Summary
Peanut sensitization in diacylglycerol kinase zeta (DGKζ) deficient mice led to elevated peanut-IgE levels and severe anaphylaxis. DGKζ deficient CD4+T cells did not account for the phenotype. Future studies will determine which immune lineage caused increased food hypersensitivity.
Keywords: Peanut allergy, Diacylglycerolkinase, Toll-like receptor, IgE, Food allergy
To the Editor
Definitive causes of food allergy have been elusive. Most food allergens have properties that may aid in sensitization, such as resistance to pepsin and trypsin digestion, stability at extreme pH and temperatures, and the ability to form aggregates. More recently, the major peanut allergen, Ara h 1, was demonstrated to have Th2 skewing properties through interactions with DC-SIGN [1]. The intrinsic properties of food allergens, however, do not explain why some people develop IgE responses while the vast majority develops oral tolerance. Genetic predisposition is a critical factor for sensitization to food antigens, while other explanations, including the hygiene hypothesis and vitamin D deficiency, exist but are controversial [2].
Studies in mice have examined the role of Tregs, TLR4 signaling, and genetic background as possible causes of a heightened risk to developing food allergies. Depletion of Tregs during oral tolerance induction led to an increase in IgE level and higher mast cell degranulation on oral challenge, compared to mice with intact Tregs [3]. The role of TLR4 signaling was studied for milk and peanut allergies in C3H/HeJ and BALB/c mice [4,5]. C3H/HeJ mice are deficient in TLR4 signaling, which led to an increase in IgE production and more severe anaphylactic reactions on oral challenge. However, in TLR4 deficient BALB/c mice the Th2-skewed immune response present in C3H/HeJ mice was not found, suggesting that additional genetic factors are critical for increased food hypersensitivity. These studies in mice have potential relevance to the human condition as Tregs are known to increase during natural resolution of milk allergy [6], and TLR mutations have been associated with atopy.
Diacylglycerol kinase zeta (DGKζ) deficient mice have impaired responses to TLR agonists, including TLR2, TLR3, and TLR4, leading to decreased IL-12 production by antigen presenting cells [7]. Additionally, DGKζ deficient T cells are more easily activated by signaling through the TCR [8]. We hypothesized that DGKζ deficient mice would be more prone to developing a Th2-skewed immune response to peanut proteins, as indicated by IgE production, which would lead to increased allergic reactions on peanut challenge. DGKζ deficient mice [8] were backcrossed to the C57BL/6 background for greater than seven generations. Mice were used at 6–8 weeks of age and all data were collected on females. The mice were kept under pathogen-free conditions and maintained on a peanut-free diet. All procedures were approved by the Institutional Animal Care and Use Committee at Duke University.
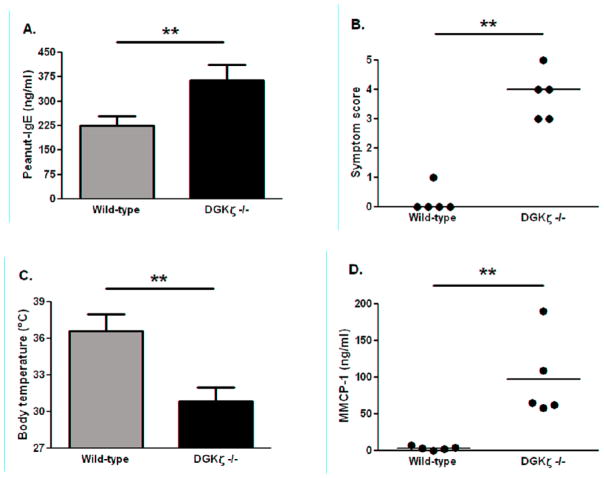
Wild-type C57BL/6 and DGKζ deficient mice on the C57BL/6 background were sensitized to peanut proteins using an established sensitization approach with aluminum hydroxide as adjuvant [9]. Following sensitization, DGKζ deficient mice produced significantly higher peanut-specific IgE than wild-type counterparts (Fig 1A). On challenge with peanut, DGKζ deficient mice experienced significantly more severe allergic symptoms (Fig 1B). The differences between the groups were obvious, as the DGKζ deficient mice reacted at a median score of 4 (cyanosis around the snout and feet with labored breathing, or no movement after prodding) compared to wild-type with a score of zero (no symptoms). Body temperature decrease is a more objective marker of anaphylaxis in mice, and the drop in DGKζ deficient mice was significantly greater than in wild-type mice (Fig 1C). Serum levels of mouse mast cell protease-1 (MMCP-1) were highly increased in DGKζ deficient mice relative to wild-type mice, indicating mast cell degranulation had occurred in DGKζ deficient but not wild-type mice (Fig 1D). These findings clearly demonstrate that DGKζ deficiency leads to increased hypersensitivity to peanuts.
Figure 1. Peanut-specific IgE production and allergic reactions in peanut-sensitized wild-type C57BL/6 and DGKζ deficient mice.
A: Peanut-specific IgE level measured in sera following peanut sensitization. B: Symptom scores following peanut challenge. C: Body temperatures at 30 minutes post-challenge. D: MMCP-1 levels in sera of mice bled 45 minutes following peanut challenge. Individual mice are represented as closed circles with lines indicating median (symptom scores) or mean (MMCP-1). Bars represent means with standard deviation shown. ** indicates p<0.01.
