track or at-risk for later reading failure. Such data can be useful in discriminating patterns of delay from deviance in the development of neural circuits for reading. In the present study, the recruitment of the reading network was examined at the beginning of kindergarten in five-year-old children either on track for reading development (OT group) or at risk for later reading difficulties (AR group) using fMRI. We further examined the changes in the recruitment of the emerging reading network at the end of the first semester of kindergarten in the same children.
It has been reported that early pre-literacy skills such as letter-name knowledge as well as phonemic awareness are important precursors to and predictors of later literacy development (Byrne and Fielding-Barnsley, 1995; Foulin, 2005; Wagner and Torgesen, 1987). In the current study, these pre-literacy skills of the children were assessed using standardized tests upon entrance to kindergarten. Although these children were too young to be diagnosed for dyslexia, those scoring below 35th percentile were considered at risk for later reading difficulties and eligible for district-supported supplemental reading instruction. Thus, the AR group received daily supplemental reading instruction with the Early Reading Intervention (Kame'enui and Simmons, 2003) from school personnel in addition to the regular kindergarten curriculum.
During fMRI data acquisition, children (and adults) performed a one-back task with letter and false font stimuli. This task allowed us to examine the neural systems supporting letter-name knowledge, which is an important predictor of reading development, in children who were not yet able to engage in word-level reading. We expected encoding and maintenance of visual information to be sufficient for task performance such that the task could be performed equally well whether or not children knew letter names. However, knowing letter names would enable encoding and maintenance of letter (but not false font) stimuli in the phonological, as well as visual, form. Therefore, comparing the activation to letter versus false font stimuli allowed us to examine the emergence of the posterior dorsal system involved in phonological processing during the earliest stage of literacy development. We predicted that the recruitment of this region for letters relative to false fonts would emerge earlier in the OT group, who started kindergarten with more letter-name knowledge, than the AR group. We also examined the involvement of the right hemisphere homologue of the posterior dorsal system in these emerging readers. We asked whether right hemisphere activation is limited to AR children, or whether OT children also recruit the right hemisphere homologue initially. As findings from previous studies suggest (Shaywitz et al., 2002; Turkeltaub et al., 2003), the right hemisphere homologue may be recruited during the early stage of literacy development of OT children but later disengaged so that the pattern of recruitment becomes more mature and specialized as children gain further literacy skills.
Method
Participants
Eighteen children and 13 adults participated in this study. Children attended one of three schools in Eugene, Oregon, and were recruited from a larger behavioral study involving the Early Reading Intervention (Kame'enui and Simmons, 2003) described below. Adults were recruited from the University of Oregon community. Both adults and children were healthy, right-handed, native English speakers with no known neurological disorders including ADHD. Fourteen of the 18 children had usable behavioral and neuroimaging data from both Sessions 1 and 2 and were included in the analysis. (See Table 1 for participant information.)
Table 1.
Participant profile and task performance
| Adults | Children | ||||
|---|---|---|---|---|---|
| Group | On-track (OT) | At-risk (AR) | t-test e | ||
| mean (SD) | mean (SD) | mean (SD) | t; p | ||
| Age (years) a | 24.6 (3.7) | 5.7 (0.3) | 5.6 (0.2) | 0.74; 0.48 | |
| Gender | 8 females/5 males | 4 females/3 males | 5 females/2 males | ||
| SES b | --- | 36.9 (6.5) | 30.2 (10.2) | 1.45; 0.17 | |
| Maternal Education c | --- | 5.1 (0.4) | 5.0 (0.0) | 1.00; 0.34 | |
| Stanford-Binet | |||||
| Nonverbal fluid reasoning d | Session 1 | --- | 11.1 (2.2) | 10.0 (3.4) | 0.75; 0.47 |
| Session 2 | --- | 12.1 (2.3) | 11.4 (3.3) | 0.47; 0.65 | |
| Verbal knowledge d | Session 1 | --- | 9.9 (3.3) | 8.6 (2.8) | 0.79; 0.45 |
| Session 2 | --- | 10.6 (1.7) | 9.1 (1.3) | 1.73; 0.11 | |
| DIBELS | |||||
| Letter Naming Fluency | Initial Screening | --- | 18.6 (3.6) | 1.1 (1.7) | 11.62; < 0.001 |
| Session 1 | --- | 16.0 (5.3) | 2.9 (3.6) | 5.40; <0.001 | |
| Session 2 | --- | 23.6 (5.2) | 17.7 (12.4) | 1.15; 0.27 | |
| Initial Sound Fluency | Initial Screening | --- | 12.6 (2.8) | 3.4 (3.3) | 5.61; < 0.001 |
| Session 1 | --- | 17.3 (9.7) | 11.4 (4.7) | 1.44; 0.18 | |
| Session 2 | --- | 24.7 (8.8) | 23.4 (12.3) | 0.22; 0.83 | |
| In-scanner task | |||||
| Letter – accuracy | Session 1 | 94 (17) | 71 (25) | 60 (21) | 0.85; 0.41 |
| Session 2 | --- | 72 (22) | 66 (16) | 0.60; 0.56 | |
| False Font- accuracy | Session 1 | 94 (17) | 80 (15) | 63 (27) | 1.45; 0.17 |
| Session 2 | --- | 77 (17) | 65 (25) | 1.05; 0.31 | |
| Number of usable runs | Session 1 | 4.00 (0.00) | 3.86 (0.38) | 3.00 (1.00) | 8.35; < 0.01 |
| Session 2 | --- | 3.71 (0.76) | 3.00 (1.00) | 5.82; < 0.01 | |
| Absolute motion (mm) | Session 1 | 0.10 (0.02) | 0.19 (0.03) | 0.27 (0.22) | −0.96; 0.36 |
| Session 2 | --- | 0.16 (0.06) | 0.23 (0.06) | −2.05; 0.06 | |
| Relative motion (mm) | Session 1 | 0.08 (0.03) | 0.17 (0.07) | 0.18 (0.11) | −0.09; 0.93 |
| Session 2 | --- | 0.15 (0.08) | 0.23 (0.06) | −2.05; 0.06 | |
At Session 1 for children
Four-factor total score, Hollingshead, 1975
Score on the level of school completed, Hollingshead, 1975
Scaled scores
OT group versus AT group
Of the 14 children, seven were considered to be on track for reading development (OT) and seven were considered to be at (some) risk for later reading difficulties (AR). Children’s grouping was based on standard school screening procedures conducted at the beginning of the kindergarten year (initial screening), using the Letter Naming Fluency (LNF) and Initial Sound Fluency (ISF) subtests of the Dynamic Indicators of Basic Early Literacy Skills (DIBELS) (Good et al., 2002). DIBELS is a standardized assessment tool for early (pre-) literacy development. LNF is a timed measure of children’s ability to name upper- and lower-case letters presented visually in random order. ISF is a timed measure of phonemic awareness, assessing children’s ability to recognize and produce the initial phoneme of auditorily presented words.
