Abstract
Galectin-1 (Gal-1), a carbohydrate-binding protein whose secretion is enhanced by hypoxia, promotes tumor aggressiveness by promoting angiogenesis and T cell apoptosis. However, the importance of tumor versus host Gal-1 in tumor progression is undefined. Here we offer evidence that implicates tumor Gal-1 and its modulation of T cell immunity in progression. Comparing Gal-1 deficient mice as hosts for Lewis lung carcinoma cells where Gal-1 levels were preserved or knocked down, we found that tumor Gal-1 was more critical than host Gal-1 in promoting tumor growth and spontaneous metastasis. Enhanced growth and metastasis associated with Gal-1 related to its immunomodulatory function, insofar as the benefits of Gal-1 expression to Lewis lung carcinoma growth were abolished in immune-deficient mice. In contrast, angiogenesis, as assessed by microvessel density count, was similar between tumors with divergent Gal-1 levels when examined at a comparable size. Our findings establish that tumor rather than host Gal-1 is responsible for mediating tumor progression through intratumoral immune modulation, with broad implications in developing novel targeting strategies for Gal-1 in cancer.
Keywords: Galectin-1, Lung cancer, immune-modulation, apoptosis, hypoxia
Introduction:
Galectins (Gal) are members of a large carbohydrate binding lectins that share a highly conserved carbohydrate recognition domain (CRD) that is responsible for β–galactoside binding 1. Recently, several galectins, specifically Gal-1, -3 and -9 have been implicated to play a role in cancer progression 2. Although included in the same family, these three galectins are different from each other in size, tissue distribution and function 3. Gal-1 is considered to be the ‘proto-type’ galectin that has one CRD and can form homodimers, whereas Gal-3 and -9 are part of different galectin subgroups 3,4. While the majority of Gal-1 is secreted into the extracellular matrix through a unconventional pathway, independent of the endoplasmic reticulum/Golgi route 4-7, it can also be found in the cytoplasm and the nucleus 2,8. Gal-1 is expressed in many tumor types including astroctyoma, melanoma, protstate, thyroid, colon, bladder and ovary carcinomas 8,9, and its expression correlates with tumor aggressiveness and metastasis 10-13. In lung cancer, increased Gal-1 expression is closely associated with larger tumors, more nodal metastasis and lower overall survival 14.
Gal-1 is well known for its roles in modulating T-cell homeostasis, especially during development in the thymus and after stimulation at the periphery 15,16. However, the exact mechanism by which this protein promotes tumor aggressiveness is not well elucidated. It has been suggested that Gal-1 is involved in various pathological processes such as tumor cell proliferation 17, aggregation 18, adhesion 19, migration 10, cytoskeletal reorganization 20 and apoptosis 21,22. More recently, two exciting new functions of Gal-1 in tumor cells have emerged. These include upregulation of tumor angiogenesis and promotion of tumor immune privilege by modulating intratumoral T-cell survival 23-25. Thijssen et al. has demonstrated that an anti-angiogenic peptide (anginex) binds to Gal-1 in endothelial cells and this interaction resulted in a reduction of endothelial cell proliferation and migration 24. Another study showed that tumor growth and in-vivo angiogenesis are impaired in Gal-1 null mice, suggesting that angiogenesis is a major function of Gal-1 in tumor development 25. In contrast, Rubinstein et al. demonstrated that the level of Gal-1 secretion in cultured supernatant correlated with the extent of tumor-induced T-cell death in both murine and human melanoma cells. Targeted inhibition of Gal-1 expression in-vivo rendered the mice resistant to tumor challenge, which is a process that requires functional CD4+ and CD8+ T-cell response 23. These results suggest that Gal-1 contributes to tumor immune privilege by modulating survival of T-cell subsets.
