Abstract
Numerous animal studies have demonstrated that ultrasound bursts combined with a microbubble-based ultrasound contrast agent can temporarily disrupt the blood-brain barrier (BBB) with little or no other apparent effects to the brain. As the BBB is a primary limitation to the use of most drugs in the brain, this method could enable a noninvasive means for targeted drug delivery in the brain. This work investigated whether BBB disruption and vessel damage when overexposure occurs can be influenced by choice of anesthesia protocol, which have different vasoactive effects. Four locations were sonicated transcranially in each brain of 16 rats using an unfocused 532 kHz piston transducer. Burst sonications (10 ms bursts applied at 1 Hz for 60 s) were combined with intravenous Definity (10 μl/kg) injections. BBB disruption was evaluated using contrast-enhanced MRI. Half of the animals were anesthetized with i.p. ketamine and xylazine, and the other half with inhaled isoflurane and oxygen. Over the range of exposure levels tested, MRI contrast enhancement was significantly higher (P<0.05) for animals anesthetized with ketamine/xylazine. Furthermore, the threshold for extensive erythrocyte extravasation was lower with ketamine/xylazine. These results suggest that BBB disruption and/or vascular damage can be affected by vascular or other factors that are influenced by different anesthesia protocol. These experiments may also have been influenced by the recently reported findings that the circulation time for perfluorocarbon microbubbles is substantially reduced when oxygen is used as the carrier gas.
Keywords: Ultrasound, brain, blood-brain barrier, drug delivery, anesthesia
INTRODUCTION
Numerous studies have shown that ultrasound bursts combined with a microbubble-based ultrasound contrast agent (USCA) can temporarily disrupt the blood-brain barrier (BBB) in animals (Hynynen et al. 2001; Hynynen et al. 2006; Choi et al. 2007; Liu et al. 2009; Xia et al. 2009). This barrier, which normally serves to protect the brain, is one of the primary hurdles to the effective delivery of drugs to the central nervous system (Pardridge 2007). With an ability to target BBB disruption at desired locations combined with the development of systems that can effectively focus ultrasound through the human skull (Clement and Hynynen 2002; McDannold et al. 2010), a noninvasive method to target drug delivery in the brain may be possible. This technology could enable the use of existing drugs that are currently ineffective in the brain, allow for the development of new drugs without the restraints imposed by the BBB, and make possible the targeted delivery of drugs to desired brain regions, reducing systemic toxicity.
The exact physical and/or physiological mechanisms through which the interactions between the ultrasound field, the microbubbles, and the brain microvasculature result in BBB disruption are not known. Existing data suggest that bulk heating (Hynynen et al. 2001) and inertial cavitation (McDannold et al. 2006; Tung et al. 2010) are not required for the disruption. Excluding these effects, it is likely to be the result of mechanical forces impinged on the blood vessel walls, such as that produced by radiation force on the microbubbles (Zheng et al. 2007), bubble oscillation (stable cavitation) (Qin and Ferrara 2006; Zhong et al. 2001), or shear stress induced by acoustic microstreaming of the fluid around the bubbles (Collis et al. 2010).
These mechanical interactions are likely to be influenced by the underlying state of the local vasculature. Vascular parameters such as blood flow, blood pressure, and vessel dilation/constriction may all have an impact on these interactions. Changes in these factors could potentially alter the local bubble concentration, change vessel compliance, increase or decrease the distance between the microbubbles and the blood vessel walls, or change the dynamics of bubble oscillation over time. The constraints imposed by small vessels, as well as vessel compliance, may also influence the bubble oscillation. Simulation studies have suggested that the diameter and elastic properties of small vessels influence the resonant frequency of the bubble oscillation (Sassaroli and Hynynen 2005; Martynov et al. 2009) and that the diameter influences the inertial cavitation threshold (Sassaroli and Hynynen 2007).
Physiological effects may also be involved in the BBB disruption produced by the ultrasound-induced mechanical stimulation and could be influenced by the underlying vascular state. Electron microscopy evaluations have shown that circulating tracers leak out of vasculature both passively through widened tight junctions that exist between endothelial cells in the brain, and actively across endothelial cells through vesicular trafficking (Sheikov et al. 2008; Sheikov et al. 2006). Other work with in vivo multiphoton microscopy has shown that temporary vasospasm can occur during the ultrasound bursts used for BBB disruption (Raymond et al. 2007). While the role of these responses in the BBB disruption is not known, these works suggest that the sonications may not be simply physically modifying the vessel walls, but that physiological factors may also play an important role.
Anesthesia agents are known to induce different vasoactive effects, such as vasodilation/vasoconstriction and changes in blood flow, and these effects might influence the BBB disruption. Such changes could influence the local bubble concentration, the ultrasound/bubble interactions, or how the vasculature responds to the mechanical stimulation. They could also influence the amount of agent that leaks from the blood stream after sonication. Indeed, prior research testing intraarterial infusion of hyperosmotic substances such as mannitol to disrupt the BBB has found that the amount of drug delivered to the brain can depend on anesthesia agent (Gumerlock and Neuwelt 1990; Remsen et al. 1999).
Two recent papers have also shown that the medical gas used with isoflurane can have a profound effect on how long microbubbles circulate in the bloodstream. In one study, the decay rate on time-intensity curves measured with ultrasound imaging was almost three times higher when oxygen was used as the carrier gas for isoflurane than when medical air was used (Itani and Mattrey 2011). Another study also found a statistically significant improvement in persistence in microbubble circulation with medical air compared to oxygen as the carrier gas (Mullin et al. 2011). These differences are likely due to gas diffusion considerations and to the role that nitrogen plays in stabilizing the microbubbles (Mullin et al. 2011; Itani and Mattrey 2011).