Children scoring between the 50–75th percentile were identified as the OT group, and children scoring below the 35th percentile on either subtest were identified as AR group in the current study. Predictive validity coefficients of kindergarten DIBELS subtest scores and first grade reading ability measures (e.g., Test of Word-Reading Efficiency, Woodcock Reading Mastery Test-Revised) have been reported to range from .29 to .46 for ISF and .48 to .73 for LNF (Dynamic Measurement Group, 2008). The two groups had similar gender ratios and did not differ significantly in age, socio-economic status (Hollingshead, 1975), level of maternal education, or Stanford-Binet non-verbal fluid reasoning or verbal knowledge, as shown in Table 1.
All children received the school’s regular half-day kindergarten curriculum, which included early literacy instruction. In addition, AR children were given 30 minutes of supplemental reading instruction outside of the regular school day using the Early Reading Intervention (ERI) (Kame'enui and Simmons, 2003), followed by 15 minutes of non-literacy activities, including puzzles and small group activities. The ERI focused on the development of phonological awareness and alphabetic skills.
In-scanner task and procedure
Participants performed a one-back task with letters and letter-like stimuli (false fonts) in the scanner (Figure 1). The stimuli included 10 lower case letters and 10 false fonts each created by rearranging all parts of the corresponding lower-case letter. Stimuli were displayed using a DLP video projector illuminating a rear projection screen located near the head end of the magnet bore and viewed through an adjustable mirror attached to the RF head coil.
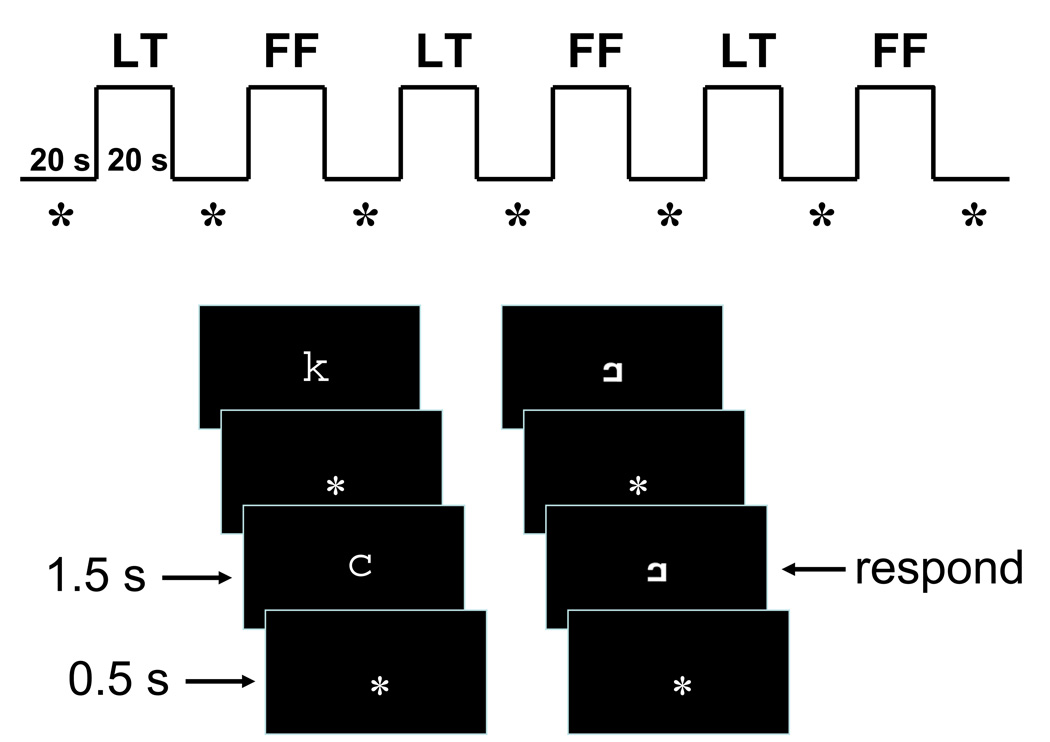
Figure 1.
In-scanner one-back task. Letters (LT) and false fonts (FF) were presented separately in six alternating blocks, interleaved by asterisk fixation blocks (*). Each block lasted for 20 s. Within each task block, ten letter or false font stimuli were presented one at a time. False font stimuli were created by rearranging the parts of corresponding lower-case letter. Each stimulus appeared for 1.5 s, separated by a 0.5 s presentation of a fixation asterisk. Participants were asked to respond by pressing a button with the thumb when the same stimulus was repeated twice in a row.
Letters and false fonts were presented separately in six alternating blocks, interleaved by fixation blocks during which participants were asked to fixate their eyes on an asterisk at the center of the screen. Each block lasted for 20 seconds. Within each task block, ten letter or false font stimuli were presented one at a time at the center of the screen. Each stimulus appeared for 1.5 s, separated by a 0.5 s presentation of an asterisk (fixation point). Participants were asked to respond by pressing a button with the thumb when the same stimulus was repeated twice in a row. Three out of ten stimuli in each block were repeated stimuli.
Each scanning session contained four runs, with each run lasting 4 minutes and 20 seconds. In two of the runs, letters were presented first, and in the other runs, false fonts were presented first. After entering the scanner, child participants underwent two runs of functional scanning and an eight-minute structural scan before a short break outside the scanner. Two more runs of functional scanning were performed after the break. The order of the runs and response hands were counter-balanced across participants. The same task with the same set of stimuli was performed at both sessions. The response hand was also kept the same across sessions.
Prior to the first scanning session, all child participants visited the neuroimaging center for a practice session. During this session, children entered an MRI simulator equipped with a speaker that presented recordings of scanner noise and permitted practice of the in-scanner task (with a different set of stimuli from those used during actual scanning sessions) and staying still. Actual scanning was conducted on different days from the practice session. To make the MRI environment inviting to young children, the simulator and the scanner were covered with a castle façade. In the scanner room, children were accompanied by an experimenter who sat next to them throughout the experiment. The experimenter ensured children were comfortable with the procedure and monitored their compliance to the task demands including minimizing their head motion. An MR-compatible eye tracker, which provided an enlarged image of one eye, was used to monitor alertness during the functional runs.
In addition to the MRI sessions, children completed standardized assessments at three time points using the DIBELS measures described above. DIBELS tests were administered by trained testers who were blind to the grouping of children. After the initial testing for evaluation at the beginning of the fall term, children were tested two more times. These testing times overlapped the time period of MRI Sessions 1 and 2. The mean interval between the two MRI sessions was 69.4 days (9.9 weeks, SD = 12.0 days).
In-scanner task data analysis
Accuracy of the one-back task performance was computed using the following formula: ((hit/total number of repeated trials)-(false alarm/total number of non-repeated trials))*100. The score for chance performance would be 0.
fMRI data acquisition
A 3T Siemens Allegra MRI system with a volume (birdcage) head coil was used for image acquisition. Functional imaging was conducted with a gradient echo, echo planar imaging (EPI) sequence (TR = 2.36 s, TE = 30 ms, flip angle = 90°, field of view (FOV) = 200 × 200 mm (64 × 64 matrix), axial slice thickness = 3.5mm, contiguous 32 slices with interleaved acquisition order). Functional imaging also incorporated Prospective Acquisition Correction (PACE) (Thesen et al., 2000), which adjusts slice position in real time prior to acquisition of each whole brain image compensating for head motion. At the first scanning session, high-resolution T1-weighted gradient echo images were also acquired (MP-RAGE, TR = 2.5 s, TE = 4.38 ms, flip angle = 8°, voxel size = 1 × 1 × 1 mm).
fMRI data processing and analysis
Data processing and analysis were performed using FMRI Expert Analysis Tool (FEAT, v. 5.63), a part of FMRIB's Software Library (FSL; www.fmrib.ox.ac.uk/fsl). The following pre-statistics processing was applied to all fMRI data: motion correction using FMRIB’s Linear Image Registration Tool (Jenkinson et al., 2002); non-brain removal using Brain Extraction Tool (Smith, 2002); spatial smoothing using a Gaussian kernel of 6mm at FWHM; mean-based intensity normalization of all volumes by the same factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 40.0s); and spatial normalization to the Montreal Neurological Institute template (MNI152). Time-series statistical analysis was carried out using FMRIB’s Improved Linear Model with local autocorrelation correction (Woolrich et al., 2001) and including the six motion parameters (x, y, z rotation and translation) as regressors in the model to account for the effects of motion remaining after application of PACE.