We have previously found that Gal-1 secretion is significantly enhanced under hypoxia in tumor cells and its tissue expression could be used to assess treatment outcome in a large cohort of head and neck cancer patients. In addition, we noted an inverse relationship between tumor Gal-1 protein expression and the level of intratumoral T-cells in these tumors, suggesting that Gal-1 is a negative regulator of T-cell activation and survival in human tumors 26. In this current study, we investigate the role of tumor versus host Gal-1 on tumor progression and metastasis. Additionally, the contribution of Gal-l’s immunomodulatory versus proangiogenic function on tumor progression is also evaluated. Lewis lung carcinoma (LLC) tumors cells with high (control) and low Gal-1 (shGal-1) expression were implanted into Gal-1 wild-type (WT) and Gal-1 null (Gal-1−/−) mice. We found that the expression of tumor Gal-1, rather than that of the host, was essential for tumor growth and spontaneous metastasis. Lung metastasis was only found in mice bearing Gal-1 expressing tumors and not in the mice bearing shGal-1 tumors. These results remained consistent even when the all tumors were allowed to reach to a comparable size. The effect of Gal-1 on intratumoral T-cell apoptosis and angiogenesis were also examined. We showed that the effect of Gal-1 on tumor growth and metastasis is dependent on the host immune function and its pro-angiogenic effect is no longer evident when T- or B-cell functions are removed in immune-deficient mice.
Materials and methods:
Generation of stable shGal-1 cells
A pLKo.1 plasmid with a shRNA sequence: CCGGCCTACACTTCAATCCTCGC TTCTCGAGAAGCGAGGATTGAAGTGTAGGTTTTT was use to generate shGal-1 lentivirus (Thermo Scientific Open Biosystems, Hunstville, AL). Another pLKo.1 shRNA plasmid with a scramble (Scr) sequence (Addgene Inc., Cambridge, MA): CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGG GCGACTTAACCTTAGG was used as a negative control. Generation of Scr and shGal-1 lenti-viral particles were performed using the Trans-Lentiviral Packaging system (Thermo Scientific Open Biosystems, Huntsville, AL) following the manufacturer’s suggested protocol. Gal-1 expression is detected by Western Blot analysis. Equal amounts of total protein were electrophoresed on 12.5% SDS polyacrylamide gel and incubated overnight at 4°C with goat anti-Gal-1 antibody (1:1000; R&D Systems, Minneapolis, MN). Following this incubation, membranes were probed with the HRP-conjugated anti-goat (1:10000; Invitrogen, Carlsbad, CA) and ECL detection (Amersham Biosciences, NJ. USA). The cell lysis buffer is used as a negative control and recombinant mouse Gal-1 is used as a positive control (1 μg; R&D Systems, Minneapolis, MN). The membranes were also stained with mouse anti-β-actin antibody (1:1000; Sigma-Aldrich, St. Louis, MO) as a loading control. The secondary detection for β-actin with HRP-conjugated anti-mouse (1:10000; Invitrogen, Carlsbad, CA) followed the same procedures as mentioned above.
In addition, Scr cells and shGal-1 cells were plated in triplicates of 5000 cells/plate and allowed to proliferate for 6 days, the cell numbers were quantified every 2 days.
Mouse tumor models
All animal procedures were approved by the Institutional Animal Care and Use Committee at Stanford University. Lgals1 null (Gal-1−/−) breeder mice were obtained from the Consortium for Functional Glycomics (Scripps Research Institute). C57BL/6 mice (Jackson Laboratory, Sacramento, CA) were bred with Gal-1−/− to generate wild-type littermates (WT). The genotype of the mice was determined by polymerase chain reaction (PCR) reactions using the antisense primer:AAACTTCAGCCGGGAGAAAGG; wild-type primer: GACCCCATCCCTACACCCCAG and Gal-1 null primer: CTATCAGGACATAGCGTTGG. Wild-type allele is indicated by 380 bp fragment while a 280 bp fragment will appear for the Gal-1−/− allele on a 2% ethidium bromide agrose gel.
