Abstract
Despite using prescribed pain medications, patients with neuropathic pain continue to experience moderate to severe pain. There is a growing recognition of a potent peripheral opioid analgesia in models of inflammatory and neuropathic pain. The goal of this study was to characterize the temporal and spatial expression of mu opioid receptor (mOR) mRNA and protein in primary afferent neurons in a rat L5 spinal nerve ligation model of persistent neuropathic pain. Bilateral L4 and L5 dorsal root ganglia (DRGs), L4 and L5 spinal cord segments, and hind paw plantar skins were collected on days 0 (naïve), 3, 7, 14, and 35 post-spinal nerve ligation or post-sham surgery. We found that expression of mOR mRNA and protein in primary afferent neurons changed dynamically and site-specifically following L5 spinal nerve ligation. Real-time RT-PCR, immunohistochemistry, and Western blot analysis demonstrated a down-regulation of mOR mRNA and protein in the injured L5 DRG. In contrast, in the uninjured L4 DRG, mOR mRNA transiently decreased on day 7 and then increased significantly on day 14. Western blot analysis revealed a persistent increase in mOR protein expression, although immunohistochemistry showed no change in number of mOR-positive neurons in the uninjured L4 DRG. Interestingly, mOR protein expression was reduced in the skin on days 14 and 35 post-nerve injury and in the L4 and L5 spinal cord on day 35 post-nerve injury. These temporal and anatomically specific changes in mOR expression following nerve injury are likely to have functional consequences on pain-associated behaviors and opioid analgesia.
Keywords: mu opioid receptors, expression, dorsal root ganglion, spinal cord, skin, peripheral nerve injury
1. Introduction
Neuropathic pain can result from various etiologies with different anatomical locations of the lesion—from peripheral nociceptors to the central nervous system. It is characterized by ongoing and/or evoked pain and impairs patients’ emotional functioning, mobility, and the ability to work (O'Connor, 2009). Neuropathic pain is estimated that about half of patients with neuropathic pain do not get any relief from their symptoms despite taking prescribed analgesics (O'Connor, 2009). Several randomized control trials have shown that opioid analgesics provide greater pain relief than placebo (Wu et al., 2008), and comparable pain relief to gabapentin and tricyclic antidepressants (Raja et al., 2002; Wu et al., 2008). However, treatment of neuropathic pain with opioids is often discouraged because of their lower efficacy and unwanted side effects (such as tolerance and addiction) caused by long-term and high-dose opioid therapy. Thus, it is important to understand dynamic temporal and spatial regulation of opioid receptor expression in nervous system following nerve injury.
In recent years, a growing body of evidence has suggested that after tissue injury and inflammation there is a potent opioid analgesia mediated by peripheral opioid receptors (Lewanowitsch et al., 2006; Shinoda et al., 2007). Opioid receptors, synthesized in dorsal root ganglion (DRG) neurons, are expressed on cell bodies of sensory neurons and are transported to their central terminals in the superficial dorsal horn and peripheral terminals in peripheral tissues (Coggeshall et al., 1997; Truong et al., 2003). Opioids increase antinociceptive potency during inflammation and that this increased potency is most pronounced for mu opioid receptor (mOR) agonists (Stanfa et al., 1992). Activation of the endogenous opioid system during inflammation in the periphery and in the spinal cord contributes to the enhanced sensitivity to opiates (Hassan et al., 1993; Stein et al., 2009). As in inflammatory pain, opioid receptors expressed on peripheral afferent axons may also contribute to the opioid-induced antinociception in neuropathic pain. Peripheral mOR activation attenuated neuropathic pain with minimal central nervous system side effects (Obara et al., 2004; Obara et al., 2007; Kabli and Cahill, 2007; Guan et al., 2008; Obara et al., 2009), suggesting that peripherally acting mORs may provide an alternative strategy for the treatment of neuropathic pain. However, the dynamic changes in mOR expression in the peripheral nervous system after peripheral nerve injury have not been carefully studied.
In the present study, using real-time reverse transcriptase-polymerase chain reaction (RT-PCR), Western blot analysis, and immunohistochemistry, we characterized the temporal and spatial expression of mORs at the levels of mRNA and protein in primary afferent neurons in a rodent L5 spinal nerve ligation (SNL) model of persistent neuropathic pain.
2. Experimental procedures
2.1. Animals
Male Sprague-Dawley rats (250–300 g, Harlan Bioproducts for Science, Indianapolis, IN) were housed in cages on a standard 12:12 h light/dark cycle. Water and food were available ad libitum until rats were transported to the laboratory approximately 1 h before experiments. The animals were used in accordance with protocols that were approved by the Animal Care and Use Committee at the Johns Hopkins University and at the University of South Carolina’s School of Medicine. All animal procedures were consistent with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Spinal nerve ligation (SNL)-induced neuropathic pain model
Rats (n = 143) were anesthetized with isoflurane, and a dorsolateral skin incision was made on the lower back. The fifth lumbar transverse process was identified and freed of its muscle attachment and then removed. The underlying fifth lumbar nerve root was isolated, ligated with a 4-0 silk suture, and transected just distal to the ligature according to the method described previously (Kim and Chung, 1992; Zhang et al., 2003; Singh et al., 2009). After appropriate hemostasis, the skin and muscle layers were closed with a silk suture. In the sham group, the surgical procedure was identical to that described above, except that the spinal nerve was not ligated and transected.
2.3. Behavioral testing
Mechanical paw withdrawal thresholds (PWTs) were measured with the up-down testing paradigm 1 day before surgery and on days 3, 7, 14, and 35 after nerve injury or sham surgery (Dixon, 1980; Chaplan et al., 1994; Singh et al., 2009). Behavioral testing was performed by experimenters blinded to the rats’ surgical group. Each animal was placed in a Plexiglas chamber on an elevated mesh screen. At least 30 min were allotted for behavioral accommodation before testing was begun. Von Frey hairs in log increments of force (3.61, 3.84, 4.08, 4.31, 4.56, 4.74, 4.93, 5.18 g) were applied to the plantar surface of the rats’ left and right hind paws. The 4.31-g stimulus was applied first. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next higher von Frey hair was used. The test was ended when (i) a negative response was obtained with the 5.18-g hair, (ii) four stimuli were applied after the first positive response, or (iii) nine stimuli were applied to one hind paw.