Only participants with at least two runs of usable data were included in analysis. The following exclusion criteria were employed in determining data quality: First, individual volumes were examined for motion artifacts. Volumes with estimated remaining head motion greater than 1 mm or with greater than 2.5 % difference in average signal intensity, sampled within a 100 × 100 × 84 mm cuboid around the center of the image, from the mean signal intensity of the run were judged to be contaminated by motion artifacts. Runs with more than 15% of volumes contaminated by motion were excluded from subsequent analyses. All 13 adults had 4 useable runs, and 14 of 18 children had at least 2 useable runs. All four children excluded from the analysis were in the AR group.
The mean number of usable runs and motion for each group at each session and statistical comparison of the groups are summarized in Table 1. The mean number of usable runs was greater for the OT group than for the AR group for both sessions. Neither absolute nor relative distance of head motion in the usable runs differed between the two kindergartener groups at Session 1. However, the head motion of the AR group tended to be greater than that of the OT group at Session 2.
Images from multiple runs from a single session of a single subject were re-sampled to 2 × 2 × 2 mm voxels and analyzed using a fixed-effects model, by forcing the random effects variance to zero. Group-level analyses were carried out for the letter versus false font contrast with a mixed-effects model using FMRIB's Local Analysis of Mixed Effects (stage 1 only) (Beckmann et al., 2003; Woolrich et al., 2004). Given the limited statistical power inherent to fMRI analyses (Yarkoni and Braver, 2010) and the small sample sizes necessitated by the age and reading ability status of the children as well as the longitudinal nature of this study, different statistical thresholds were applied to balance the likelihood of both Type I and Type II errors. To provide as complete a picture as possible of session-level data, a threshold of Z = 1.96 (uncorrected) was applied to data for each group at each session. The statistical images for direct group contrasts was thresholded at Z = 2.57 (uncorrected), and for the Session 1 versus Session 2 contrast within each kindergartener group, the statistical image was thresholded at Z = 2.33 (uncorrected). In all analyses, only clusters with 10 or more contiguous voxels (>= 80 ml) are reported, and peak Z and p-values are provided for all areas reported in the Supplementary Tables. In Figures 2 and 3 the lateral and medial renderings were divided at the MNI coordinates of ×= +/−26. Subcortical activations were not rendered. Anatomical labels for activations were determined by referencing the Harvard-Oxford cortical and subcortical structural atlases, which are based on adult brains (Flitney et al., 2007).
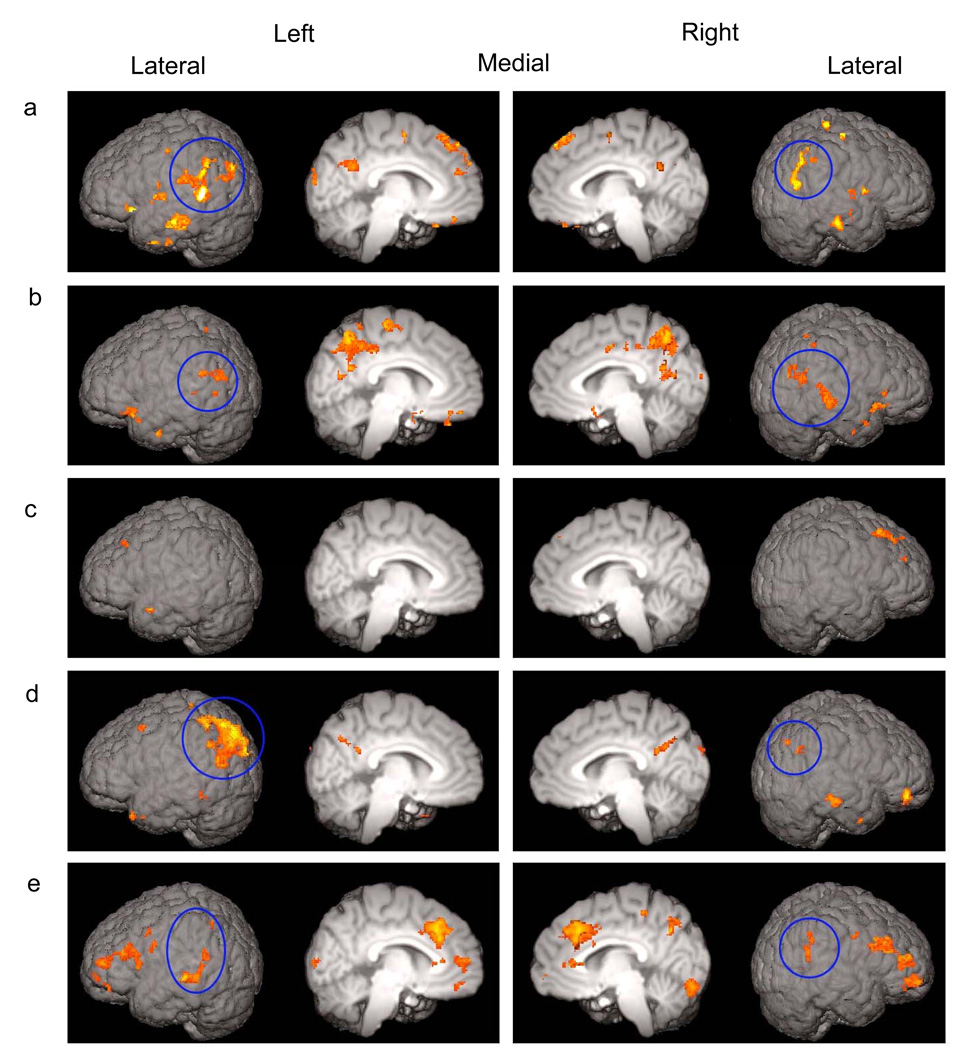
Figure 2.
Letter activations in (a) adults; (b) OT group and (c) AR group at Session 1; and (d) OT group and (e) AR group at Session 2. Suprathreshold (Z > 1.96) voxels for the letter > false font contrast were surface rendered. All regions of activations are listed in Supporting Information - Tables 1–5 online. The activations in the posterior dorsal region are circled. These activations are left lateralized in adults and OT group at Session 2 but bilateral in OT group at Session 1 and AT group at Session 2.