The WT and Gal-1 null mice were inoculated subcutaneously with either 2×105 Scr cells or shGal-1 cells at 2 month of age. A total of 60 mice were used for this study: Gal-1 null/Scr (n = 15), Gal-1 null/shGal-1 (n = 15), WT/Scr (n = 15) and WT/shGal-1 (n = 15). The animals were euthanized when the tumor volume reached ~ 1.0 – 2.0 cm3 or 10% of the total body weight. Separate experiments were also performed to collect tissue at earlier time-points post-implantation.
2 month old Nod.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (Nod-Scid; n = 15), B6.Cg-Foxn1nu/J (T cell−/−; n = 15) and B6.129S2-Ign-6tm1Cgn/J (B cell−/−; n = 15) mice were purchased from Jackson Laboratory (Sacramento, CA) to study the effects of immunity on tumor growth. 7 animals from each mouse strain were inoculated subcutaneously with Scr control cells (1.5 × 105 cells), while 8 animals from each mouse strain were implanted with shGal-1 cells (1.5 × 105 cells). Animals were euthanized when the tumor volume reached ~ 1.0 – 2.0 cm3 or 10% of the total body weight.
Immunohistochemistry
Paraffin embedded sections of tumor were deparaffinized and incubated at 4 °C overnight with a goat anti-Gal-1 antibody (1:50 dilution; R&D Systems, Minneapolis, MN) or rabbit anti-CD3 (1:100 dilution; Cell Marque, Rocklin, CA) rabbit anti-Ki67 (1:100 dilution; Cell Marque, Rocklin, CA). Secondary detection was performed using either an anti-goat or anti-rabbit peroxidase polymer detection kit (Vector Laboratories, Burlingame, CA). The staining signal was detected by using the 3,3′-daiminobenzidine (DAB) substrate kit (Vector Laboratories, Burlingame, CA). The sections were counter stained with hematoxylin (Sigma-Aldrich, St. Louis, MO). Images were acquired by Leica DM6000 B microscope (Leica Microsystems Inc., Bannockburn, IL). The images of tumor sections were taken at 400x magnification.
Tumor samples collected at 22 days post-implantation and at >25 days when tumors reached its maximum allowable volume (10% of body mass, ~1.0 – 2.0 cm3) were stained for angiogenic marker CD31. Scr tumors from wildtype animals were also collected at an early timepoint (approximately day 14) when these tumors were of comparable size as that of shGal-1 tumors at day 22 (~0.35 cm3) and stained for CD31. Paraffin embedded sections were deparaffinized and incubated at 4°C overnight with goat anti-CD31 (1:100 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA), followed by secondary detection as described above. Images were taken both 100x and 200x magnification. Images of CD31 staining at 100x magnification were quantified using ImageJ software. A total of 4 visual fields of 1.26 mm2 area from each tumor section were quantified.
Hematoxylin and Eosin (Sigma-Aldrich, St. Louis, MO) staining of paraffin lung sections was use to determine the number of lung metastases in each animal. A total of 3 lung sections per mouse lung sample were quantified under 50x magnification. Paraffin lung sections were also stained for Gal-1 as mentioned above. The staining signal was detected by using the 3-amino-9-ethylcarbazole (AEC) substrate kit (Vector Laboratories, Burlingame, CA). The images of lung sections were taken at 100x magnification.
Frozen tissue sections were warmed to room temperature and fixed in acetone on ice for 20 min. After fixation, slides were washed in phosphate buffered saline (PBS) solution. Slides were incubated at 4 °C overnight with rat anti-CD4 or rat anti-CD8 (1:100 dilution; BD Biosciences, San Jose, CA). After overnight incubation the slides were rinsed with PBS and then incubated with Alexa Fluor 594 anti-rat secondary antibody (1:200 dilution; Invitrogen, Carlsbad, CA) at room temperature for 1 hour. The labeled sections were fixed in 1% paraformaldehyde (PFA) solution for 15 min at room temperature, proceeded by TUNEL labeling with an in situ cell death detection kit (Roche Applied Science, Indianapolis, IN) using the method suggested by the manufacturer. All sections were mounted with medium containing 4′, 6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA). Immunofluorescence images were acquired at 400x magnification using a Leica TCS SP2 confocal microscope (Leica Microsystems Inc., Bannockburn, IL). Total positive TUNEL nuclei and TUNEL positive cells colocalized with CD4 and CD8 membrane staining were quantified with ImageJ software. 4 visual fields at 400x magnification per tumor section were quantified.