2.4. Quantitative real-time RT-PCR
Thirty minutes to an hour after behavioral testing, rats were anesthetized by isoflurane and decapitated. Then the L4 and L5 DRGs were rapidly dissected and collected. To obtain enough RNA, unilateral L4 or L5 DRGs from three rats per time point were pooled (n = 12 rats/time point). Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA, USA), treated with RNase-free DNase I (2 U/20 µl; Promega Corp., Madison, WI) and reverse-transcribed with Omniscript kit (Qiagen, Valencia, CA) and Oligo-dT. cDNA was amplified by real-time PCR by using the probe for mOR (5’ - /56 - FAM/ AGGCAGAAACTGCTCCATTGCCCTA/3BHQ_1/ - 3’; Integrated DNA Technologies, Coralville, IA) and glyceraldehyde-3-phosphate dehydrogenase (GADPH) (Ref: Rn99999916_s1; Applied Biosystems, Foster City, CA) as an internal control for normalization. The mOR PCR primer sequences were 5′-AACATCCCTCCACGGCTAATAC-3' (forward) and 5′-AGAGCCTCCCACACACCCTG-3' (reverse). Real-time PCR for each sample was run in quadruplicate in a 20-µl reaction with TaqMan Universal PCR master mix kit (Applied Biosystems). Reactions were performed in an ABI 7500 Fast real-time PCR system (Applied Biosystems). The amplification protocol was: 3 min at 95°C, followed by 45 cycles of 10 s at 95°C for denaturation and 45 s at 58°C for annealing and extension. Ratios of ipsilateral-side mRNA levels to contralateral-side mRNA levels were calculated by using the ΔCt method (2−ΔΔCt) at a threshold of 0.02. All data were normalized to GAPDH, which has been demonstrated to be stable even during peripheral nerve injury (Jankowski et al., 2009; Guan et al., 2009).
2.5. In vitro transfection of HEK 293 cells
To examine the specificity and selectivity of the mOR antibody used, we transfected the mammalian expression vectors encoding full-length cDNAs of mOR, delta opioid receptor (dOR), or kappa opioid receptor (kOR) into the human embryonic kidney (HEK) 293 cells (GenHunter, Nashville, TN). Briefly, cDNA were added to the cells in 1:1 ratios for a total of 4 µg. Four to 6 h later, the cells were treated with a 15% glycerol solution in the buffer (50 mM BES(N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid), 280 mM NaCl, 1.5 mM Na2HPO4) for 30 s. The cells were collected 48 h after transfection and resuspended in 100ul of chilled lysis buffer [50 mM Tris, 150 nM NaCl, 1% NP-40, 1 mM EDTA, 1% Triton X-100 and Protease Inhibitor Cocktail at 1:100 (Sigma P8340)]. After the sonication, the samples were centrifuged at 15,000g for 30 min at 4°C. The supernatants were collected for Western blot analysis as described below.
2.6. In vivo tissue collection and preparation
After behavioral testing, the rats were deeply anesthetized by isoflurane and decapitated. Hind paw plantar skin, L4 and L5 DRGs, and L4 and L5 spinal cord on the ipsilateral and contralateral sides were removed and frozen on dry ice and stored at −80°C for use in Western blot analysis (n = 4/time point). The frozen skin was placed inside a 1.5 ml tube and shredded using a Dremel tool (with tip #191) for 15 seconds on ice. All tissues including skin, DRG, and spinal cord were incubated in the chilled lysis buffer on ice for 1 h. During the incubation period, the samples were sonicated twice for 10 seconds each time. All of the samples were centrifuged at 15,000g for 30 min at 4°C. The supernatants were collected.
2.7. Western blot analysis
Protein concentrations in in vivo and in vitro samples were measured by BCA protein assay. The samples were diluted in lysis buffer and combined with SDS sample buffer. After being heated at 95°C for 10 min, the samples were loaded into a BioRad Criterion Tris-HCl gel for SDS-PAGE electrophoresis. The gels were transferred onto a PVDF membrane with a BioRad SemiDry Transfer. Blotting membranes were blocked with 5% powdered milk in phosphate buffered saline (PBS) with 0.05% Tween 20 (PBST) for 1 h at room temperature. The membranes were then incubated with rabbit anti-mOR antibody (1:1,500, ImmunoStar #24216) overnight at 4°C or mouse anti-β-actin antibody (1:20,000, Sigma A5441) for 2 h at room temperature. After three washes in PBST, the membranes were placed in HRP-labeled secondary donkey anti-rabbit antibody (1:4000, Santa Cruz, Santa Cruz, CA sc2313) or anti-mouse antibody (1:5000, Cell Signaling Technologies, Beverly, MA #7076) for 1 h at room temperature. The membranes were washed several times and the bands were visualized using ECL (Pierce 32209). Band densities were determined by using Scion Image (Scion Corporation, Gaithersburg, MD).
To further indentify the specificity and selectivity of mOR antibody used, we carried out another experiment: the immunoadsorption of the primary antiserum with excess the corresponding antigen. Briefly, 5 µl of rabbit anti-mOR antibody (1:1,500) was incubated with 10 µg/ml mOR peptide (Immunostar Cat# 24335) at 4°C for 24 h. After the mixture was centrifuged at 16,000g at 4°C for 30 min, the supernatant was collected and substituted for rabbit anti-mOR antibody.
2.8. Immunohistochemistry
Rats were deeply anesthetized by isoflurane after behavioral testing and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Bilateral L4 and L5 DRGs were collected, post-fixed in the same fixative solution for 2–4 h, cryoprotected by immersion in 30% sucrose overnight at 4°C, and frozen-sectioned at 20 µm. Every third section was collected (at least four sections/DRG). The sections were blocked for 1 h at 37°C in 0.01 M phosphate-buffered saline (PBS) containing 10% normal goat serum plus 0.3% Triton X-100. Sections were incubated with primary rabbit anti-mOR serum (1:2000; ImmunoStar Inc., Hudson, WI) overnight at 4°C. After being washed in PBS, sections were incubated with Cy3-conjugated goat anti-rabbit secondary antibody (1:300; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. Control sections lacking primary antiserum were stained in parallel. The immunofluorescence-labeled sections were rinsed in 0.01 M PBS and mounted onto gelatin-coated glass slides. Cover slips were applied with a mixture of 50% glycerin and 2.5% triethylene diamine in 0.01 M PBS. The labeled sections were observed with a Nikon TE2000E fluorescence microscope (Nikon Co., Japan). The images were captured with a CCD spot camera. The number of mOR-positive and -negative DRG cells in each section was counted. The mean percentage of mOR-positive cells per L4 or L5 section at each time point was calculated from at least 12 sections (n = 3 rats/time point).
2.9. Anterograde transport
Anterograde transport of mORs in the sciatic nerves was measured in normal uninjured animals (n = 6) and in animals that were 14 days post-L5 SNL (n = 6) (Raivich et al., 1991). Animals were anesthetized with intraperitoneal urethane (2 g/kg) for the duration of the experiment. The sciatic nerve was exposed at mid-thigh level. Two silk ligatures (5-0) were tied around the sciatic nerve 0.5 cm apart. The incision was closed with silk ligatures on the fascia and wound clips to the skin. Animals were given Ringer’s to maintain hydration and monitored on a heating pad to maintain normal body temperature. At 6 h post-ligation, animals were asphyxiated with CO2. Three 0.5-cm sections of sciatic nerve were removed from between the two ligatures, distal to the ligatures, and proximal to the ligatures. The distal, proximal, and ligated sections of sciatic nerve were frozen on dry ice and stored at −80°C until being homogenized for Western blot analysis. The sections were homogenized in 150 µl of chilled lysis buffer and processed as described above for Western blot analysis of mOR expression. The intermediate nerve segment was used as a control for local synthesis, while the mOR expression in the proximal nerve segment was used as a measure of anterograde transport.