The purpose of this study was to investigate whether anesthesia protocol can influence BBB disruption and vessel damage induced by ultrasound bursts combined with an ultrasound contrast agent. In experiments in rats, the BBB disruption and onset for extensive erythrocyte extravasation in animals anesthetized with ketamine/xylazine was compared to that in animals anesthetized with isoflurane/oxygen. BBB disruption was evaluated with contrast-enhanced MRI and leakage of Trypan blue. A secondary goal of this work was to evaluate whether the BBB disruption can be achieved reliably with a low-frequency planar transducer that might be useful for disrupting larger volumes to facilitate drug delivery tests in animal tumor models.
MATERIALS AND METHODS
Animals
The experiments were approved by our institutional animal care and use committee. Sonications were targeted at two locations in each hemisphere in the brains of male rats (approximately 250 g) as described previously (Treat et al. 2007). The animals were divided into two groups based on anesthesia. One group of 8 animals was anesthetized with i.p. injection of a cocktail of ketamine (90 μg/kg) and xylazine (10 μg/kg) administered hourly or as needed. The other 8 animals were anesthetized with 2% isoflurane and oxygen through a nosecone. Respiratory rate was continuously monitored throughout the procedure and used to modify the isoflurane delivery between 1–2%. Before the experiments, the animals were anesthetized, the fur on the scalp was removed with clippers and depilatory cream (Nair, Church & Dwight Co., Inc., Princeton, NJ), and a catheter was placed in the tail vein.
Equipment
An air-backed piston transducer with a diameter of 3 cm operating at 532 kHz generated the ultrasound field. In order to use this transducer inside a small animal MRI system (see below) and to not operate in the near-field of the transducer where the acoustic pressure field is spatially inhomogeneous, the ultrasound beam was transmitted through a 16 mm diameter circular aperture constructed of 6 mm thick rubber. The resulting acoustic pressure distribution in water is shown in Figure 1. This distribution was measured with a needle hydrophone (spot diameter 0.2 mm, Precision Acoustics, Dorchester, UK) at the Fresnel distance of the transducer (approximately 2 cm from the face of the rubber) in a plane perpendicular to the ultrasound beam direction. In the animal experiments, this distance was approximately 5 mm from the brain surface. Here, the beam half-width of the pressure amplitude was measured to be approximately 2 cm. The acoustic field in water at other depths was estimated using planar projection (Clement and Hynynen 2000). The efficiency of the transducer was measured with a radiation force balance consisting of an absorbing brush target and a digital scale (model XS205, Mettler Toledo, Columbus, OH, USA). The spatial peak, temporal peak acoustic intensity (ISPTP) was estimated at the Fresnel distance using the acoustic field distribution and the acoustic power (Herman and Harris 1982). The spatial peak, temporal peak pressure amplitude (PSPTP) was estimated from ISPTP assuming plane waves (i.e. assuming ISPTP = PSPTP2/2ρc, using ρ=998.2 kg/m3 and c=1498 m/s). This estimate was confirmed using a using a 4 mm diameter calibrated hydrophone (model TC4038, Reson, Slangerup, Denmark). The measurements made with the calibrated hydrophone were 3–5% lower than those estimated with the acoustic field maps, perhaps due to a small amount of inhomogeneity in the acoustic field around over the dimensions of the hydrophone.
Figure 1.
Plots of the normalized acoustic pressure distribution for the 532 kHz unfocused transducer. A rubber absorber with a circular aperture was used to move the Fresnel distance to approximately 2 cm away from the rubber. This aperture enabled the transducer to be operated within the animal MRI system with the brain in this region and not in the near field. (A): Map of the pressure distribution perpendicular to the beam direction at this depth. Contours at 10 to 50% of the peak pressure amplitude are shown. (B): Map along the direction of beam propagation. (C) and (D): Pressure distributions perpendicular and parallel to the beam direction. Lines through the peak and at ±0.7 and 1.3 mm away are shown.
The insertion loss for the rat skull was measured using the calibrated hydrophone. Each skull sample was approximately 1 cm2 and was placed 1–3 mm in front of the hydrophone. The pressure amplitude was measured with and without the skull to calculate the insertion loss. The skull sample was repositioned (displaced a few mm and rotated by a few degrees to mimic small differences in the rat positioning between experiments), and this measurement was repeated. Overall, measurements were obtained in six locations in each of three formalin-fixed skull samples. The average drop in pressure amplitude was 25% ± 10%. These measurements are in agreement with a 27% insertion loss reported for the rat skull at 558 kHz (O’Reilly et al. 2010).
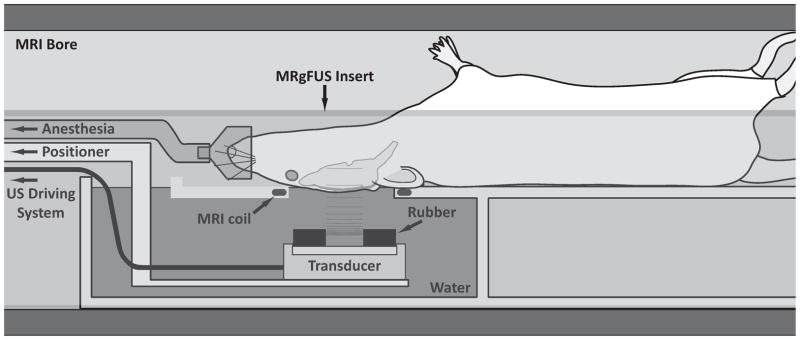
The sonications were performed in a 33 cm diameter Bruker Biospec 4.7 T MRI scanner (Bruker Biospin, Ettlingen, Germany) equipped with a 12-cm actively shielded gradient set that operates at 20 G/cm. Images were obtained using an elliptical (4 ×5.5 cm) transmit/receive surface coil, which was constructed in-house. This coil was integrated in a focused ultrasound insert constructed in-house for this MRI. This insert, which was constructed from a plastic pipe cut lengthwise, contained a small water tank and a platform for the rat to lay supine with its head placed within the opening of the surface coil. The transducer was attached to a MRI-compatible, manually-operated 3-axis positioning system which was coupled to the end of the insert and located at the opening of the MRI bore. The beam fired upward through the surface coil into the brain. The experimental setup is diagrammed in Figure 2. Intravenous injections of ultrasound and MRI contrast agents into the tail vein were made with the animal within the MRI scanner via extension tubing.