In-vitro T-cell study
Splenocytes of wild-type C57BL/6 animals were collected by mechanical disruption of the spleens 23 and T-cell isolation was performed using the EasySep mouse T-cell enrichment kit (STEMCELL Technologies Inc., Vancouver, BC). Concanavalin A (ConA, 5 μg/ml, Sigma-Aldrich, St. Louis, MO) was use in this study to activate cultured T-cells. T-cells were cultured in RPMI 1640 medium. T-cells were divided into 4 treatment groups: control, recombinant mouse Gal-1 (rmGal-1:10 μg/ml, R&D Systems, Minneapolis, MN), ConA, and ConA + rmGal-1. After 48 hours of treatments, the cells were fixed in 1% PFA at room temperature for 20 min and permeabilized with 70% ethanol on ice for 30 min. The cells were labeled for TUNEL using the Apo-Direct kit (BD Biosciences, San Jose, CA). The cells were analyzed with the BD LSR II flow cytometer (BD Biosciences, San Jose, CA). The FACS data was processed with Flowjo software (Tree Star Inc., Ashland OR).
The effects of Gal-1 on T-cell proliferation was detected through carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen, Carlsbad, CA) labeling. Freshly isolated T-cell were diluted to 1×106 cells/ml and labeled with 1 μM CFSE as suggested by the manufacturer’s protocol. CFSE labeled T-cells were divided into the 4 treatment groups and cultured under the same conditions as mentioned above. Following 48 hours of treatment, the T-cells were collected and analyzed through FACS.
Hypoxprobe-1 (pimonidazole) detection
A separate group of WT (n = 5) and Gal-1 null (n = 5) mice were used to detect hypoxic regions and T-cell death in Scr and shGal-1 tumors. WT mice were inoculated subcutaneously with 2×105 Scr cells while Gal-1 null mice were inoculated with the same number of shGal-1 cells and the tumors were allow to grow for 2 weeks. Intraperitoneal injection of Hypoxyprobe-1 (60 mg/kg, HPI Inc., Burlington, MA) 90 mins prior to euthanasia was use to detect hypoxic regions in the tumor.
Frozen tumor tissue sections were incubated at 4 °C overnight with rat anti-CD4 or rat anti-CD8 as described above. After overnight incubation the slides were rinsed with PBS and then incubated with Alexa Fluor 647 anti-rat secondary antibody (1:200 dilution; Invitrogen, Carlsbad, CA) at room temperature for 1 hour. All sections were also incubated with mouse anti-hypoxyprobe-1 (1:50; HPI Inc., Burlington, MA) and then incubated with Alexa Fluor 594 anti-mouse secondary antibody (1:200 dilution; Invitrogen, Carlsbad, CA). The labeled sections were fixed in 1% paraformaldehyde (PFA) solution for 15 min at room temperature, proceeded by TUNEL labeling using the method suggested by the manufacturer. All sections were mounted with medium containing DAPI. Immunofluorescence images were acquired at 400x magnification using a Leica confocal imaging system.
Statistical analyses
Data are expressed as mean ± SEM. Statistical analysis was performed using IBM SPSS 19 software (SPSS Inc., Chicago, IL). A two-way ANOVA and post-hoc Tukey’s test was used determine the significant differences of the tumor growth rates between mice strain and treatment groups. Lung metastases, TUNEL and CD31 measurements was analyzed with the unpaired Student’s T-test. A p-value ≤ 0.05 is considered to be statistically significant.