2.10. Statistical analysis
The results from the behavioral tests, RT-PCR, Western blot, and immunohistochemistry were statistically analyzed with a one-way or two-way analysis of variance (ANOVA). Data are presented as mean ± SEM. When ANOVA showed significant difference, pairwise comparisons between means were tested by the post hoc Tukey method. Significance was set at p < 0.05. The statistical software package SigmaStat (Systat, Chicago, IL) or GraphPad Prism 4.0 (GraphPad Software Inc., La Jolla, CA) was used to perform all statistical analyses.
3. Results
3.1. SNL-evoked mechanical hypersensitivity in rats
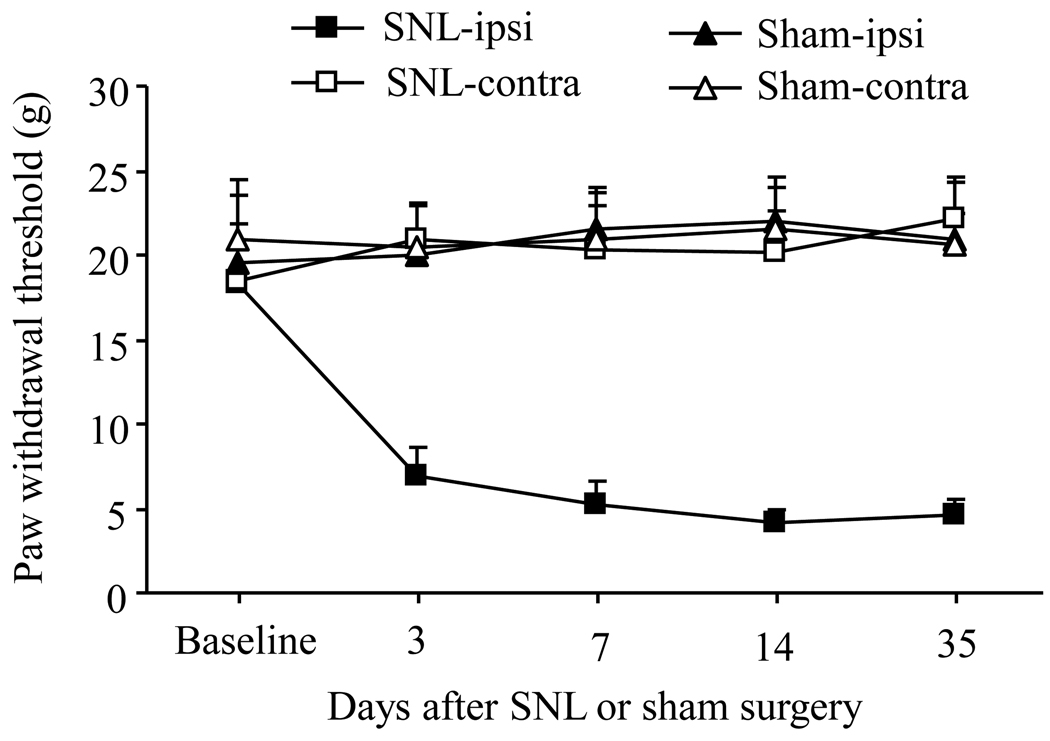
To ensure that all SNL rats used in the study showed pain hypersensitivity, we measured the PWTs in response to mechanical stimuli. Consistent with our previously published data (Zhang et al., 2003; Guan et al., 2008; Singh et al., 2009; Guan et al., 2009), SNL induced mechanical hypersensitivity, which was evident by a significant decrease in PWT on the ipsilateral side (but not the contralateral side) compared with pre-surgery baseline values (Fig. 1, p < 0.01). This decrease occurred on day 3 post-SNL and persisted for at least 35 days post-SNL (Fig. 1). The SNL rats that failed to develop neuropathic pain and/ or showed evidence of motor paralysis were excluded from the study. As expected, sham operation did not change PWTs on either the ipsilateral or contralateral side (Fig. 1, p > 0.05).
Fig. 1.
Spinal nerve ligation (SNL)-induced mechanical allodynia. SNL led to a significant decrease in paw withdrawal threshold in response to mechanical stimuli on the ipsilateral (ipsi), but not contralateral (contra), side on days 3, 7, 14, and 35 after L5 SNL. Sham surgery did not produce a significant change in paw withdrawal threshold on either the ipsilateral or contralateral side during the observation period.