Figure 2.
Experimental setup.
Sonications
Burst sonications (10 ms bursts applied at 1 Hz for 60 s) were delivered transcranially at acoustic power levels ranging from 1.1–6.6 W. The burst parameters were determined from prior studies (McDannold et al. 2008b; Chopra et al. 2010); the acoustic power was determined in a pilot study with this transducer (data not shown). The acoustic power levels corresponded to estimated peak intensities and pressure amplitudes in water of 0.5–3.2 W/cm2 and 0.13–0.31 MPa, respectively. The highest exposure level was not tested in the ketamine/xylazine animals because extensive erythrocyte extravasation was observed at lower values (see below). Sonication was synchronous to an I.V. bolus injection of an ultrasound contrast agent (Definity, Lantheus Medical Imaging, N. Billerica, MA) at a dosage of 10 μl/kg (diluted 10x in PBS). Four locations were targeted in each animal, with two sonications of equal power delivered to each hemisphere. The targets were centered in the thalamus and in the putamen and were selected based on geometrical considerations. These locations are near the midline (so the overlying skull was relatively flat and normal to the ultrasound beam propagation), and they present relatively large structures that do not include large blood vessels or ventricles. While this targeting resulted in non-overlapping spots of BBB disruption of 3–6 mm in diameter, it is possible that cumulative effects occurred due to the comparatively large diameter of the ultrasound beam. In addition, effects from reflections or standing waves (see Results) may have influenced in situ pressure amplitude, and thus the BBB disruption, at any particular location. However, since the sonications in the two anesthesia groups were delivered identically, it is unlikely that the outcome was influenced by these factors.
MRI
MRI procedures were similar to previous studies (Hynynen et al. 2001; Hynynen et al. 2006; McDannold et al. 2008a; McDannold et al. 2008b). Before each experiment, the location of the ultrasound beam in the MRI coordinate-space was found visualizing ultrasound-induced heating a silicone phantom in a T1-weighted fast spin echo (FSE) images. Then the animal was placed on the focused ultrasound system, and standard MR imaging sequences were used to select the brain targets. Contrast-enhanced T1-weighted FSE images (TR/TE: 500/18.3 ms; echo train length: 4; field of view: 6 cm; matrix: 256×256, slice thickness: 1.5 mm; bandwidth: ± 32 kHz; 2 averages) were used to evaluate the BBB disruption. Images were acquired before and after administration of an MRI contrast agent (Magnevist®, Berlex Laboratories, Inc., Wayne, NJ, USA) at a dose of 0.125 mmol per kg of body weight as a bolus injection through the tail vein.
Histology
Within 1–2 hours of the last sonication, the animals were injected with Trypan blue, a vital dye used to visualize the BBB disruption after euthanasia. 0.08g Trypan blue powder (MP Biomedicals, Solon, OH, USA) was dissolved in 2.5 ml of 0.45% NaCl and heated until boiling. This solution was then passed through a filter (MILX GV.22UM PVDF, Millipore Cor. Bedford, MA, USA) and then slowly injected intravenously at a dose of 0.1 g Trypan Blue per kg of body weight (Bakay et al. 1956).
Four hours after the sonications, the animals were deeply anesthetized with ketamine/xylazine, sacrificed, and the brains fixed via transcardial perfusion (0.9% NaCl, 250 mL; 10% buffered formalin phosphate, 500 mL). The brains were removed and placed in 10% buffered formalin phosphate for immersion fixation. The brains were cut into four approximately 2 mm blocks with a rat brain matrix (model RBM-4000DV, ASI Instruments, Warren, MI, USA). Macrophotographs were obtained for both sides of each block. The sonicated locations were categorized using these photographs as having (i) no or minimal or (ii) extensive extravasation of erythrocytes. Blocks from representative examples of these categories and for the two anesthesia agents were then embedded in paraffin and serially sectioned at 5 μm. Every 50th section (every 250 μm) was stained with hematoxylin and eosin (H&E) for light microscopy evaluation. The author who evaluated the histology was blind to the exposure parameters and anesthesia group. The histology procedures were similar that used in prior studies by our group (Hynynen et al. 2001; Hynynen et al. 2006; McDannold et al. 2008a; McDannold et al. 2008b).
Data and statistical analysis
In each brain, a 5×5 voxel region of interest (ROI) was defined at each targeted location in a horizontal contrast-enhanced T1-weighted image (perpendicular to the direction of the ultrasound beam). For each ROI, the percent change in signal intensity relative to the pre-contrast imaging signal intensity was found. An additional 5×5 voxel ROI in each hemisphere was selected in non-enhancing brain regions. The percent increase in this ROI was subtracted from the value obtained in the targeted location to exclude any MR signal enhancement from the contrast agent within the vasculature.
The mean MRI signal enhancement (± standard deviation) was calculated for each exposure level for the two anesthesia protocols. To determine whether the greater enhancement evident in the ketamine/xylazine animals compared to the isoflurane/oxygen animals was significant, a one-tailed unpaired student’s t-test was used. Equal variances were not assumed in the comparisons, and P<0.05 was considered significant. A normal distribution for each test group was confirmed with a Lilliefors test. To confirm that the anesthesia agents themselves were not affecting the BBB, the percent increase in the non-targeted regions were compared for the two anesthesia groups using an unpaired, two-tailed student’s t-test.