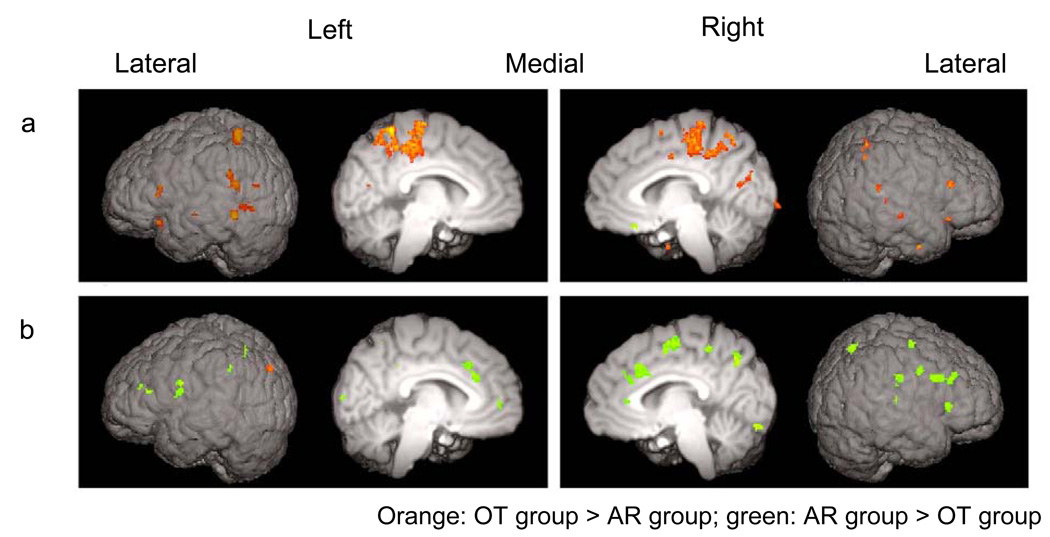
Figure 3.
Comparison between the OT and AR groups at (a) Session 1 and (b) Session 2. Suprathreshold (Z > 2.57) voxels from the direct group comparisons for the letter > false font contrast were surface rendered. For the list of all suprathreshold clusters, see Supporting Information - Tables 6 and 7 online.
The laterality of the spatial extent of activations was examined by counting the number of voxels with z > = 1.96 in the left and right posterior dorsal ROIs for each subject. These ROIs included the posterior STG, anterior and posterior SMG, AG and sLOC. The sLOC was included in view of inconsistencies in labeling of the region anterior to the intraparietal sulcus. This region is typically labeled as the lateral occipital cortex in the Harvard-Oxford atlas. However, the same region is labeled as either the angular gyrus or inferior parietal lobule in other frequently used atlases (Duvernoy et al., 1991; Mai et al., 2004). Areas with >= 5% probability of being one of the above were included in the ROIs. A laterality quotient (LQ) was computed using the following formula: (R−L)/(R+L)*100. The possible range of LQ was from −100 (complete left lateralization) to +100 (complete right lateralization).
Results
Pre-literacy skills
Table 1 presents scores on the Letter Naming Fluency (LNF) and Initial Sound Fluency (ISF) subtests of the Dynamic Indicators of Basic Early Literacy Skills (DIBELS) (Good et al., 2002) for the OT and AR groups at three time points: initial screening, Session 1 (pretest), and Session 2 (post-test).
At the initial screening, conducted immediately after children entered kindergarten and used to classify children as either being on track or at risk, early literacy scores were significantly higher for the OT group as compared to the AR group. At the first fMRI session, which took place after one month of regular kindergarten curriculum, the mean LNF score was still significantly higher for the OT group than for the AR group while the two groups did not differ significantly on ISF. At the second fMRI session, which took place at the end of the first semester, the two groups did not differ significantly on either of the tests.
In-scanner task performance
As we expected, the one-back task with single letters and false fonts were easy enough for both groups of children to perform, with accuracy well above the chance level across sessions and conditions (Session 1: OT: letter, t(6) = 7.5, p < .001; false font, t(6) = 14.1, p < .001; AR: letter, t(6) = 7.7, p < .001; false font, t(6) = 6.2, p = .001; Session 2: OT: letter, t(6) = 8.8, p < .001; false font, t(6) = 12.3, p < .001; AR: letter, t(6) = 10.9, p < .001; false font, t(6) = 6.8, p = .001). As reported in Table 1, there was no significant difference in task performance between the two kindergartener groups. Within each group, accuracy did not differ between sessions for either letter (OT: p = .80; AR: p > .40) or false font (OT: p > .43; AR: p > .89) conditions or between conditions at either Session 1 (OT: p > .14; AR: p > .70) or 2 (OT: p > .30; AR: p > .85). Given the small sample size, a few of these null results may be due to insufficient statistical power. Therefore, potential differences in the in-scanner task performance will be taken into consideration in discussing the fMRI results.
Adults were also scanned while they performed the same task as the kindergarteners did to ascertain whether the one-back task with letters versus false fonts engages the posterior dorsal region of the reading network and establish the pattern of activations in mature skilled readers. Although their task performance was generally more accurate than that of the kindergartener groups, accuracy did not differ between the letter and false font conditions (p > 0.5).
fMRI
Adults
Greater activations to letters relative to false fonts were observed in the left posterior dorsal region of the putative reading network, including the supramarginal gyrus (SMG), angular gyrus (AG), and superior part of the lateral occipital cortex (sLOC) (Figure 2a). Activations in the left SMG extended ventrally to posterior middle temporal gyrus (pMTG). Activations in the left AG extended posteriorly to sLOC. Smaller areas of activations were also found in the right SMG and AG, extending to sLOC. The activations in the posterior dorsal region were more spatially extensive in the left hemisphere than in the right hemisphere (Laterality Quotient [LQ]: M = −25.60, SE = 12.29, t (12) = −2.22, p = 0.047). In addition to these activations, anteriorly a small area in the left frontal orbital cortex (ORB) had greater activation for letters relative to false fonts.
Kindergarteners – Session 1
OT group
Both the posterior dorsal and anterior regions of the reading network in the left hemisphere and the homologous areas in the right hemisphere were engaged more for letters than false fonts in this group (Figure 2b). In the left posterior dorsal region, significantly activated areas occurred in the SMG and AG, extending to the sLOC, and posterior parts of middle and superior temporal gyri (pMTG and pSTG). Furthermore, greater activation to letters relative to false fonts was also found in the right hemisphere homologues in this group. These activations were located in the pSTG extending to the pMTG, AG, SMG, and sLOC near the AG. The extent of activations in this region was similar in the left and right hemispheres, approaching a statistical trend for right lateralization (LQ: M = 14.1, SE = 7.45, t (6) = 1.90, p = 0.11). In the anterior region, the ORB was activated in both hemispheres.
AR group
In contrast to the OT group, very few areas were activated more for letters than for false fonts in this group (Figure 2c). None of these areas was located within the putative reading network. To examine whether the differential neural recruitment for letters and false fonts in the OT group reported above was due to a larger number of runs included in the analysis for this group, an additional analysis for the OT group was performed. In this analysis, the number of runs and motion in the data were matched to those of the AR group, yet the OT group still showed suprathreshold activations to letters as compared to false fonts in the bilateral posterior dorsal region as well as in the ORB. (See Supporting Information - Note and Supporting Information - Figure 1 online for details.)