3.2. Effect of SNL on the expression of mOR mRNA in injured and intact DRGs
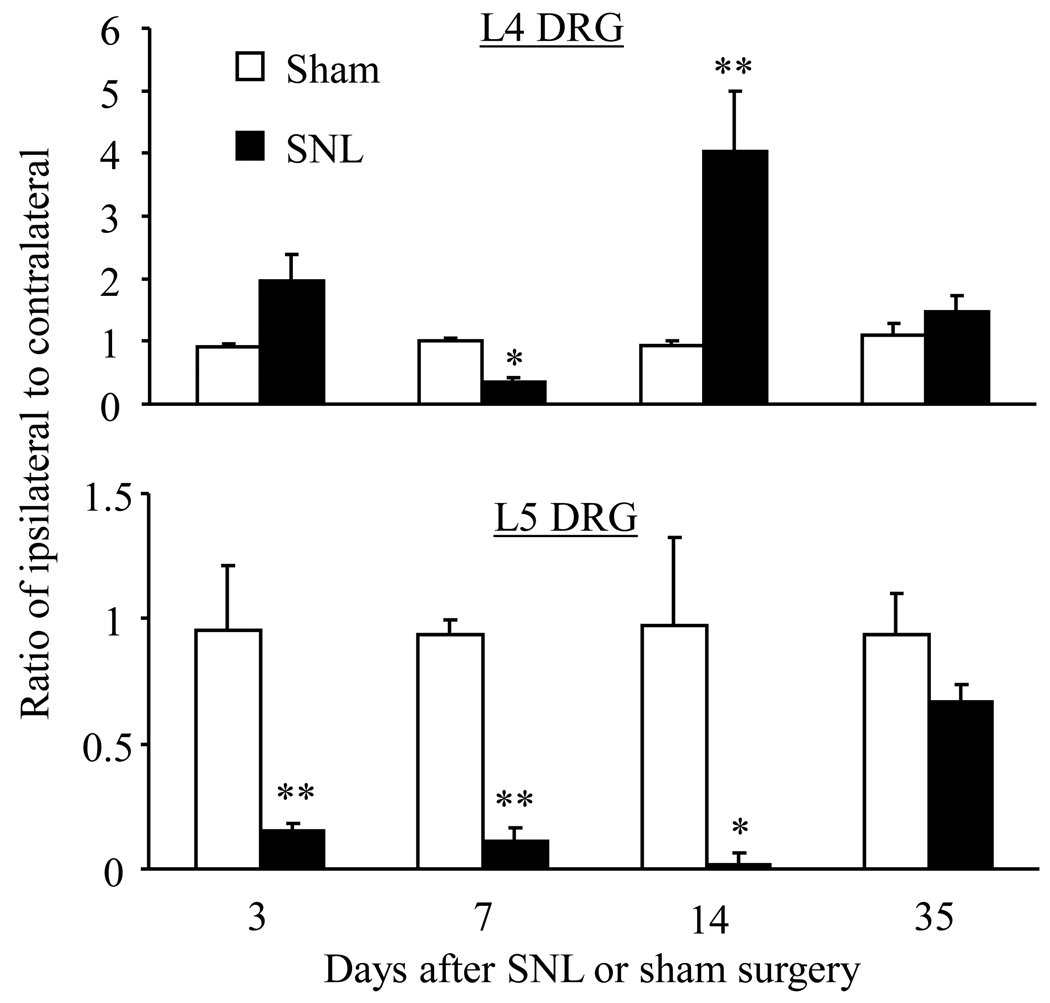
We first used a quantitative real-time RT-PCR approach to examine the changes in mOR mRNA in injured (ipsilateral L5) and intact (L4) DRGs after SNL in rats. We found that SNL time-dependently altered mOR mRNA expression in both the injured and intact DRGs (Fig. 2). SNL significantly reduced expression of mOR mRNA in the ipsilateral L5 DRG on days 3 to 14, but returned back towards levels observed in the sham group by day 35, after SNL. The ratios of ipsilateral to contralateral mOR mRNA in L5 DRGs after SNL were decreased by 84% on day 3 (p < 0.01), 88% on day 7 (p < 0.01), 97% on day 14 (p < 0.01), and 29% on day 35 (p > 0.05) compared to the corresponding time points in sham groups (Fig. 2). The pattern of changes in expression of mOR mRNA in the ipsilateral L4 DRGs was different from that in the ipsilateral L5 DRGs. The ratios of ipsilateral to contralateral mOR mRNA in L4 DRGs after SNL were significantly decreased on day 7 (by 64.5%, p < 0.05), but increased on day 14 (by 4.34 fold, p < 0.01) compared to the corresponding time points in sham groups (Fig. 2). The levels of mOR mRNA in the ipsilateral L4 DRG on days 3 and 35 post-SNL were not significantly different from those on days 3 and 35 post-sham surgery (Fig. 2).
Fig. 2.
Dynamic changes in mOR mRNA expression in L4 and L5 DRGs after SNL. Relative expression levels of mOR mRNA in the L4 (Top) and L5 (bottom) DRGs on days 3, 7, 14, and 35 after L5 SNL or sham surgery. * p < 0.05 or ** p < 0.01 compared to the corresponding sham groups.
3.3. Effect of SNL on mOR protein expression in injured and intact DRGs
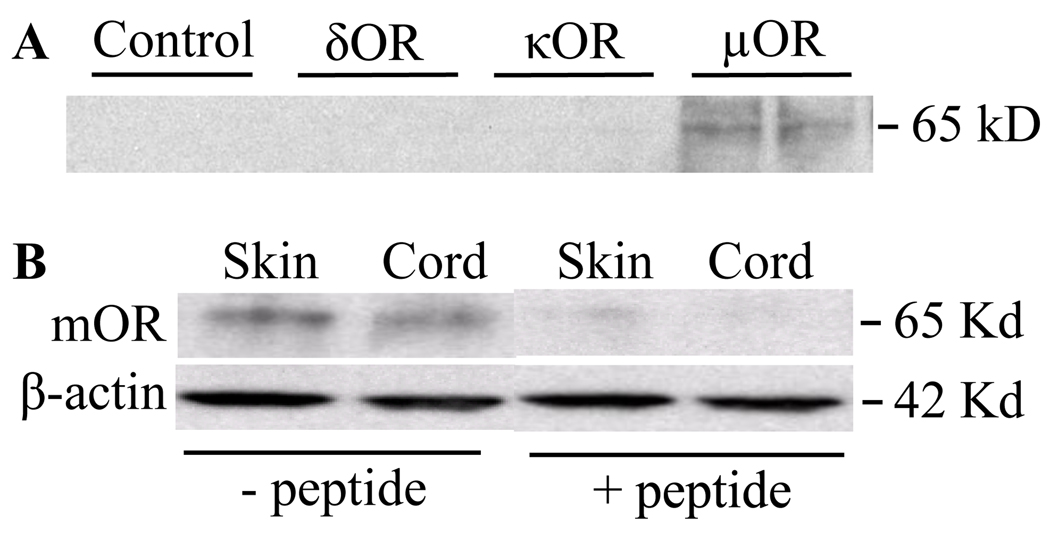
To determine whether SNL-induced changes in mOR mRNA alters mOR protein expression, we used both immunohistochemistry and Western blot analysis. Immunohistochemistry was used to determine the percentage of cells that expressed mOR, whereas Western blot analysis was used to determine how much mOR protein was expressed within the tissue as a whole. The specificity and selectivity of the mOR antibody used were determined first by in vitro HEK 293 cells transfected with mOR, δOR, and κOR and pre-immunoadsorption of the primary antibody with excess of the corresponding antigen. We found that only HEK 293 cells transfected with mOR showed a band at 65 kD (Fig. 3A) and that the band at 65kD isolated from rat lumbar spinal cord and hind paw skin was competed away when the mOR antibody was pre-adsorbed with a mOR blocking peptide (Fig. 3B). Our findings indicate that the mOR antibody used specifically and selectively recognizes the mOR protein. This antibody has also been used in the previous studies (Kohno et al., 2005; Zhang et al., 2008).
Fig. 3.
Specificity and selectivity of rabbit anti-mOR antibody. (A) Expression of mOR protein in HEK 293 cells transfected only with mOR, but not dOR, kOR, or un-tranfected controls. (B) Expression of mOR protein in skin and spinal cord tissues could be detected when the rabbit mOR antibody was preadsorbed with excess of mOR peptide.