Results:
Tumor growth and spontaneous metastasis in the in-vivo mouse tumor models
Wild-type LLC cells were infected with either a lentiviral control scramble construct (Scr) or a shGal-1 construct to create a knockdown Gal-1 cell line (shGal-1, Fig. S1a). To assess the effects of host Gal-1 in tumor growth and metastasis, Gal-1 null (Gal-1−/−) mice were bred with C57BL/6 mice to generate Gal-1 wild-type (WT) littermates. The wild-type (380 bp) and knockout (280 bp) genotypes were confirmed by PCR analysis (Fig. S1b). Scr and shGal-1 LLC cells were implanted in the flank of either WT or Gal-1 null mice to form subcutaneous tumors. Four groups of mouse/tumor were generated: Gal-1 WT/Scr (high Gal-1 expression in both host and tumor), Gal-1 null/Scr (no host Gal-1 expression, high in tumor), Gal-1 WT/shGal-1 (normal host Gal-expression, low in tumor) and Gal-1 null/shGal-1 (low Gal-1 expression in both). The mice were sacrificed in two different cohorts. In the first cohort, all four groups were euthanized at the same time, around day 22 when the difference in tumor size between the groups was the largest. In the second cohort, the tumors were allowed to reach the maximum comparable volume of approximately 1.0 – 2.0 cm3 for all 4 groups then sacrificed. This means that four groups were sacrificed at different time points due to differential tumor growth rate.
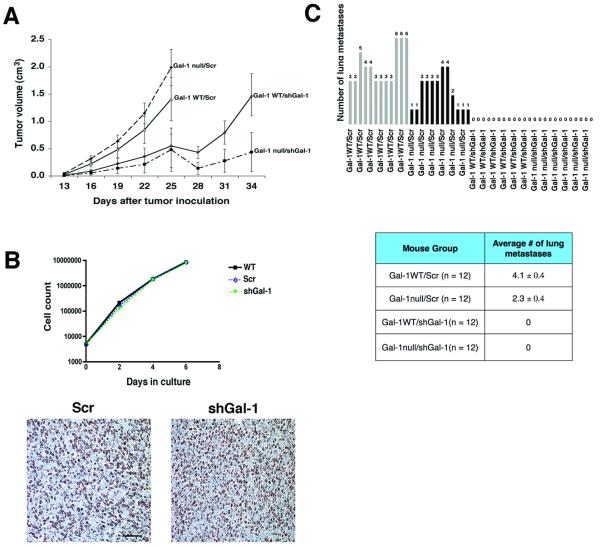
Fig. 1a shows that the rate of tumor growth is significantly delayed by knocking down tumor but not host Gal-1 (this experiment was performed two times). In fact, scramble control tumors (Scr) grew slightly faster in Gal-1 null mice than in Gal-1 WT mice. However, shGal-1 tumors grew significantly slower than Scr tumors in either host with the slowest rate noted in the null mice. The difference in the tumor growth rate in-vivo was not due to an aberrant effect of knocking down Gal-1 on cell proliferation since in-vitro growth curves showed similar growth rate between parental, Scr and shGal-1 cells (Fig. 1b). Moreover, Ki67 staining of the tumor showed similar level of Ki-67 positive cells in Scr and shGal-1 tumors (Fig. 1b).
Figure 1. Silencing Gal-1 expression in LLC cells delayed tumor growth and metastasis.
Panel A: Average tumor volume over time for the 4 different groups of mice. The shGal-1 tumors were significantly smaller compared to Scr tumors regardless of mouse phenotype (p = 0.01). Tumor growth was impaired when mice (WT and Gal-1 null mice) were inoculated with shGal-1 cells. The Scr tumors in WT and Gal-1 null mice showed similar growth rate. Panel B upper: In-vitro growth curves showing similar growth rate between WT (wild-type parental), Scr and shGal-1 tumors. Panel B lower: Ki67 staining (200x magnification; scale bar = 100 μm) showing similar number of Ki-67 positive cells in excised Scr and shGal-1 tumors. Panel C: the upper graph shows the absolute number of spontaneous lung metastasis quantified with light microscopy. The lower table shows the average number of lung metastases/section for each mouse group. Note that Gal-l WT/Scr mice had the highest number of lung metastases (n = 12, 4.1 ± 0.4 mets/lung section) followed by Gal-1 null/Scr mice (n = 12, 2.3 ± 0.4 mets/lung section). None of the mice with shGal-1 tumors developed spontaneous lung metastasis (Gal-1 WT and Gal-1 null: n = 12, 0 ± 0 mets/lung section).