Comparison between OT group and AR group
Activation for the letter versus false font contrast was significantly greater for the OT group than for the AR group in the areas of the reading network (Figure 3a). Within the posterior dorsal region, left SMG and sLOC and, to a lesser extent, right SMG and pSTG were engaged more in the OT group as compared to the AR group. In the anterior region, the OT group engaged bilateral IFG and ORB more than the AR group did.
Kindergarteners – Session 2
OT group
A large cluster of activation extending from the AG to sLOC was found in the left posterior dorsal region for the letter > false font contrast (Figure 2d). This cluster contained an area near the border between the AG and sLOC which overlapped with the activation found for the same contrast in Session 1. At Session 1, the peak Z voxel within this area was found at the MNI coordinates of x: −42, y: −66, z: 18 (see Table 2 of the Supporting Information). At Session 2, although the peak Z voxel of the entire cluster was located at x: −42, y: −76, z: 46 (see Table 4 of the Supporting Information), a local peak Z voxel (Z = 2.7) in the overlapping area was located at x:−46, y: −64, z: 16. In the right hemisphere, small areas of activation were located near the border between the AG and sLOC. The extent of activations tended to be greater in the left hemisphere as compared to the right hemisphere in the posterior dorsal region at Session 2 (LQ: M = −40.61, SE = 17.07, t (6) = −2.38, p = 0.055). From Session 1 to Session 2, activations for the letter > false font contrast shifted from a more right-hemisphere dominant to left-hemisphere dominant pattern in this region (LQ - Session 1 vs. Session 2: t (6) = 3.78, p = 0.009). The activations in the anterior region observed at Session 1 were reduced to subthreshold.
AR group
The posterior dorsal and anterior regions of the reading network were now recruited for letters versus false fonts (Figure 2e). In the posterior dorsal region, areas of greater recruitment for letters were found bilaterally in the SMG, extending to pMTG, and AG in the left hemisphere and the SMG extending to AG in the right hemisphere. The extent of activations in this region did not differ between the two hemispheres (LQ: M = 8.17, SE = 14.65, t (6) = 0.56, p = 0.60). In the anterior region in the left hemisphere, the large area of activation extending from inferior frontal region to frontal pole included the inferior frontal gyrus (IFG)-pars triangularis and pars opercularis. The activations in the right frontal region were primarily in the middle frontal gyrus and frontal pole. In addition, anterior cingulate (ACC) and paracingulate cortices were activated more for letters as compared to false fonts. Thus, although the recruitment of the bilateral posterior dorsal region in this group at Session 2 was similar to that found in the OT group at Session 1, the AR group additionally recruited large areas in the frontal region including the ACC.
Comparison between OT group and AR group
In contrast to Session 1, significantly greater activation for the letter > false font contrast was revealed in a number of areas for the AR group as compared to the OT group (Figure 3b). Within the posterior dorsal region of the reading network, the AR group engaged the right SMG to a greater degree than the OT group. No suprathreshold group difference (AR > OT) was found in the posterior dorsal region in the left hemisphere. In the anterior region, the engagement of the left IFG and precentral gyrus was significantly greater in the AR group. The engagement of the frontal region, in particular bilateral paracingulate and right ACC and the right middle frontal gyrus, as expected from the within-group results reported above, was significantly greater in the AR group as compared to the OT group. The only area that was engaged to a greater degree in the OT group was in the left sLOC.
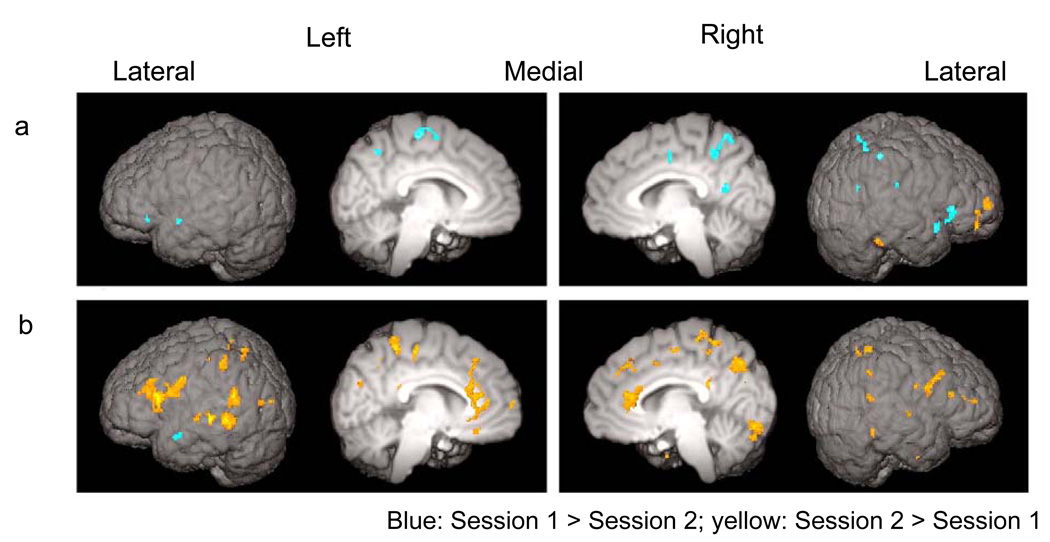
Comparison between Session 1 and Session 2
A direct contrast between Sessions 1 and 2 for the letter > false font contrast was conducted for the OT and AR groups separately. In the OT group, this analysis revealed decreases in activations in areas in the right-hemisphere homologue of the reading network (Figure 4a). These areas included the right SMG as well as bilateral ORB. In contrast, the AR group had more areas showing significant increases than decreases in activation for the same contrast across sessions (Figure 4b). These areas were found in the left-hemisphere reading network and their right hemisphere homologue. Within the posterior dorsal region, increased activation was observed in the left AG, extending to SMG, and pSTG and the right AG and SMG. Within the anterior region, increases were observed in the left IFG and ORB and, to a lesser extent, in the right IFG. A large area of the ACC also exhibited increased activation from Session 1 to Session 2. This area was more anterior ventral to the area of the ACC recruited for the letter > false font contrast in the AR group at Session 2.
Figure 4.
Changes in letter activations from Session 1 to Session 2 in (a) OT group and (b) AR group. Suprathreshold (Z > 2.33) voxels from the direct comparison between Session 1 and Session 2 for the letter > false font contrast were surface rendered. For the list of all suprathreshold clusters, see Supporting Information - Tables 8 and 9 online.
Discussion
The present study provided neuroimaging evidence for the emergence of a reading circuit in five-year-old beginning readers and illustrated both similarities and differences in this process for children on track (OT) in literacy development versus at risk (AR) for later reading difficulty. A single-symbol, one-back task with letter versus false font stimuli was used to track the engagement of the posterior dorsal (phonological) system across the first semester of formal reading instruction.
At the beginning of kindergarten (Session 1), the OT group, with average or higher levels of letter-name knowledge, recruited this phonological system to a greater degree during letter processing relative to false font processing. The increased activity to letters was also found in the homologous region in the right hemisphere. In contrast, children in the AR group, who scored below the 35th percentile on the tests of pre-literacy skills, did not show similar recruitment in spite of equivalent chronological age, socio-economic status, and IQ to the OT group. Thus, these results demonstrated that the different levels of pre-literacy skills with which children enter kindergarten are reflected in differential recruitment of the phonological system in the posterior dorsal region during letter processing.