Since DGKζ is expressed in many cell types, we aimed to investigate its role in T cells. CD4+T cells are highly relevant to allergic sensitization, as a Th2-biased response to food allergens is required to drive production of IgE from B cells. CD4+ T cells were isolated from wild-type and DGKζ deficient mice and transferred to T cell deficient mice (TCRβ−/−δ−/−). Briefly, CD4+ T cells were isolated by depletion of non-T cells from mouse spleens using MACS technology, followed by positive selection with anti-CD4 microbeads (Miltenyi). Purity of CD4+ T cells was greater than 95%. One million CD4+ T cells were injected into the retro-orbital vein of TCRβ−/−δ−/− mice (Jackson Laboratory) at 5 weeks of age. CD4+ T cells were allowed to expand for 10 days, and then the mice underwent the same peanut sensitization protocol. Mice were challenged with peanut and then sacrificed to ascertain the presence of CD4+ and CD8+ T cells in spleens and lymph nodes. Both groups of mice had approximately equal numbers of CD4+ T cells in the spleen (data not shown); demonstrating that the T cell transfer was effective and survived the course of the peanut sensitization and challenge.
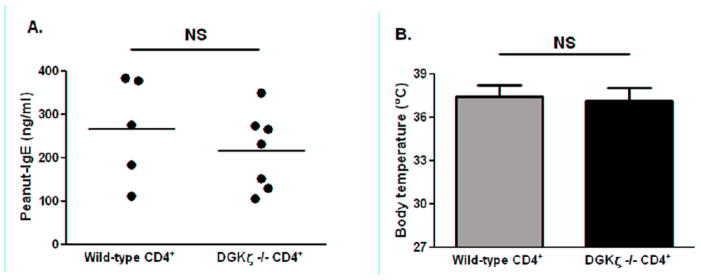
No differences in peanut-specific IgE levels in recipient mice reconstituted with wild-type or DGKζ deficient T cells were found (Fig 2A). Upon challenge with peanut, TCRβ−/−δ−/− mice reconstituted with either DGKζ deficient or wild-type CD4+ T cells had no visible symptoms of anaphylaxis and were given symptom scores of zero (data not shown). Body temperatures measured at 30 minutes post-challenge averaged 37.1°C for mice reconstituted with DGKζ deficient CD4 T cells and 37.5°C for mice reconstituted with wild-type CD4+ T cells (Fig 2B). These findings suggest that a compartment of the immune system other than CD4+ T cells is likely causing the increased allergic responses in DGKζ deficient mice. The most likely contributor is the innate immune system. Generating lineage specific deletion of DGKζ will be helpful to determine the contribution of individual immune lineages in the development of food allergy in DGKζ deficient mice.
Figure 2. Peanut-specific IgE production and allergic reactions to peanut in TCRβ−/−δ−/− mice reconstituted with CD4+ T cells from wild-type or DGKζ deficient mice.
A: Peanut-specific IgE level measured in serum following sensitization. B: Body temperatures at 30 minutes post-challenge. Individual mice are represented as closed circles with lines at the mean. Bars represent means with standard deviation shown. NS: not significant.
In conclusion, we have shown that DGKζ deficient mice have increased hypersensitivity to peanut with severe anaphylactic reactions owing to an immune response which does not involve or depend upon CD4+T cells. It is plausible that TLR signaling deficiency is critical to the phenotype. This novel mouse model of peanut allergy will prove valuable for additional studies on contribution of various cell types in food sensitization and immunotherapy. Clearly, further research in both mice and human samples is necessary to determine the underlying contributors to peanut allergy.
Acknowledgments
Support for this study is provided by the National Institute of Allergy and Infectious Diseases to Xiao-Ping Zhong (NIH1R21-AI079873) and Food Allergy and Anaphylaxis Network to Xiao-Ping Zhong. Mike Kulis wishes to thank the National Institute of Allergy and Infectious Diseases for an NRSA training grant (#F32AI084332).
Abbreviations
- DGKζ
diacylglycerol kinase zeta
- TLR
Toll-like receptor
- MMCP-1
mouse mast cell protease-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Food allergy: separating the science from the mythology. Nat Rev Gastroenterol Hepatol. 2010;7:380–400. doi: 10.1038/nrgastro.2010.80. [DOI] [PubMed] [Google Scholar]
- 3.van Wijk F, Wehrens EJ, Nierkens S, Boon L, Kasran A, Pieters R, Knippels LM. CD4+CD25+ T cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin Exp Allergy. 2007;37:572–581. doi: 10.1111/j.1365-2222.2007.02681.x. [DOI] [PubMed] [Google Scholar]
- 4.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61:64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 5.Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, Li AM. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111:1122–1128. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- 6.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43–52. e47. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 7.Liu CH, Machado FS, Guo R, Nichols KE, Burks AW, Aliberti JC, Zhong XP. Diacylglycerol kinase zeta regulates microbial recognition and host resistance to Toxoplasma gondii. J Exp Med. 2007;204:781–792. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 9.Pons L, Ponnappan U, Hall RA, Simpson P, Cockrell G, West CM, Sampson HA, Helm RM, Burks AW. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004;114:915–921. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]