Interestingly, tumor Gal-1 expression also influenced the development of spontaneous lung metastases when the tumors were grown in the flank of these mice, as quantified by light microscopy. In this experiment, the tumors in the four groups were allowed to reach the maximal allowable size size (see above). The animals were then sacrificed and the lungs were examined for spontaneous metastasis. None of the mice inoculated with shGal-1 tumor cells developed lung metastasis (even when the tumor size were > 1.0 cm3) whereas Scr cells developed on the average 4.1 ± 0.4 metastases/lung section in the WT mice and 2.3 ± 0.4 metastases/lung section in Gal-1 null mice. We also confirmed that shGal-1 cells continued to maintain low level of Gal-1 expression in vivo by immunoblot and immunohistochemical staining of tumor tissue (Fig. S1c). Furthermore Gal-1 staining of the lung sections from WT mice bearing Scr tumors showed that Gal-1 protein expression is significantly more intense in the lung metastases than in the adjacent normal lung tissues (Fig. S4). Our result showed that reduction of Gal-1 protein in the tumor but not the host suppressed both tumor growth and distant metastasis.
Mechanism of tumor Gal-1 on cell growth and metastasis
Immunomodulation via T-cell apoptosis
Gal-1 has been shown to promote tumor growth through several mechanisms. One major mechanism focuses on its inhibitory effect of immune tumor surveillance by promoting intratumoral T-cell apoptosis 23, blocking T-cell activation 27 and inhibiting secretion of pro-inflammatory cytokines. Another major mechanism invokes its pro-angiogenic effect 25. Here we aimed to elucidate some of these mechanisms in our model
Like others, we found that exposing splenocytes to recombinant Gal-1 resulted in increased T-cell apoptosis (by TUNEL) and suppressed T-cell division (measured by CSFE), especially when these cells were activated by Concavalin A (Fig. S2). We then proceeded to examine tumor growth and spontaneous metastasis for both Scr and shGal-1 tumor cells in several immune-compromised mouse models, hence eliminating the immunomodulatory effect of Gal-1. These included Nod.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (Nod-Scid; lacking B/T/macrophage and natural killer cell function), B6.Cg-Foxn1nu/J (lacking T cell function) and B6.129S2-Ign-6tm1Cgn/J (lacking B cell function). As shown in Fig. 2a, both Scr and shGal-1 tumors grew at the same rate in these immune-compromised mice. Likewise, the number of spontaneous lung metastases was similar for Scr and shGal-1 tumors (Fig. 2b: 19.8 ± 3.8 metastases for Scr versus 17.4 ± 2.8 mets for shGal-1). These data strongly indicated that the Gal-1’s enhancing effect on tumor growth and metastasis that was previously noted in immune-competent mice was most likely due to its immunomodulatory function.
Figure 2. The effects of Gal-1 on tumor growth and metastasis are eliminated in immuno-compromised mouse models.
Panel A: The average tumor volume over time of Scr and shGal-1 tumors grown in Nod.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (Nod-Scid; n = 15), B6.Cg-Foxn1nu/J (T cell−/−; n = 15) and B6.129S2-Ign-6tm1Cgn/J (B cell−/−; n = 15) mice. Note the growth rate was similar for both tumor types. Panel B: Average number of spontaneous lung metastases for Scr (n = 5) and shGal-1 (n = 7) in Nod-Scid mice. The mice inoculated with Scr (19.8 ± 3.8 mets/lung section) and shGal-1 (17.4 ± 2.8 mets/lung section) tumors show similar number of lung metastasis.