RESULTS
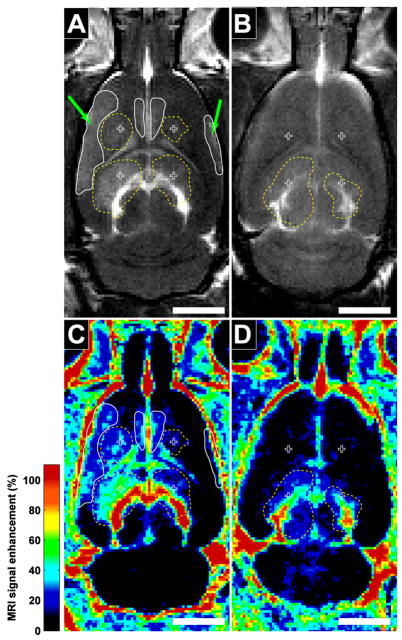
With the unfocused transducer, approximately 3–6 mm diameter regions with BBB disruption were observed as enhancing regions in contrast-enhanced MRI (Figure 3). Substantially more enhancement in the ketamine/xylazine animals was clearly evident at each exposure level tested. In addition to the contrast enhancement at the target locations, the brain ventricles were also found to be enhancing as a result of the sonication, and this enhancement was generally at a substantially higher magnitude than the enhancement in the brain parenchyma. In many cases where disruption occurred at the targets, enhancement at additional regions at the brain periphery or near the midline was also observed.
Figure 3.
Examples of contrast-enhanced T1-weighted FSE images in the rat brain after sonication with an unfocused transducer in animals under ketamine/xylazine (A) and isoflurane/oxygen (B) anesthesia. Four locations were targeted in each animal (crosses). Left locations were sonicated at 4.1 W and right locations at 2.8 W (corresponding to peak pressures in water of 250 and 230 kPa). MRI contrast-enhancement at the sonication targets (dotted outlines) is clearly evident for animals anesthetized with ketamine/xylazine at all four locations. With isoflurane/oxygen, only minor enhancement at the two inferior locations was observed. With ketamine/xylazine, additional enhancement away from the targets (solid outlines) was also evident at peripheral brain regions (arrows) and near the midline, presumably from reflections or standing waves within the cranium. (bar: 5 mm)
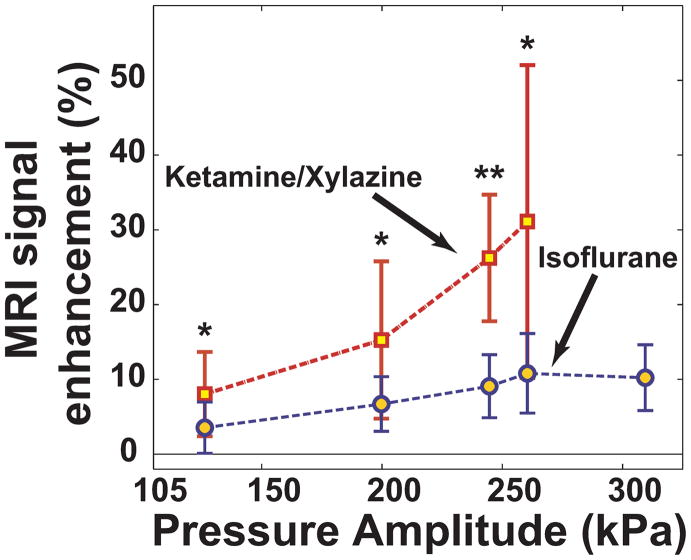
Analysis of the contrast enhancement for all animals confirmed our observations that isoflurane/oxygen produced less enhancement. At each power level tested, sonication in the ketamine/xylazine animals produced significantly (P<0.05) higher contrast enhancement than the isoflurane/oxygen animals (Figure 4). Substantial variation from location-to-location was observed, especially in the ketamine/xylazine animals. Differences in the percent enhancement relative to the pre-contrast images in non-targeted regions (i.e. any changes potentially due to the anesthesia agents alone) were not significant (P=0.2).
Figure 4.
MRI signal enhancement as a function of the peak negative pressure amplitude (measured in water) for animals anesthetized with ketamine/xylazine and isoflurane/oxygen (mean ± standard deviation shown). The enhancement was significantly higher for the ketamine/xylazine animals (*P<0.05, **P<0.001).
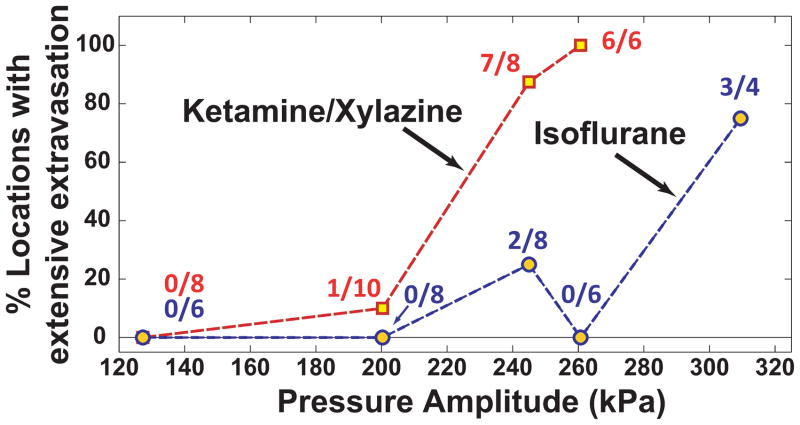
The appearance of Trypan blue that leaked into the brain parenchyma in the post mortem examination agreed with the MRI findings (Figure 5). At higher exposure levels, large areas with extravasations of erythrocytes were observed in addition to the leakage of the blue dye. Intense blue staining was often observed at the dorsal brain surfaces and adjacent to the skull base, and in several cases extensive extravasation of red blood cells was observed at these locations. The onset for extensive erythrocyte extravasation occurred at lower exposure levels with ketamine/xylazine than for isoflurane/oxygen (Figure 6, left). MRI signal enhancement was significantly higher in the ketamine/xylazine animals for both cases with and without severe erythrocyte extravasation (P=0.045, 0.003 for no/minor and extensive extravasation, respectively; Figure 6, right; left, right bars show results with ketamine/xylazine and isoflurane/oxygen, respectively).
Figure 5.