The AR children in this study started receiving daily supplementary reading instructions after Session 1. When examined at the end of the first semester (Session 2), the AR group, like the OT group, recruited the posterior dorsal system. The recruitment was observed in the right as well as left hemisphere. Thus, in both the AR and OT groups, the recruitment of the posterior dorsal region was bilateral in the early stage of pre-literacy development. In the OT group, this system continued to be engaged at Session 2, but the pattern of engagement had become left lateralized as seen in adults.
Accuracy in the in-scanner task performance was well above the chance level for both the OT and AR groups, indicating that both groups were engaged in and capable of performing the one-back task with the simple stimuli used in this study. Although no significant differences in the accuracy rates were observed between letters and false fonts at either session for either group, nor were there significant differences in accuracy rates between the OT and AR groups in either condition at either session, statistical power was limited in detecting behavioral differences. The OT group overall performed the task more accurately (but not significantly) than the AR group; however, the relative accuracy of task performance between groups and conditions changed little across sessions. Thus, any undetected differences are unlikely to account for the significant differences in fMRI results between the OT and AR groups or the changes in the fMRI results from Session 1 to Session 2 in these groups. Similarly, the mean accuracy rates suggested better performance for the false font relative to the letter stimuli in the OT group. This could indicate that, at this early stage of development, children with better pre-literacy skills may not have a processing advantage for the letter stimuli in terms of performance accuracy – and perhaps even a relative disadvantage. This pattern of behavioral performance could emerge due to phonological confusion with letters, which was absent for false fonts, or interference by not fully automatized retrieval process of phonological information somewhere in the information processing stream before making overt manual responses. However, since the pattern of the task performance was similar across sessions, it is unlikely to account for the shift from bilateral (with a trend for right-lateralization) to left-lateralized recruitment of the posterior dorsal region from Session 1 to Session 2.
The current results converge with the findings from a longitudinal magnetic source imaging (MSI) study of older children with high and low risk for developing reading problems (Simos et al., 2005). In that study, children were examined twice, at the end of kindergarten and at the end of Grade 1, while performing tasks that required mapping letters to phonemes. In the low risk children, the number of activity sources found in the posterior dorsal region was greater in the left than the right hemisphere at both grades. In contrast, no significant hemispheric difference was found in the high-risk group at either grades although the children in this group received reading interventions at Grade 1 and their performance on reading achievement test improved to be within the average range (> 24th percentile). These results raise the question of whether or not the recruitment of the posterior dorsal region in at-risk children, including the AR children in the current study, would become left lateralized as observed in skilled adult readers. The findings from another MSI study with older children (Grades 2 and 3) (Simos et al., 2007) suggest that the recruitment of this region may eventually become more adult-like (i.e., left lateralized) in high-risk children if they benefit sufficiently from interventions.
In the current study, the OT group initially recruited the posterior dorsal system bilaterally. By Session 2, the engagement of the right-hemisphere homologue was significantly reduced, especially in the right SMG. Together with previous findings in which a higher level of reading ability was associated with disengagement of the right hemisphere homologue of the posterior ventral region of the reading network (Shaywitz et al., 2002; Turkeltaub et al., 2003), these results provide further evidence that the development of the reading network involves disengagement of the right hemisphere homologous regions including the posterior dorsal region. The current results also suggest that the engagement of the right, in addition to the left, posterior dorsal region is a part of normal early literacy development.
The role of the right-hemisphere homologous region during the early stages of literacy development has not been investigated systematically. In several studies reporting involvement of the right-hemisphere homologue in adults and children with dyslexia or at risk for reading problems, this recruitment was speculated to be a compensatory mechanism for the insufficient engagement of the left posterior dorsal system (Eden et al., 2004; Pugh et al., 2000; Rumsey et al., 1999; Simos et al., 2005). Indeed, the right hemisphere homologue may play a similar role in young typically developing children and those with dyslexia. Concomitant changes in cortical activation with skill acquisition have been well documented (Kelly and Garavan, 2005). Petersen and colleagues (1998) proposed that the regions that show greater activity during initial unskilled performance are recruited to cope with task demands (i.e., “scaffolding”). For instance, in the motor cortex where unimanual finger movements activate the region contralateral to the hand used, activity in the ipsilateral region has been reported during the performance of complex and untrained pattern of finger movements (Verstynen et al., 2005). In the language domain, a shift from a bilateral to left-lateralized pattern of recruitment during auditory word comprehension has been reported in infants from 13 to 20 months in age (Mills et al., 1997; Mills et al., 1993). Bilateral or greater right hemisphere recruitment has also been reported in groups of adults with less expertise in a particular domain, including late learners (Pakulak and Neville, in press; Weber-Fox and Neville, 1996) and low proficiency native speakers (Pakulak and Neville, 2010) during language processing and deaf signers during written English processing (Neville et al., 1998). Therefore, the right hemisphere homologue of the posterior dorsal region of the reading network may be a scaffolding mechanism that is recruited in unskilled readers including both individuals with dyslexia and emerging readers. Compared to normal readers, adults and children with dyslexia show hypoactivation in the posterior dorsal region in the left hemisphere (Aylward et al., 2003; Eden et al., 2004; Hoeft et al., 2006; Meyler et al., 2008; Shaywitz et al., 1998; Temple et al., 2001). Therefore, the lack of engagement of the left posterior dorsal system seems to be a hallmark of reading disability, and the greater engagement of the right posterior dorsal region may be a consequence of the insufficient engagement of the left hemisphere system either due to immaturity or disability.
By Session 2, the AR group’s pre-literacy skills measured by DIBELS were comparable to those of the OT group at Session 1. Similarly, the AR group at Session 2 recruited the posterior dorsal region bilaterally as did the OT group at Session 1. With respect to the pattern of recruitment of the left hemisphere posterior dorsal system, the AR group appeared to be following the normal, but delayed, developmental trajectory in light of the evidence on older children and adults with developmental dyslexia that under-activation of this system does not ultimately catch up. However, the whole-brain pattern of activations of the AR group showed evidence of atypical recruitment not seen in the OT group, even at the earlier time point. In addition to the bilateral posterior dorsal regions, the AR children also recruited large areas in the frontal lobe including the left IFG, a part of the anterior system of the reading network, and the ACC.i An increase in the recruitment of the bilateral or right IFG and ACC after intervention has been reported in several studies (Meyler et al., 2008; Shaywitz, 2003; Simos et al., 2007; Temple et al., 2003). The areas of the IFG reported in these studies are found in the posterior part sometimes extending to the premotor cortex. This area is involved in articulatory recoding and has been hypothesized to be one of the compensatory mechanisms recruited when the left posterior dorsal region does not function sufficiently in individuals with dyslexia (Pugh et al., 2001). ACC activity has been associated with modulation of attention or executive functions, motivation, and working memory (Bush et al., 2000). This structure is also reciprocally connected to lateral prefrontal cortex (Bush et al., 2000), which was also recruited in the AR children at Session 2. The increase in activity levels in these regions may reflect increases in attentional or motivational allocation to letters, which was encouraged throughout the supplemental reading intervention they received. These compensatory mechanisms may be eventually disengaged as the AR children acquire greater reading abilities.