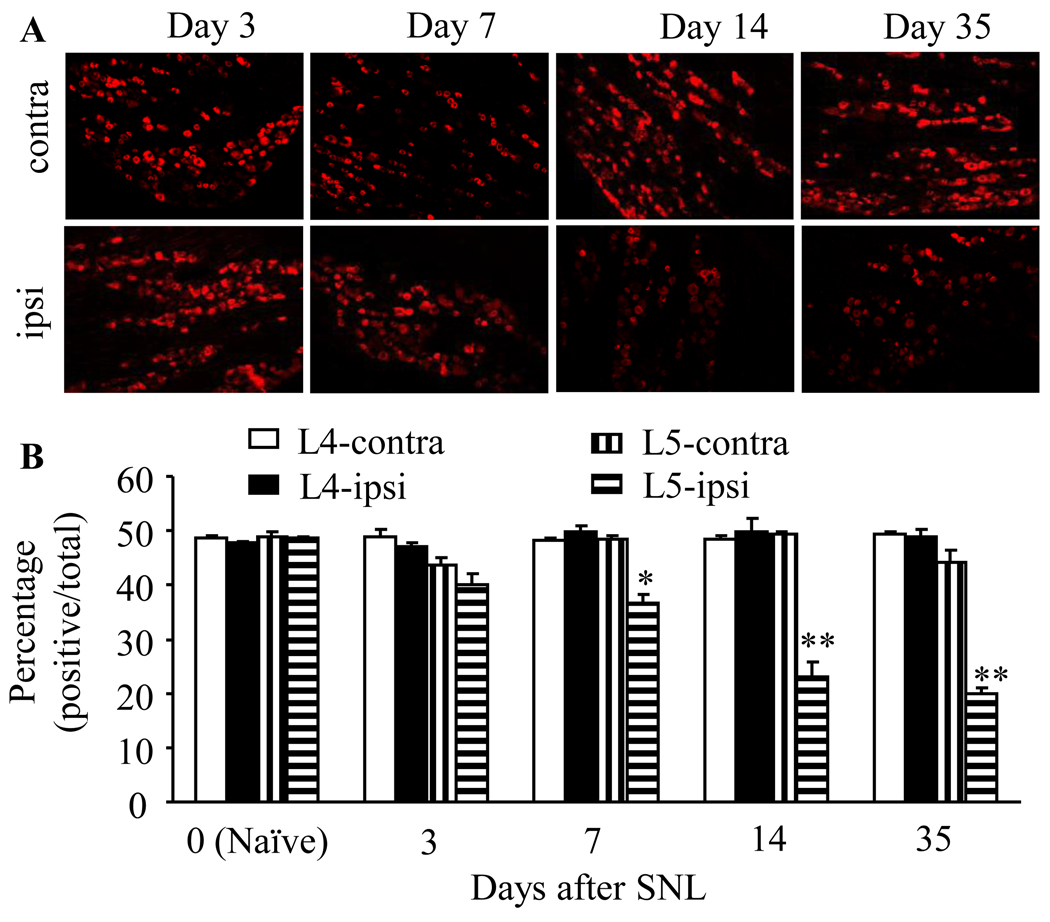
Immunohistochemical analysis was completed in ipsilateral L4 (intact) and L5 (injured) DRGs as well as contralateral L4 and L5 DRGs after SNL. Consistent with previous studies (Zhang et al., 1998; Kohno et al., 2005), mORs were expressed predominantly in small DRG neurons, and about half of DRG neurons were mOR-positive in naïve rats (Fig. 4). As expected, the number of mOR-positive neurons in ipsilateral and contralateral L4 and L5 DRGs was not altered dramatically after sham surgery at the observed time points (data not shown). SNL time-dependently reduced the number of neurons expressing mORs in L5 DRG on the ipsilateral, but not contralateral, side (Fig. 4). The number of mOR-positive neurons in the ipsilateral L5 DRGs after SNL was decreased by 17.5% on day 3 (p > 0.05), 24.5% on day 7 (p < 0.05), 52.7% on day 14 (p < 0.01), and 58.8% on day 35 (p < 0.01) compared to that in naïve rats (Fig. 4).
Fig. 4.
Dynamic changes in the number of mOR-positive neurons in L4 and L5 DRGs after SNL. Top panels: representative mOR immunohistochemical staining in the ipsilateral (ipsi) and contralateral (contra) L5 DRGs on days 3, 7, 14, and 35 post-SNL. Bottom: statistical summary of the percentage of mOR-positive neurons in L4 and L5 DRGs on both ipsilateral and contralateral sides on days 3, 7, 14, and 35 post-SNL. Data are presented as mean ± SEM (n = 3/group). * p < 0.05 or ** p < 0.01 compared to the corresponding naïve rats.
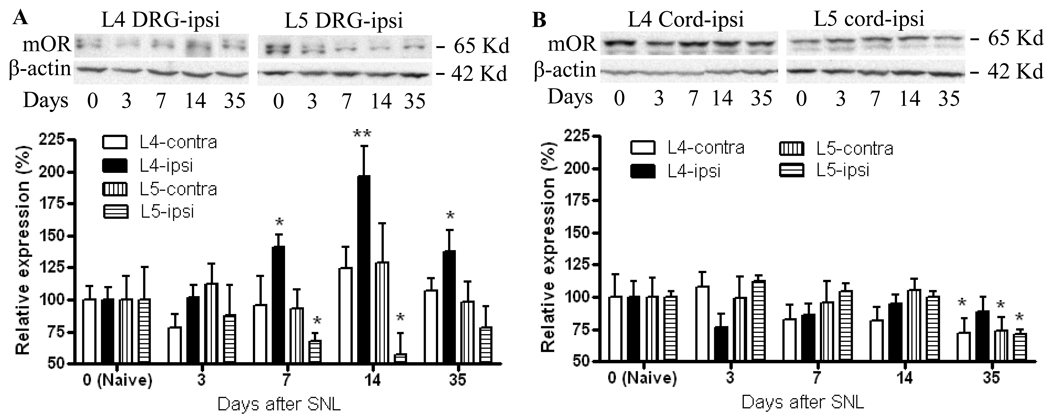
Similarly, by Western blot analysis, mOR expression in the ipsilateral L5 DRG was significantly reduced by 32.2% on day 7 (p < 0.05) and 42.8% on day 14 (p <0.05) post-SNL compared to that in naïve rats (Fig. 5A). No change in mOR expression in the contralateral L5 DRG was observed at any time point post-SNL (Fig. 5A). By immunohistochemistry, SNL did not significantly change the number of mOR-positive neurons in L4 DRG on either the ipsilateral or contralateral side during the observation period (Fig. 4). In contrast, by Western blot analysis, mOR expression in the ipsilateral L4 DRG was time-dependently increased by 40.1% on day 7 (p < 0.05), 96.7% on day 14 (p < 0.01), and 37.4% on day 35 (p < 0.05) post-SNL compared to expression in the naïve rats (Fig. 5A). As expected, Western blot analysis revealed no a significant change in mOR expression in the contralateral L4 DRG during the observation period (Fig. 5A).
Fig. 5.