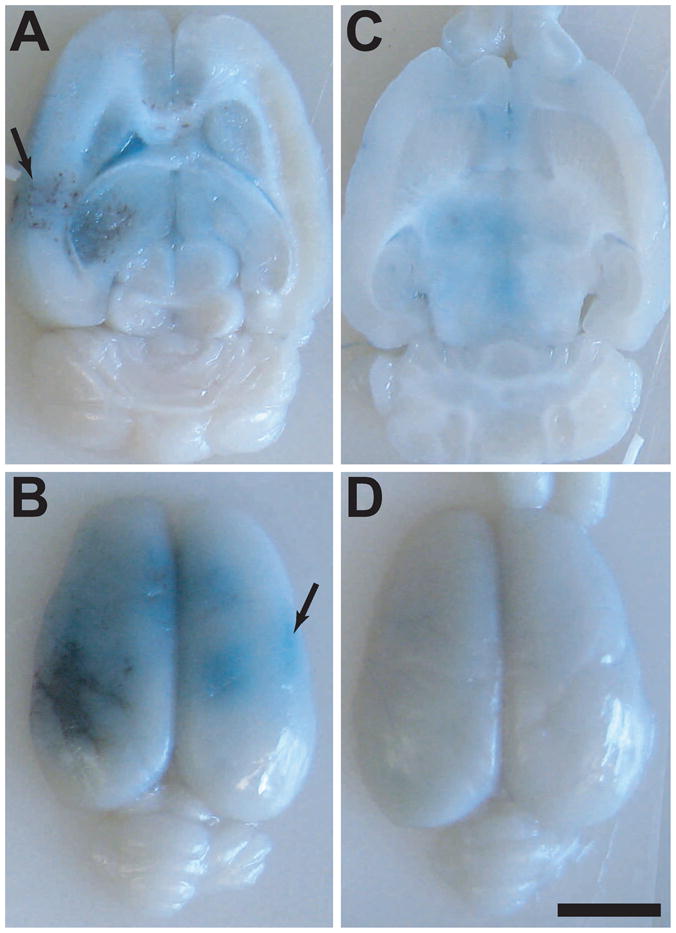
Macrophotographs showing gross tissue effects in the formalin-fixed brains from the two examples shown in Figure 3. With ketamine/xylazine (A and B), BBB disruption, as evidenced by leakage of Trypan blue, was observed at the target depth and on the brain surface. Regions with extensive extravasation of erythrocytes were produced by sonication at 4.1W (left), and additional blue-stained regions were observed areas at the peripheral regions and on the brain midline (arrows). With isoflurane/oxygen (C and D), the Trypan blue leakage was substantially less, and the extravasated erythrocytes were not seen. (bar: 5 mm)
Figure 6.
Left: Number of sonicated locations where extensive erythrocyte extravasation was observed as a function of peak negative pressure amplitude. The threshold for extensive extravasation appeared at a lower exposure level with ketamine/xylazine compared to isoflurane/oxygen. Right: MRI contrast enhancement for locations with and without extensive erythrocyte extravasation (mean ± standard deviation shown). In both cases, sonication with ketamine/xylazine (left bars) resulted in significantly more enhancement (*P<0.05).
Examples from histology are shown in Figure 7. In cases where examination of tissue blocks showed negligible or minor effects, a few small clusters of erythrocytes were observed outside the capillaries. The surrounding neurons appeared normal. In cases where more severe effects were observed, substantially more erythrocyte clusters were found, and they were larger. There was an accumulation of red blood cells in the brain ventricles and sulci in some cases. Parenchymal damage was observed in the region of the erythrocyte clusters. Overall, the histological findings were consistent with prior work on ultrasound-induced BBB disruption, but with more severe effects in the animals anesthetized with ketamine/xylazine.
Figure 7.
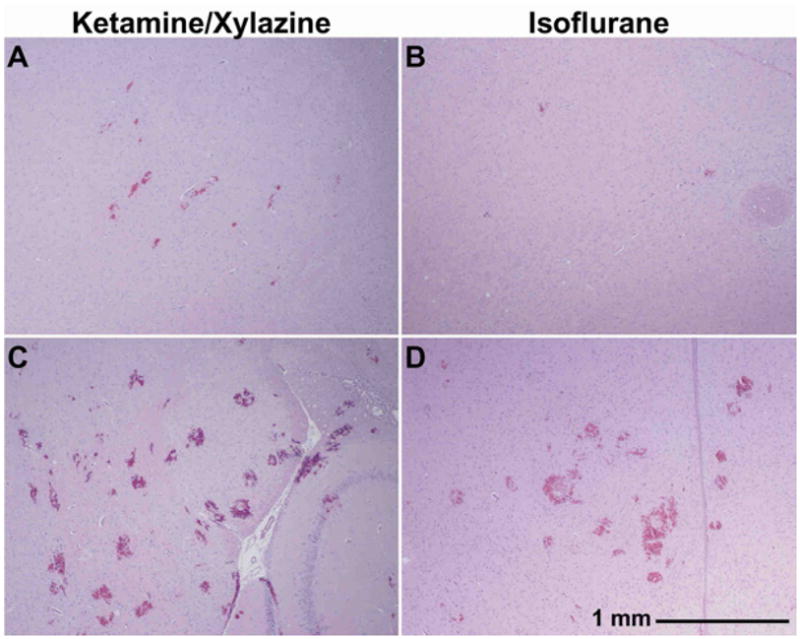
Microphotographs of H&E stained sections (40×) acquired four hours after sonication for ketamine/xylazine- and isoflurane/oxygen-anesthetized animals. Examples are shown from animals classified as having negligible (A–B) and extensive (C–D) red blood cell extravasation.
DISCUSSION
The use of focused ultrasound and an USCA to temporarily disrupt the BBB offers a potential noninvasive method to overcome one of the primary hurdles to the treatment of many brain diseases. The results of this study demonstrate that the leakage of tracers and histological effects can be strongly influenced by the anesthesia agent employed or perhaps the use of oxygen as a carrier gas for isoflurane. The leakage of both the MRI contrast agent and Trypan blue were greater in animals anesthetized with ketamine/xylazine than with isoflurane/oxygen, and red blood cell extravasations at higher exposure levels appeared to be more severe with ketamine/xylazine. The threshold for extensive erythrocyte extravasation also appeared to be lower with ketamine/xylazine. Presuming such damage was caused by inertial cavitation, these results also suggest that the bubble distribution and/or the inertial cavitation threshold may be influenced by the anesthesia procedure.