In addition to entering kindergarten with less letter knowledge, there was evidence to suggest that children in the AR group also had poorer attention and self-regulation skills. For example, children in the AR group had significantly fewer fMRI runs usable for analysis due to excessive motion in some runs. Furthermore, the four children who were excluded from analyses due to excessive motion were all from the AR group. To stay still during scanning requires the abilities to monitor and regulate one’s body movement, which is a component of attention and self-regulation (Rueda et al., 2005). Attention and self-regulation have been reported to be a significant predictor of future academic achievement, including reading, in young children (Blair, 2002; Blair and Razza, 2007; Duncan et al., 2007; Lewit and Schurrman Baker, 1995; McClelland et al., 2000). A study conducted in our lab (Stevens et al., in press) found that, upon entrance to kindergarten, these same at-risk children did not display the typical effects of attention on event-related potentials (ERPs), whereas the OT children did. However, following the first semester of kindergarten plus the intervention described above, the AR children did display the typical effects of attention, as did the OT children. Therefore, this is consistent with the hypothesis that the AR children in this study might have been at risk for later reading difficulties in part because they had difficulties in self-regulation and attention.
Unlike the majority of the neuroimaging studies on reading in which words were presented visually, the current study used single letters. Using single symbol stimuli, as compared to strings of symbols, is likely to reduce working memory load in performing a one-back task, especially for pre-reading children. This point was particularly important in the current study since the task needed to be equally easy for five-year old children with different pre-literacy skills to perform so that all children would be engaged in, but not overwhelmed by, the task. Using these stimuli, differential recruitment of the posterior dorsal region for letter processing was revealed between children with higher and those with lower pre-literacy abilities. However, since the stimuli employed were single letters and letter knowledge alone cannot be equated with the ability to engage in word-level reading, this raises the question as to whether the neural circuits recruited for letter processing in this study could be considered as an early reading network.
Maurer and colleagues (2006), for example, hypothesize that the different patterns of hemispheric lateralization indexed by the ERP N1 effects for words at different ages reflect different states of the underlying neural systems. Using a similar one-back task, they examined the changes in the specialization of the N1 component for words in pre-reading children in kindergarten and in the same children in the 2nd grade. The N1 component elicited over the posterior scalp region in adult readers has been reported to be larger and left-lateralized for words as compared to strings of symbols, and the source of this component has been localized in the posterior ventral temporal region near the visual word form area (Maurer et al., 2005). In kindergarten, the N1 word effect was found over the right-hemisphere scalp region only in children with higher letter knowledge. When the same children were tested in the 2nd grade, the N1 enhancement for words was found over the bilateral regions and correlated with reading speed. Based on these results, they hypothesized that the right-lateralized N1 effect, reflecting letter knowledge or familiarity with words, indicates a precursor state which is distinct from the later, more reading-specific state indexed by the bilaterally distributed N1 effect correlating with reading speed. Thus, the neural circuits revealed in the letter condition in the current study may be an early, precursor state of the reading network. Longitudinal follow-up of these children as they acquire word-level reading skills would provide evidence bearing on this hypothesis.
In this study, we did not find greater recruitment for letters versus false fonts in the posterior ventral temporal region (the “visual word form area”) in any of the groups, including adults. In adults, single letters may not be the optimal stimuli to recruit this area. Some studies with adults report that visually presented single letter processing does not activate the word form area but instead activates distinct areas that are more anterior and/or lateral to the visual word form area (Flowers et al., 2004; James et al., 2005). In contrast, recent studies with young children report differential activations to single letters in the visual word form area for dyslexic and non-dyslexic children or for typically developing pre-schoolers before and after a sensori-motor training (i.e., practice writing letters and words) (Blau et al., 2010; James, 2010). Children in the present study did not show greater activations in this region for the letters versus false font contrast. This result may indicate that the children in this study have not acquired sufficient expertise in visual letter processing. This result may also be attributed to the fact that the false font stimuli were constructed by rearranging the parts of corresponding letter stimuli and thus had visual features that were highly similar to the letter stimuli. The conflicting results from previous adult and child studies may suggest that the way that single letters are processed in the posterior ventral temporal region changes as the unit of processing shifts from single letters (i.e., letter-by-letter processing) in emerging readers to string of letters (i.e., word-level processing) in fluent readers. Future work examining how single letters and words are processed within the same individuals with different levels of reading proficiency would contribute a clearer picture of such developmental changes.
Since, from the perspective of evolution, reading is a recently developed ability, it has been hypothesized that some aspects of processing involved in reading are performed by the same or overlapping brain structures that perform non-reading tasks. For example, this possibility has been raised for the visual word form area (McCandliss et al., 2003). An important next step in future research would be to examine the degree of specificity of the pattern of the neural recruitment observed in this study to linguistic materials. In particular, it will be important to investigate whether the recruitment of the posterior dorsal region is exclusive for the visual-auditory conversion of linguistic input such as visually presented letters and words or is a part of the more general system also serving in the processing of non-linguistic visual input such as pictures of nameable objects (versus non-nameable figures).
The current study revealed the emergence of the neural circuit supporting early literacy development during the first semester of kindergarten, both in children on track for reading development and in children at risk for reading difficulties. The left posterior dorsal system involved in phonological processing during reading was recruited in children entering kindergarten with well-developed pre-literacy skills. The task employed in this study allowed us to investigate the emergence of the reading network in children who had not yet acquired the ability to engage in word-level reading, thus taking the first step toward identifying which subregions and related mechanisms of the reading network are under-recruited and which additional regions and mechanisms may be recruited in pre-reading at-risk children and raising specific hypotheses about which aspects of the reading process to target in early interventions.
Supplementary Material
Acknowledgments
We would like to thank L. Sabourin, S. Klein, S. Watrous, J. Fanning and D. Parisi for their contribution in data collection. We thank G. Eden for her comments on the manuscript. We also thank our participants and the Bethel School District in Eugene, Oregon for their assistance. This study was supported by Grant Number R01 DC000481 from NIH/NIDCD and the Center for Teaching and Learning at the University of Oregon. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIDCD, NIH, or the Center for Teaching and Learning, University of Oregon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
An anonymous reviewer suggested that an additional comparison of the OT group at Session 1 and the AR group at Session 2 might be informative in examining whether the children in the AR group were simply delayed or deviant in the development of the reading network. This contrast did not reveal significant hypoactivation in the left posterior dorsal region (Z = 2.57, the same threshold used for all between-group contrasts), suggesting that the development of this region was delayed in the AR children. However, the analysis revealed a number of areas in the frontal region, including bilateral frontal poles, bilateral middle frontal gyri, and right anterior cingulated cortex, showing greater activations in the AR group, suggesting the recruitment of compensatory mechanisms.