Dynamic changes in mOR protein expression in L4 and L5 DRG and lumbar spinal cord after SNL. (A) Top panels: examples of Western blots that show the expression of mOR protein in the ipsilateral L4 and L5 DRGs in naïve rats (N) and on days 3, 7, 14, and 35 post-SNL. β-actin is used as a loading control. Bottom: statistical summary of mOR protein expression in ipsilateral and contralateral L4 and L5 DRGs on days 3, 7, 14, and 35 post-SNL. Data are presented as mean ± SEM (n = 4/group). * p < 0.05 or ** p < 0.01 compared to the corresponding naïve rats. (B) Top panels: examples of Western blots that show the expression of mOR protein in ipsilateral L4 and L5 spinal cord in naïve rats (N) and on days 3, 7, 14, and 35 post-SNL. Bottom: statistical summary of mOR protein expression in ipsilateral and contralateral L4 and L5 spinal cord on days 3, 7, 14, and 35 post-SNL. Data are presented as mean ± SEM (n = 4/group). * p < 0.05 compared to the corresponding naïve rats.
3.4. Effect of SNL on expression of mOR protein in the injured and intact spinal cord and hind paw skin
In contrast with the findings in the DRG, changes in mOR expression in the spinal cord were limited following SNL. On day 35 post-SNL, mOR expression was reduced in ipsilateral (29.1%, p < 0.05) and contralateral (26.0%, p < 0.05) L5 spinal cord compared to that in naïve rats (Fig. 5B). Similarly, on day 35 post-SNL, there was a 27.8% reduction in mOR expression in the contralateral L4 spinal cord (p < 0.05, Fig 5B). In contrast, mOR expression in ipsilateral L4 spinal cord did not differ from that in naïve rats at any time point. Sham surgery did not alter mOR expression in the L4 and L5 spinal cord on either the ipsilateral or contralateral side during the observation period (data not shown).
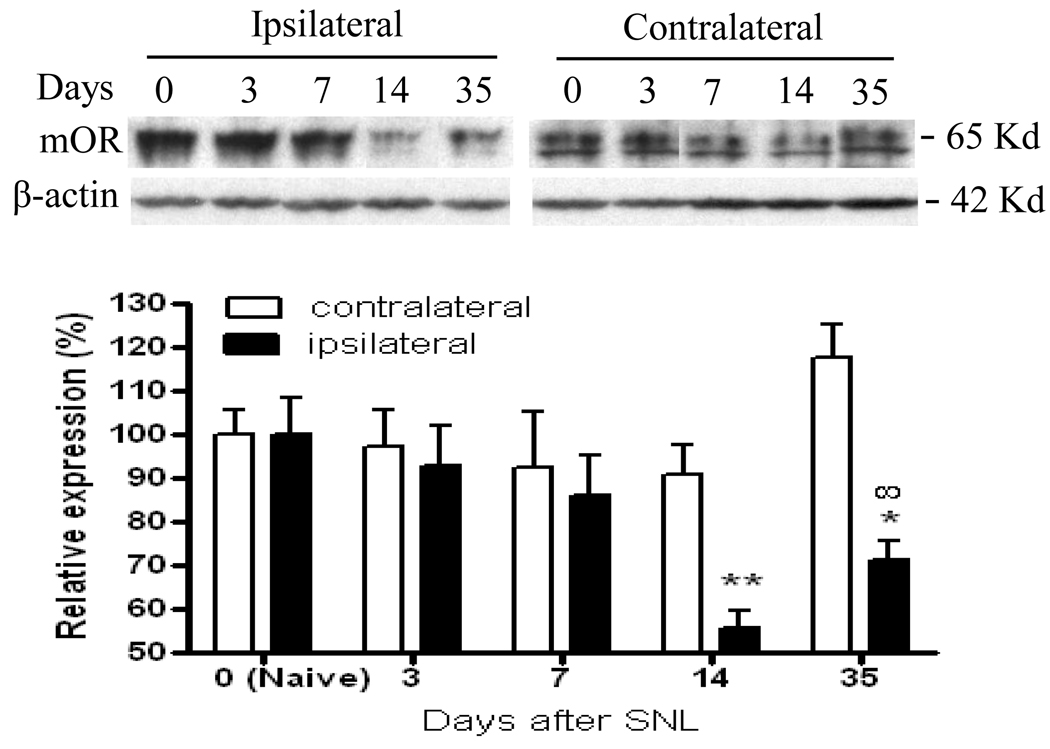
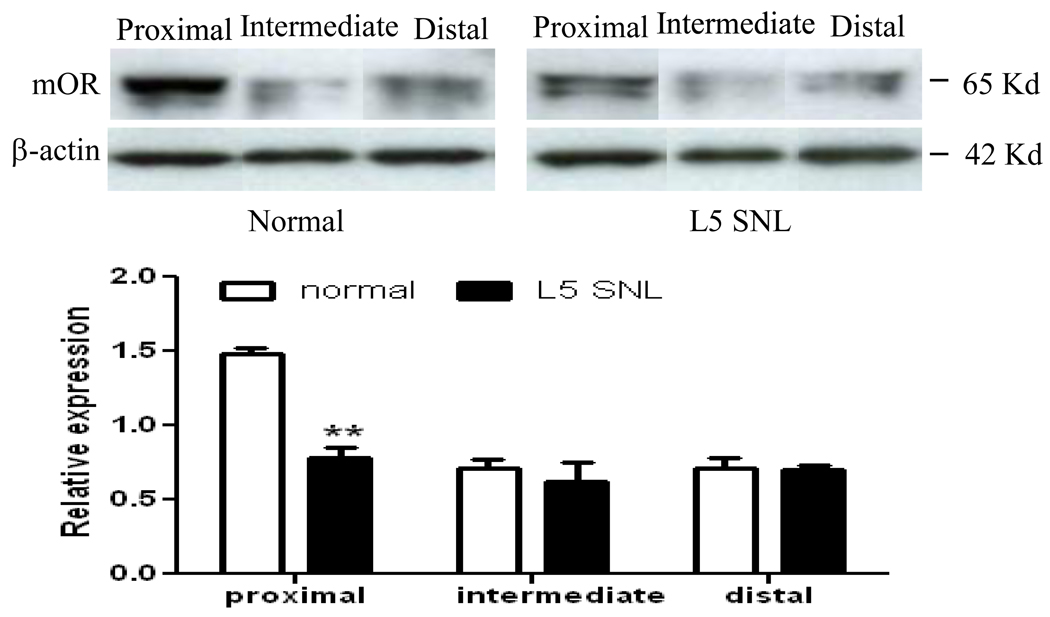
No change in mOR expression was observed in the contralateral hind paw skin (Fig. 6). However, we found a reduction in mOR expression in the ipsilateral hind paw plantar skin (Fig. 6). The level of mOR in the ipsilateral hind paw skin was decreased by 44.4% on day 14 (p < 0.01), and 28.9% on day 35 (p < 0.05) compared to that in naïve rats. As expected, the amount of mOR protein in the ipsilateral hind paw skin on day 14 post-sham surgery was similar to that in naïve rats (data not shown). Furthermore, mOR expression on day 14 post-SNL was lower in the proximal nerve segment of rats subjected to double ligature of the sciatic nerve than in normal rats (Fig. 7). This finding suggests reduced axonal transport of mOR protein on day 14 post-SNL.