Other groups have successfully used isoflurane anesthesia for focused ultrasound BBB disruption (Choi et al. 2010; Alonso et al. 2010; Bing et al. 2009; Lin et al. 2009; Howles et al. 2010b). Here, ketamine/xylazine appeared more effective in producing BBB disruption than isoflurane/oxygen, but it was also associated with a lower threshold for severe vessel damage. It is not clear which agent is “better” for this application, but it is clear that such effects can make it challenging to compare results of different studies. If the BBB disruption and sensitivity to vascular damage is influenced by vascular properties, it will also be challenging to predict in patients what ultrasound exposure levels are appropriate, as patients will likely have different histories and will be taking different therapeutic agents that might influence brain vascular properties. It will thus be critical to develop methods to guide and control the exposures used for BBB disruption.
The results of the present study may have been due to the vascular action of different anesthetics. Ketamine, which blocks N-methyl-D-aspartate (NMDA) receptors, can increase and decrease cerebral metabolism and cerebral blood flow (CBF), depending on brain structure (Cavazzuti et al. 1987). Xylazine, a sedative and muscle relaxant used to minimize effects of ketamine, decreases the heart rate, intracranial pressure, blood pressure, and CBF (Greene and Thurmon 1988; Lei et al. 2001). Isoflurane, a GABAergic anesthetic, is generally known as a vasodilator (Eger 1984), although in some vessels it may act as a vasoconstrictor (Park et al. 1995). At high doses (>1.6%), it increases CBF (Eger 1984). Isoflurane is expected to produce higher CBF than ketamine/xylazine (Franceschini et al. 2010) due to the action of xylazine on systemic blood flow.
Changes in local vessel diameter, physical vessel properties, and local blood flow caused by different anesthetics could potentially effect the local bubble concentration, the interaction of the microbubbles with the ultrasound field and the vessel walls, and perhaps the amount of tracer that leaks out of the vessels after the BBB disruption. Different agents are also known to affect neurovascular coupling (Franceschini et al. 2010), which may influence how the vessels respond to mechanical stimulation and how they behave after BBB disruption occurs.
Previous studies investigating intraarterial infusions of hyperosmotic mannitol to disrupt the BBB have also observed a strong dependence on anesthesia agent on the amount of tracers delivered to the brain. Gumerlock and Neuwelt compared five different anesthesia agents in rats during osmotic BBB disruption (Gumerlock and Neuwelt 1990). Unlike our findings, they noted that both ketamine/xylazine and isoflurane both produced similar leakage of Evan’s Blue dye, but that two of the other agents they tested (methoxyflurane and fentanyl/droperidol) resulted in leakage of only trace amounts. However, when these two agents were combined with a beta-blocker (propanolol) to normalize pulse and blood pressure, the results were the same as the other agents tested. This led the researchers to posit that normal cardiac output may be optimal for osmotic BBB disruption.
In a subsequent study, the same group found that propofol anesthesia produced significantly more delivery of [3H]-methotrexate to inoculated tumors and the surrounding brain compared to isoflurane, while controlling for several factors, including PaC02, blood pressure, and heart rate (Remsen et al. 1999). They concluded that parameters related to the drug infusion and manipulation of PaC02 were of secondary importance in comparison to anesthesia in determining the success of the BBB disruption, and that differences were likely due to differences between the two anesthesia agents in regional blood flow. They also found increased toxicity when propofol was used during chemotherapy delivery after osmotic BBB disruption, presumably due to increased drug delivery (Fortin et al. 2000).
Another factor that could explain our results was the use of oxygen as a carrier gas for isoflurane. Two recent studies (published after the completion of these experiments) have shown that use of oxygen as a carrier gas for isoflurane profoundly reduces the circulation time of perfluorocarbon microbubbles compared to medical air (Mullin et al. 2011; Itani and Mattrey 2011). These findings would be consistent with the present study, where the isoflurane/oxygen-anesthetized animals were supplied pure oxygen through a nosecone and the ketamine/xylazine animals breathed ambient air. A reduced clearance time may be similar to a reduction in microbubble dose. Some studies have shown a strong dependence on microbubble dose on the resulting BBB disruption and vascular damage (Treat et al. 2007; Yang et al. 2007), while others found no significant effects of increasing the microbubble dose (McDannold et al. 2008b). Future work will be necessary to evaluate the carrier gas used on BBB disruption.
In addition to studying the effects of anesthesia protocol, this study also tested the use of a non-focused transducer with an aim of providing a large area of BBB disruption using a clinically-relevant ultrasound frequency to facilitate our ongoing studies on delivering anticancer agents to inoculated tumors in the rat brain. Prior studies with a non-focused, higher-frequency transducer were successful in wide disruption in mice (Howles et al. 2010b). Here, BBB disruption was produced at peripheral brain areas in addition to disruption at the center of the ultrasound beam. This finding was presumably due to reflections or standing waves within the intact rat cranium. Combination of the beam with the reflected field may have also increased the pressure amplitude in the brain. Indeed, the peak pressure amplitude in water at which BBB disruption and red blood cell extravasation were observed were substantially lower than what one would expect based on a prior study of the frequency dependence for BBB disruption (McDannold et al. 2008a), suggesting that the pressure amplitude in the brain may have been higher than expected based on measurement in water due to additive effects of the reflections. Furthermore, the area of the disruption was only 3–6 mm in diameter, which is small in comparison to the half-width of the acoustic field. Finally, vessel damage was sometimes observed at the brain surfaces, which may have been due to increased bubble activity in and damage to large blood vessels on the brain surface and/or shielding effects bubbles in the near-field of the ultrasound beam. Overall, these findings suggest that such an approach is not optimal, as effects appear to occur at unwanted locations and the in vivo exposure levels will be difficult to estimate due to standing waves. Standing waves may be mitigated by a pulsing scheme designed to avoid interference between the transmitted and reflected wave (O’Reilly et al. 2010), or through the use of a higher frequency (Howles et al. 2010a).