References
- Aylward EH, Richareds TL, Berninger VW, Nagy WE, Field KM, Grimme AC, Richards AL, Thomson JB, Cramer SC. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Blair C. School Readiness: Integrating cognition and emotion in a neurobiological conceptualization of children's functioning at school entry. American Psychologist. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza R. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Blau V, Reithler J, van Atteveldt N, Seitz J, Gerretsen P, Goebel R, Blomert L. Deviant processing of letters and speech sounds as proximate cause of reading failure: a functional magnetic resonance imaging study of dyslexic children. Brain. 2010;133:868–879. doi: 10.1093/brain/awp308. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Byrne B, Fielding-Barnsley R. Evaluation of a program to teach phonemic awareness to young children: A 2- and 3-year follow-up and a new preschool trial. Journal of Educational Psychology. 1995;87:488–503. [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex. 2008;18:2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff M-A, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Duncan G, Claessens A, Huston A, Pagani L, Englel M, Sexton H, Dowsett C, Magnuson K, Klebanov P, Feinstein L, Brooks-Gunn J, Duckworth K, Japel C. School readiness and later achievement. Developmental Psychology. 2007;43:1428–1446. doi: 10.1037/0012-1649.43.6.1428. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Cabanis EA, Iba-Zizen MT, Tamraz J, Guyot J. Three-Dimensional Sectional Anatomy and MRI. New York: Springer-Verlag Wien; 1991. The Human Brain: Surface. [Google Scholar]
- Dynamic Measurement Group. DIBELS 6th Edition Technical Adequecy Information (Tech. Rep. No. 6) Eugene, OR: Dynamic Measurement Group; 2008. [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NAE, Agnew JA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Flitney D, Webster M, Patenaude B, Seidman L, Goldstein J, Gutierrez DT, Eickhoff S, Amunts K, Zilles K, Lancaster J, Haselgrove C, Kennedy D, Jenkinson M, Smith S. Anatomical brain atlases and their application in the FSLView visualisation tool. Thirteenth Annual Meeting of the Organization for Human Brain Mapping. 2007 [Google Scholar]
- Flowers DL, Jones K, Noble K, VanMeter J, Zeffiro TA, Wood FB, Eden GF. Attention to single letters activates left extrastriate cortex. Neuroimage. 2004;21:829–839. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Foulin JN. Why is letter-naming knowledge such a good predictor of learning to read? Reading and Writing. 2005;18:129–155. [Google Scholar]
- Good RH, Gruba J, Kaminiski R. Best practices in using dynamic indicators of basic early literacy skills (Dibels) in an outcomes-driven model. In: Thomas A, Grimes J, editors. Best Practices in School Psychology. Washington DC: National Association of School Psychologists; 2002. pp. 699–720. [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, Whitfield-Gabrieli S, Gabrieli JDE. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. Journal of Neuroscience. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, Connecticut: Yale University; 1975. [Google Scholar]
- James KH. Sensori-motor experience leads to changes in visual processing in the developing brain. Developmental Science. 2010;13:279–288. doi: 10.1111/j.1467-7687.2009.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong AC-N, Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kame'enui E, Simmons D. Early Reading Intervention. Scott-Foresman Publishing. 2003 [Google Scholar]
- Kelly AMC, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cerebral Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Lewit EM, Schurrman Baker L. School readiness. The Future of Children. 1995;5:128–139. [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Maurer U, Brem S, Bucher K, Brandeis D. Emerging neurophysiological specialization for letter strings. Journal of Cognitive Neuroscience. 2005;17:1532–1552. doi: 10.1162/089892905774597218. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Kranz F, Bucher K, Benz R, Halder P, Steinhausen H-C, Brandeis D. Coarse neural turning for prints peaks when children learn to read. NeuroImge. 2006;33:749–753. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaeane S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McClelland MM, Morrison FJ, Holmes DL. Children at risk for early academic problems: The role of learning-related social skills. Early Childhood Research Quarterly. 2000;15:327–329. [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JDE, Just MA. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: a longitudinal study of neuroplasticity. Neuropsychologia. 2008;46:2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DL, Coffey-Corina S, Neville HJ. Language comprehension and cerebral specialization from 13 to 20 months. Developmental Neuropsychology. 1997;13:397–445. [Google Scholar]
- Mills DL, Coffey-Corina SA, Neville HJ. Language acquisition and cerebral specialization in 20-month-old infants. Journal of Cognitive Neuroscience. 1993;5:317–334. doi: 10.1162/jocn.1993.5.3.317. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Bavelier D, Corina D, Rauschecker J, Karni A, Lalwani A, Braun A, Clark V, Jezzard P, Turner R. Cerebral organization for language in deaf and hearing subjects: Biological constraints and effects of experience. Proceedings of the National Academy of Sciences. 1998;95:922–929. doi: 10.1073/pnas.95.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakulak E, Neville H. Maturational constraints on the recruitment of early processes for syntactic processing. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21586. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakulak E, Neville H. Proficiency differences in syntactic processing in native speakers indexed by event-related potentials. Journal of Cognitive Neuroscience. 2010;22:2728–2744. doi: 10.1162/jocn.2009.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, Frith CD. Is developmental dyslexia a disconnection syndrome? evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Mier HV, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proceedings of the National Academy of Sciences. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and developmental disabilities research reviews. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Rueda M, Posner MI, Rothbart MK. The development of executive attention: contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King C, Hamburger SD, Pikus A, Rapoport JL, Cohen RM. Failure to activate the left temporoparietal cortex in dyslexia: an oxygen 15 positron emission topographic study. Archives of Neurology. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace KL, Maisog JM, Andreason P. A functional lesion in developmental dyslexia: left angular gyral blood flow predicts severity. Brain and Language. 1999;70:187–204. doi: 10.1006/brln.1999.2158. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Paul A. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarskie P, Mencl WE, Constable RT, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Overcoming Dyslexia: A New and Complete Science-based Program. New York: Alfred A. Knopf; 2003. [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, Davis RN, Fitzgerald M, Papanicolaou AC. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Denton C. Altering the brain circuits for reading through intervention: a magnetic source imaging study. Neuropsychology. 2007;21:485–496. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Francis DJ, Castillo EM, Pataraia E, Denton C, Papanicolaou AC. Early development of neurophysiological processes involved in normal reading and reading disability: a magnetic source imaging study. Neuropsychology. 2005;19:787–798. doi: 10.1037/0894-4105.19.6.787. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Harn H, Chard D, Currin J, Parisi D, Neville H. Examining the role of attention and instruction in at-risk kindergarteners: Electrophysiological measures of selective auditory attention before and after an early literacy intervention. Journal of Learning Disabilities. doi: 10.1177/0022219411417877. in press. [DOI] [PubMed] [Google Scholar]
- Temple E. Brain mechanisms in normal and dyslexic readers. Current Opinion in Neurobiology. 2002;12:178–183. doi: 10.1016/s0959-4388(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proceedings of the National Academy of Sciences. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospestive acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- van Atteveldt N, Formisano E, Goebel R, Blomert L. Integration of letters and speech sounds in the human brain. Neuron. 2004;43:271–282. doi: 10.1016/j.neuron.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements related to task complexity. Journal of Neurophysiology. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- Weber-Fox C, Neville HJ. Maturational constraints on functional specializations for language processing: ERP and behavioral evidence in bilingual speakers. Journal of Cognitive Neuroscience. 1996;8:231–256. doi: 10.1162/jocn.1996.8.3.231. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Braver T. Cognitive neruroscience approaches to individual differences in working memory and executive control: Conceptual and methodologial issues. In: Gruszka A, Matthews G, Szymura B, editors. Handbook of Individual Differences in Cognition. New York: Springer; 2010. pp. 87–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.