Fig. 6.
Dynamic changes in mOR protein expression in the hind paw plantar skin after SNL. Top panels: examples of Western blots that show the expression of mOR protein in the ipsilateral hind paw skin in naïve rats (N) and on days 3, 7, 14, and 35 post-SNL. β-actin is used as a loading control. Bottom: statistical summary of mOR protein expression in ipsilateral hind paw skin on days 3, 7, 14, and 35 post-SNL. Data are presented as mean ± SEM (n = 4/group).* p < 0.05 or ** p < 0.01 compared to the corresponding naïve rats. # p < 0.05 compared to day 14 post-SNL.
Fig. 7.
Decreased axonal transport of mOR on day 14 post-SNL. Injured animals have less mOR receptor in the nerve that is proximal to the double ligature as compared to normal uninjured animals demonstrating decreased axonal transport of mOR. Top panels: examples of Western blots that show the expression of mOR protein in the sciatic nerve proximal, intermediate, and distal to two ligations in normal and SNL rats. Bottom: statistical summary of mOR protein expression in the sciatic nerve proximal, intermediate, and distal to two ligations in normal and SNL rats. Data presented as average arbitrary density normalized to β-actin ± SEM (n=6 animals/group). ** p < 0.01 compared to the corresponding normal rats.
4. Discussion
Peripheral nerve injury leads to persistent pain hypersensitivity in animals that mimics chronic neuropathic pain states in humans. We recently reported that a peripherally acting mu-opioid receptor agonist attenuated neuropathic pain in rats after L5 SNL (Guan et al., 2008). This finding suggests that peripherally acting opioids may provide an alternative strategy for the treatment of neuropathic pain with minimal central nervous system-mediated side effects. However, evidence from preclinical studies and clinical trials indicate that mOR agonists have lower efficacy in the treatment of neuropathic pain when compared to nociceptive pain (Benedetti et al., 1998; Wu et al., 2008). This suggests that peripheral nerve injury might produce the dynamic changes in mOR expression in the peripheral nervous system and the spinal cord. The present study provides biochemical evidence that L5 SNL down-regulates mOR mRNA and protein in the injured L5 DRG and produces temporally specific changes in mOR mRNA and protein in uninjured L4 DRG. In addition, mOR protein expression is reduced in skin and spinal cord after L5 SNL. These temporal and anatomically specific changes in mOR expression after peripheral nerve injury are likely to have functional consequences on neuropathic pain-associated behaviors and opioid analgesia.
In our study, peripheral nerve injury down-regulated expression of mORs in the injured L5 DRG. We found time-dependent reduction of mOR at both mRNA and protein levels in the injured L5 DRG after L5 SNL. The reduction in mOR mRNA occurred before down-regulation of protein expression. Immunohistochemical and Western blot analysis correlated a decrease in the total amount of mOR protein with a decrease in the number of mOR-positive neurons in the injured L5 DRG on days 7 and 14 post-SNL. In line with our observations, investigators have reported a decrease in the abundance of mOR mRNA and/or protein in the injured DRG of rats at one time point (day 14) after SNL (Kohno et al., 2005) and after axotomy (Zhang et al., 1998), partial sciatic nerve ligation (Rashid et al., 2004), and chronic constriction injury (Obara et al., 2009). The mechanisms by which mOR mRNA is down-regulated in injured DRG neurons after peripheral nerve injury are not completely understood, but a recent study suggests that neuron-restrictive silence factor (NRSF), a transcriptional repressor that binds to the neuron-restrictive silencer element within the mOR gene, might be involved in nerve injury-induced downregulation of mOR mRNA in the DRG. Blocking nerve injury-induced up-regulation of NRSF in the DRG neurons attenuates nerve injury-evoked down-regulation of mOR in the injured DRG (Uchida et al., 2010).
One important finding in our study was that mOR RNA and protein underwent dynamic changes in the uninjured L4 DRG after L5 SNL. The ipsilateral L4 DRG showed a transient decrease in mOR mRNA on day 7 followed by a dramatic increase on day 14. Western blot analysis also showed a significant increase in mOR protein in the ipsilateral L4 DRG on days 14 and 35 post-SNL. Interestingly, immunohistochemistry did not reveal a significant change in the number of mOR-positive neurons in the ipsilateral L4 DRG after L5 SNL. These findings indicate a change in mOR protein concentration per neuron as opposed to an increase in the number of neurons expressing mORs in the uninjured L4 DRG. Thus, the change in mOR expression in the uninjured ipsilateral L4 DRG is likely a result of increased protein expression within DRG neurons and not an increased population of mOR-expressing DRG neurons. A previous study showed no change in mOR mRNA and protein in the ipsilateral L4 DRG on day 14 post-L5 SNL (Kohno et al., 2005). The reason for the discrepancy between the previous and present results is unclear but may be related to the use of different methodologies (in situ hybridization vs real time RT-PCR), primary antibodies (Chemicon vs ImmunoStar), and comparisons between groups.
How L5 SNL changes mOR expression in the uninjured L4 DRG is unclear. Several proteins involved in nociception, such as the neuropeptide CGRP, the channels NaV1.8, TRPV1, and TRPA1, and growth factor BDNF are also up-regulated in the uninjured L4 DRG and nerves after L5 SNL (Fukuoka et al., 1998; Fukuoka et al., 2001; Fukuoka et al., 2002; Gold et al., 2003). Electrophysiological recording showed spontaneous activity development and enhanced activity-dependent slowing of conduction in uninjured C-fibers of the L4 spinal nerve after L5 SNL (Wu et al., 2001; Wu et al., 2002; Shim et al., 2007). The changes, including those of mORs, observed in this study might be driven by injury-induced alterations in the milieu surrounding the uninjured nerve and nerve terminals. For example, nerve injury-induced Wallerian degeneration of the axons distal to the injury site involves invasion of the nerves by macrophages and the release of cytokines and chemokines. However, we observed a decrease of mOR expression in paw skin and spinal cord and reduced axonal transport of mOR after L5 SNL. Thus, the significance in an increase of mOR protein expression in uninjured L4 DRG neurons after L5 SNL is unknown and will remain to be elucidated in future studies.