This study had several limitations. Due to the constraints of working in the MRI, we were not able to measure the animals’ physiological parameters that may shed light on why the two anesthesia agents produced different results. Future work should consider differences in blood pressure, heart rate, and metrics obtained from blood gas analysis to understand whether they or some other reason was responsible for these findings. More techniques such as MRI-based estimation of cerebral blood flow or ultrasound imaging-based evaluation of bubble concentration may also provide useful information. Only limited light microscopy evaluation of the tissue effects was performed. It would be beneficial to evaluate the tissue under electron microscopy to evaluate the effects of the anesthesia agents on the tight junctions and on vesicular trafficking (Sheikov et al. 2006) and in vivo microscopy to evaluate whether similar vascular reactions occur for the different agents (Raymond et al. 2007). Finally, only a limited range of acoustic parameters were performed in this study. Future work should investigate whether the window of acoustic parameters in which good BBB disruption can be achieved without producing extensive erythrocyte extravasation or parenchymal damage is similar for different agents. Results in non-anesthetized animals and in tumors should also be considered. Future work should also investigate whether these results were due to the anesthesia or to our use of oxygen as a carrier gas for isoflurane (Mullin et al. 2011; Itani and Mattrey 2011).
It should also be emphasized that it cannot be determined from these results whether the increased leakage of MRI contrast agent and Trypan blue in the ketamine/xylazine animals was the result of increased BBB disruption, increased vessel damage resulting from inertial cavitation, or both. Both BBB disruption and vessel damage will result in leakage of agents into the brain parenchyma, and we did not have enough information in this study to distinguish between the two effects. Future work with more detailed histological examination and acoustic emission monitoring to detect wideband emission is necessary to determine which effect is dominant. Such work was beyond the scope of this study. It would also be interesting to explicitly measure the BBB disruption and inertial cavitation/vascular damage thresholds for the two anesthesia protocols. If one threshold were shifted independent of the other, it could point to ways to increase the safety window of the procedure.
Despite these limitations, the results demonstrate the difficulty in predicting the extent of BBB disruption based on acoustic parameters alone. Changes resulting from the use of different anesthesia agents or perhaps from the use of oxygen produced a profound effect on the resulting BBB disruption, and they need to be taken into account in any treatment planning for ultrasound-induced BBB disruption or in comparing different studies that use different anesthesia agents. Most importantly, these findings demonstrate the need for feedback and control of the procedure, both to ensure that sufficient BBB disruption occurs, and to avoid overexposure.
Acknowledgments
This work was funded by NIH grant RC2NS069413 and from a gift from Betty Brudnick.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Alonso A, Reinz E, Jenne JW, Fatar M, Schmidt-Glenewinkel H, Hennerici MG, Meairs S. Reorganization of gap junctions after focused ultrasound blood-brain barrier opening in the rat brain. J Cereb Blood Flow Metab. 2010;30:1394–1402. doi: 10.1038/jcbfm.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay L, Hueter TF, Ballantine HT, Sosa D. Ultrasonically produced changes in the blood-brain barrier. Arch Neurol. 1956;76:457–467. doi: 10.1001/archneurpsyc.1956.02330290001001. [DOI] [PubMed] [Google Scholar]
- Bing KF, Howles GP, Qi Y, Palmeri ML, Nightingale KR. Blood-brain barrier (BBB) disruption using a diagnostic ultrasound scanner and Definity in Mice. Ultrasound Med Biol. 2009;35:1298–1308. doi: 10.1016/j.ultrasmedbio.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzuti M, Porro CA, Biral GP, Benassi C, Barbieri GC. Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab. 1987;7:806–811. doi: 10.1038/jcbfm.1987.138. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Spatio-temporal analysis of molecular delivery through the blood-brain barrier using focused ultrasound. Phys Med Biol. 2007;52:5509–5530. doi: 10.1088/0031-9155/52/18/004. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Selert K, Gao Z, Samiotaki G, Baseri B, Konofagou EE. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. J Cereb Blood Flow Metab. 2010;31:725–737. doi: 10.1038/jcbfm.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood-brain-barrier opening using transcranial ultrasound exposures. ACS Chem Neurosci. 2010;1:391–398. doi: 10.1021/cn9000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement GT, Hynynen K. Field characterization of therapeutic ultrasound phased arrays through forward and backward planar projection. J Acoust Soc Am. 2000;108:441–446. doi: 10.1121/1.429477. [DOI] [PubMed] [Google Scholar]
- Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47:1219–1236. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- Collis J, Manasseh R, Liovic P, Tho P, Ooi A, Petkovic-Duran K, Zhu Y. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics. 2010;50:273–279. doi: 10.1016/j.ultras.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Eger EI. The pharmacology of isoflurane. Br J Anaesth. 1984;56 (Suppl 1):71S–99S. [PubMed] [Google Scholar]
- Fortin D, McCormick CI, Remsen LG, Nixon R, Neuwelt EA. Unexpected neurotoxicity of etoposide phosphate administered in combination with other chemotherapeutic agents after blood-brain barrier modification to enhance delivery, using propofol for general anesthesia, in a rat model. Neurosurgery. 2000;47:199–207. doi: 10.1097/00006123-200007000-00041. [DOI] [PubMed] [Google Scholar]
- Franceschini MA, Radhakrishnan H, Thakur K, Wu W, Ruvinskaya S, Carp S, Boas DA. The effect of different anesthetics on neurovascular coupling. Neuroimage. 2010;51:1367–1377. doi: 10.1016/j.neuroimage.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SA, Thurmon JC. Xylazine--a review of its pharmacology and use in veterinary medicine. J Vet Pharmacol Ther. 1988;11:295–313. doi: 10.1111/j.1365-2885.1988.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Gumerlock MK, Neuwelt EA. The effects of anesthesia on osmotic blood-brain barrier disruption. Neurosurgery. 1990;26:268–277. doi: 10.1097/00006123-199002000-00014. [DOI] [PubMed] [Google Scholar]
- Herman BA, Harris B. Calibration of miniature ultrasonic receivers using a planar scanning technique. J Acoust Soc Am. 1982;72:1357–1363. [Google Scholar]
- Howles GP, Bing KF, Qi Y, Rosenzweig SJ, Nightingale KR, Johnson GA. Contrast-enhanced in vivo magnetic resonance microscopy of the mouse brain enabled by noninvasive opening of the blood-brain barrier with ultrasound. Magn Reson Med. 2010a;64:995–1004. doi: 10.1002/mrm.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howles GP, Qi Y, Johnson GA. Ultrasonic disruption of the blood-brain barrier enables in vivo functional mapping of the mouse barrel field cortex with manganese-enhanced MRI. Neuroimage. 2010b;50:1464–1471. doi: 10.1016/j.neuroimage.2010.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood–brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurgery. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- Itani M, Mattrey RF. The Effect of Inhaled Gases on Ultrasound Contrast Agent Longevity In Vivo. Mol Imaging Biol. 2011 doi: 10.1007/s11307-011-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Grinberg O, Nwaigwe CI, Hou HG, Williams H, Swartz HM, Dunn JF. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913:174–179. doi: 10.1016/s0006-8993(01)02786-x. [DOI] [PubMed] [Google Scholar]
- Lin KJ, Liu HL, Hsu PH, Chung YH, Huang WC, Chen JC, Wey SP, Yen TC, Hsiao IT. Quantitative micro-SPECT/CT for detecting focused ultrasound-induced blood-brain barrier opening in the rat. Nucl Med Biol. 2009;36:853–861. doi: 10.1016/j.nucmedbio.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Liu HL, Hsu PH, Chu PC, Wai YY, Chen JC, Shen CR, Yen TC, Wang JJ. Magnetic resonance imaging enhanced by superparamagnetic iron oxide particles: usefulness for distinguishing between focused ultrasound-induced blood-brain barrier disruption and brain hemorrhage. J Magn Reson Imaging. 2009;29:31–38. doi: 10.1002/jmri.21599. [DOI] [PubMed] [Google Scholar]
- Martynov S, Stride E, Saffari N. The natural frequencies of microbubble oscillation in elastic vessels. J Acoust Soc Am. 2009;126:2963–2972. doi: 10.1121/1.3243292. [DOI] [PubMed] [Google Scholar]
- McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66:323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. 2008a;34:834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption. Ultrasound Med Biol. 2008b;34:930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin L, Gessner R, Kwan J, Kaya M, Borden MA, Dayton PA. Effect of anesthesia carrier gas on in vivo circulation times of ultrasound microbubble contrast agents in rats. Contrast Media Mol Imaging. 2011 doi: 10.1002/cmmi.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MA, Huang Y, Hynynen K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model. Phys Med Biol. 2010;55:5251–5267. doi: 10.1088/0031-9155/55/18/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Park KW, Dai HB, Lowenstein E, Darvish A, Sellke FW. Oxygen-derived free radicals mediate isoflurane-induced vasoconstriction of rabbit coronary resistance arteries. Anesth Analg. 1995;80:1163–1167. doi: 10.1097/00000539-199506000-00017. [DOI] [PubMed] [Google Scholar]
- Qin S, Ferrara KW. Acoustic response of compliable microvessels containing ultrasound contrast agents. Phys Med Biol. 2006;51:5065–5088. doi: 10.1088/0031-9155/51/20/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SB, Skoch J, Hynynen K, Bacskai BJ. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J Cereb Blood Flow Metab. 2007;27:393–403. doi: 10.1038/sj.jcbfm.9600336. [DOI] [PubMed] [Google Scholar]
- Remsen LG, Pagel MA, McCormick CI, Fiamengo SA, Sexton G, Neuwelt EA. The influence of anesthetic choice, PaCO2, and other factors on osmotic blood-brain barrier disruption in rats with brain tumor xenografts. Anesth Analg. 1999;88:559–567. doi: 10.1097/00000539-199903000-00018. [DOI] [PubMed] [Google Scholar]
- Sassaroli E, Hynynen K. Resonance frequency of microbubbles in small blood vessels: a numerical study. Phys Med Biol. 2005;50:5293–5305. doi: 10.1088/0031-9155/50/22/006. [DOI] [PubMed] [Google Scholar]
- Sassaroli E, Hynynen K. Cavitation threshold of microbubbles in gel tunnels by focused ultrasound. Ultrasound Med Biol. 2007;33:1651–1660. doi: 10.1016/j.ultrasmedbio.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32:1399–1409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med Biol. 2008;34:1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- Tung YS, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys Med Biol. 2010;55:6141–6155. doi: 10.1088/0031-9155/55/20/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CY, Zhang Z, Xue YX, Wang P, Liu YH. Mechanisms of the increase in the permeability of the blood-tumor barrier obtained by combining low-frequency ultrasound irradiation with small-dose bradykinin. J Neurooncol. 2009;94:41–50. doi: 10.1007/s11060-009-9812-9. [DOI] [PubMed] [Google Scholar]
- Yang FY, Fu WM, Yang RS, Liou HC, Kang KH, Lin WL. Quantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrier disruption. Ultrasound Med Biol. 2007;33:1421–1427. doi: 10.1016/j.ultrasmedbio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Zheng H, Dayton PA, Caskey C, Zhao S, Qin S, Ferrara KW. Ultrasound-driven microbubble oscillation and translation within small phantom vessels. Ultrasound Med Biol. 2007;33:1978–1987. doi: 10.1016/j.ultrasmedbio.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Zhong P, Zhou Y, Zhu S. Dynamics of bubble oscillation in constrained media and mechanisms of vessel rupture in SWL. Ultrasound Med Biol. 2001;27:119–134. doi: 10.1016/s0301-5629(00)00322-7. [DOI] [PubMed] [Google Scholar]