Dynamic temporal and spatial changes in mOR expression after peripheral nerve injury may influence pain-associated behaviors as well as opioid-induced peripheral antinociceptive effects. The relative contribution of the pre- and post-synaptic action of mOR to nociceptive transmission has been addressed in mOR knockout mice. mOR knockout mice displayed enhanced mechanical allodynia in both injured and uninjured paws under neuropathic pain conditions (Mansikka et al., 2004). This finding implies that a loss of pre- and post-synaptic mORs in injured L5 DRG and spinal cord observed in this and previous studies (Coggeshall et al., 1997; Zhang et al., 1998; Kohno et al., 2005) might participate in the development and maintenance of neuropathic pain through loss of a tonic mOR-mediated inhibitory mechanism. We recently reported that systemic and intraplantar injection of loperamide, a peripherally acting mOR-preferring agonist, produced less analgesic effect on day 14 post-SNL than on days 7, 28, and 42 post-SNL (Guan et al., 2008). This finding correlates with our present observation that mOR in the ipsilateral hind paw is lower on day 14 post-SNL than on days 3, 7, or 35 and implies that higher doses of opioids are required to achieve equal analgesic effects. Interestingly, on day 35 post-SNL, the mechanical hypersensitivity was of the same magnitude as on day 14, whereas mOR mRNA and protein synthesis in the injured L5 DRG recovered, although the levels of mRNA and protein were still lower than those in the sham group. The reason for this lack of correlation between the behaviors and biochemical change of mOR is unclear, but it might be related to the complicated mechanisms that underlie the maintenance of neuropathic pain. It is very likely that, rather than mOR-mediated inhibitory mechanism, other peripheral and central mechanisms might play a more key role at the late-phase of neuropathic pain. For example, nerve injury-induced astrocyte activation and release of neuromodulators (e.g., chemokines and cytokins) are required to maintain neuropathic pain (Kawasaki et al., 2008).
In summary, we used a combined approach of real-time RT-PCR, Western blot, and immunohistochemistry to characterize the expression of mOR mRNA and protein in primary afferent neurons following peripheral nerve injury. We demonstrated dynamic temporal and spatial changes of mOR expression in both injured and uninjured DRG neurons in a rat L5 spinal nerve ligation model of neuropathic pain. These changes might be involved in the development and maintenance of neuropathic pain symptoms as well as be responsible for the reduced efficacy of opioid analgesics in clinical treatment of neuropathic pain.
Acknowledgements
This work was supported by National Institutes of Health Grants (NS26363 and NS058886), the Blaustein Pain Research Fund, and the Patrick C. Walsh Prostate Cancer Research Fund. The authors thank Claire Levine, MS, for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
CYL carried out the animal surgery, behavioral testing, all tissue collection, immunohistochemistry and its data analysis, and wrote a draft of the manuscript. FMP was responsible for axonal transport studies, Western blot experiments, and their data analysis. WW and XG performed real-time RT-PCR experiments. XZ and YXT designed the mOR probe and primers. YG was involved in the data interpretation and critical review of the manuscript. JLF tranfected the HEK 293 cells and performed the antibody control studies. SMS, SNR, and YXT contributed to the concept and design of the study, the coordination of all experiments, the data analysis and interpretation, and critical review of the manuscript.
Reference List
- Benedetti F, Vighetti S, Amanzio M, Casadio C, Oliaro A, Bergamasco B, Maggi G. Dose-response relationship of opioids in nociceptive and neuropathic postoperative pain. Pain. 1998;74:205–211. doi: 10.1016/s0304-3959(97)00172-3. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res. 1997;764:126–132. doi: 10.1016/s0006-8993(97)00446-0. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, Lai J. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Zhu X, Tao YX. Peripheral nerve injury up-regulates expression of interactor protein for cytohesin exchange factor 1 (IPCEF1) mRNA in rat dorsal root ganglion. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:459–463. doi: 10.1007/s00210-009-0451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AH, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993;55:185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Lawson JJ, McIlwrath SL, Rau KK, Anderson CE, Albers KM, Koerber HR. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29:1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci. 2006;78:682–688. doi: 10.1016/j.lfs.2005.05.062. [DOI] [PubMed] [Google Scholar]
- Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology. 2004;100:912–921. doi: 10.1097/00000542-200404000-00022. [DOI] [PubMed] [Google Scholar]
- O'Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- Obara I, Makuch W, Spetea M, Schutz J, Schmidhammer H, Przewlocki R, Przewlocka B. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur J Pharmacol. 2007;558:60–67. doi: 10.1016/j.ejphar.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009;141:283–291. doi: 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett. 2004;360:85–89. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, Royall RM, Max MB. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59:1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- Shim B, Ringkamp M, Lambrinos GL, Hartke TV, Griffin JW, Meyer RA. Activity-dependent slowing of conduction velocity in uninjured L4 C fibers increases after an L5 spinal nerve injury in the rat. Pain. 2007;128:40–51. doi: 10.1016/j.pain.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Hruby VJ, Porreca F. Antihyperalgesic effects of loperamide in a model of rat neuropathic pain are mediated by peripheral delta-opioid receptors. Neurosci Lett. 2007;411:143–146. doi: 10.1016/j.neulet.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh OV, Yaster M, Xu JT, Guan Y, Guan X, Dharmarajan AM, Raja SN, Zeitlin PL, Tao YX. Proteome of synaptosome-associated proteins in spinal cord dorsal horn after peripheral nerve injury. Proteomics. 2009;9:1241–1253. doi: 10.1002/pmic.200800636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfa LC, Sullivan AF, Dickenson AH. Alterations in neuronal excitability and the potency of spinal mu, delta and kappa opioids after carrageenan-induced inflammation. Pain. 1992;50:345–354. doi: 10.1016/0304-3959(92)90040-I. [DOI] [PubMed] [Google Scholar]
- Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol. 2003;53:366–375. doi: 10.1002/ana.10465. [DOI] [PubMed] [Google Scholar]
- Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci. 2010;30:4806–4814. doi: 10.1523/JNEUROSCI.5541-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Agarwal S, Tella PK, Klick B, Clark MR, Haythornthwaite JA, Max MB, Raja SN. Morphine versus mexiletine for treatment of postamputation pain: a randomized, placebo-controlled, crossover trial. Anesthesiology. 2008;109:289–296. doi: 10.1097/ALN.0b013e31817f4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci. 2002;22:7746–7753. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tao F, Liaw WJ, Bredt DS, Johns RA, Tao YX. Effect of knock down of spinal cord PSD-93/chapsin-110 on persistent pain induced by complete Freund's adjuvant and peripheral nerve injury. Pain. 2003;106:187–196. doi: 10.1016/j.pain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang G, Mohammad H, Peper BD, Raja S, Wilson SP, Sweitzer SM. Enhanced peripheral analgesia using virally mediated gene transfer of the mu-opioid receptor in mice. Anesthesiology. 2008;108:305–313. doi: 10.1097/01.anes.0000299836.61785.79. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hokfelt T. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82:223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]