To support this observation, we stained the mouse tumors for CD3, a pan-T cell marker. As shown in Fig. 3a, there was more CD3 staining in the shGal-1 tumors than in the Scr tumors, suggesting less T-cell death. We then co-localized CD4 (T-helper cell marker) and CD8 (cytotoxic T cell marker) with TUNEL and quantified the number of apoptotic T-cells within the tumors. As expected, there was significantly more T-cell apoptosis in the Scr tumors than in shGal-1 tumors in the WT mice (Fig. 3b). The CD8+ T-cell death in the Scr tumors (n = 4, 61.2 ± 8.3%) was significantly greater than that in shGal-1 tumors (n = 4, 15.3 ± 4.6%). Similarly, the CD4+ T-cell death in the Scr tumors (n = 4, 46.2± 7.5%) was higher than in shGal-1 tumors (n = 4, 3.9 ± 1.5%). Similar observation was also made in tumors growing in Gal-1 null mice (Fig. S3a).
Figure 3. Silencing Gal-1 expression in tumor cells enhanced lymphocyte survival.
Panel A: CD3 staining (DAB detection, 200x magnification, scale bar = 50 μm) demonstrated greater intratumoral lymphocytes in the shGal-1 tumors than the Scr tumors grown in the Gal-1 WT host. Staining of mouse lymph nodes is used as a positive control. Panel B: colocalization of CD4 and CD8 (red) with TUNEL (green) was performed on Gal-1 WT mouse tumors (400x magnification, scale bar = 50 μm). Cell nucleus is labeled with DAPI (blue). The greatest amount of CD8 cell death is found in the Scr tumors (n = 4, 61.2 ± 8.3%) when compared to the shGal-1 tumors (n = 4, 15.3 ± 4.6%). Similarly, CD4 T-cell death in the Scr tumors (n = 4, 46.2± 7.5%) is greater than the shGal-1 tumors (n = 4, 3.9 ± 1.5%).
Since hypoxia induces Gal-1 secretion, we hypothesize that we would observe more T-cell apoptosis in the hypoxic regions of Gal-1 expressing tumors but not in shGal-1 tumors. We colocalized CD4, CD8, TUNEL and pimonidazole (a hypoxia marker) in the WT/Scr and Gal-1 null/shGal-1 tumors (Fig. S5). Although we saw minimal T-cell apoposis within the hypoxic regions of either tumors, we noted more viable CD4+ (Fig. S5 top right) and CD8+ T-cells (Fig S5 bottom right) in the hypoxic areas of shGal-1 tumors than in Scr tumors. Moreover, Scr tumors had more apoptotic CD4+ and CD8+ T-cells in the periphery of hypoxic regions. These data suggest that hypoxia enhances Gal-1 secretion and diffusion, which promotes T-cell apoptosis.
Effects of Gal-1 on Angiogenesis
Several studies have indicated that Gal-1 also has a pro-angiogenic function, which may primarily drive tumor growth and metastasis 24,25. Therefore, we evaluated the microvessel density count (MVD) by CD31 staining on tumor tissues collected at three different time points. For the first time point, both shGal-1 and Scr tumors were implanted at the same time (day 0) and sacrified at day 22 when the tumor growth differential was the largest between the two tumor groups (n = 3/group). For the second time point, we sacrified the mice when the tumors reached the maximum allowable volume (approximately 1.5 cm3, n = 3/group). The time of sacrifice for the Scr group was around day 22-25 and for shGal-1 tumor was day 31-34. This time point assesses MVD when both tumors were of comparable large size. For the third time point, we sacrified the mice bearing Scr tumors at a much earlier time when these tumors reached a volume similar to that of shGal-1 tumor at day 22 (approximately 0.3 – 0.4 cm3, n=3/group). This time point assesses MVD when both tumors were of comparable small size. At the first time point, MVD was significantly lower in the shGal-1 tumors (34.0 ± 4.6 microvessels/mm2) than in Scr tumors (62.0 ± 5.7 microvessels/mm2) in WT host (Fig. 4a I and b I). Similarly, less MVD was noted in shGal-1 (27.0 ± 2.6 microvessels/mm2) than in Scr tumors (35.2 ± 3.7 microvessels/mm2) in Gal-1 null mice, but the difference was not as marked as in WT mice (Fig. S3b, left panel). In contrast, similar MDVs were noted when shGal-1 and Scr tumors were of comparable large size (MVD: 40.9± 2.9 microvessels/mm2 vs. 40.1 ± 2.6 microvessels/mm2, Fig. 4a II and b II) or comparable small size (MVD: 33.5 ± 1.6 microvessels/mm2 vs. 34.0 ± 4.6 microvessels/mm2, Fig. 4a III and b III). Similar trend was also noted when the MVD of Scr tumors (47.5 ± 2.9 microvessels/mm2) was compared to that of shGal-1 tumors (42.4 ± 6.3 microvessels/mm2) tumors in Gal-1 null hosts (Fig. S3b, right panel: n = 3/group). Moreover, the MVD of tumors grown in immune-compromised mice (T-cell−/− and B cell−/−) showed no significant difference between Scr and shGal-1 tumors (Fig. 4c). The average MVD for Scr tumors in the B cell−/− host (43.3 ± 2.1 microvessels/mm2, n = 3) and T-cell−/− host (22.8 ± 1.3 microvessels/mm2, n = 3) did not differ from their respective shGal-1 tumors in the B cell−/− host (37.7 ± 3.7 microvessels/mm2, n = 3) and T-cell−/− host (23.9 ± 1.0 microvessels/mm2, n = 3). Note that the Scr and shGal-1 tumors showed a similar growth rate throughout the time of observation in these immune-compromised mice. These data argues that Gal-1 immunomodulatory function governs its effect on angiogenesis, which was no longer apparent when the immune function was removed in immune-compromised mice.
Figure 4. Pro-angiogenic effect of Gal-1 is related to the tumor volume.
Panel A: Quantification of microvessel density count (MVD) based on CD31 staining were performed on WT mouse tumor tissues (200x magnification, scale = 100 μm). The graph shows the timepoints when tumors were collected to examine for MVD. The MVD was examined at 22 days after tumor implantation when tumor volume between the Scr and shGal-1 tumors showed the largest difference (panels A I and B I). At 22 days post-implantation, the MVD was significantly reduced in the shGal-1 tumor (n = 3, 34.0 ± 4.6 microvessels/mm2; tumor volume: 0.35 ± 0.15 cm3) when compared to the Scr tumors (n = 3, 62.0 ± 5.7 microvessels/mm2, tumor volume: 0.85 ± 0.25 cm3). The MVD of tumors at collected at >25 days after tumor implantation when tumor volume was comparable between the two groups was also examined (panels A II and B II). There was no difference in MVD between the Scr (n = 3, 40.1 ± 2.6 microvessels/mm2, tumor volume: 1.80 ± 0.25 cm3) and shGal-1 (n = 3, 40.9± 2.9 microvessels/mm2, tumor volume: 1.46 ± 0.42 cm3) tumors. Finally, the MVD of Scr tumors collected when the tumor volume (0.36 ± 0.08 cm3, < 22 days) was comparable to that of shGal-1 tumor at day 22 was examined (panels A III and B III). The MVD of the smaller Scr tumors (n = 3, 33.5 ± 1.6 microvessels/mm2) is simlar to the MVD of shGal-1 at day 22 post implantation (34.0 ± 4.6 microvessels/mm2). Panel C: quantification of MVD in Scr and shGal-1 tumors grown in B cell−/− and T-cell−/− mice showed no significant difference between the Scr and shGal-1 tumors. The average MVD for Scr in the B cell−/− host (n = 3, 43.3 ± 2.1 microvessels/mm2) and T-cell−/− host (n = 3, 22.8 ± 1.3 microvessels/mm2) did not differ from their respective shGal-1 tumors in the B cell−/− host (n = 3, 37.7 ± 3.7 microvessels/mm2) and T-cell−/− host (n = 3, 23.9 ± 1.0 microvessels/mm2).
Discussion:
Gal-1 is a secreted protein that is expressed by both tumor and host cells from various organs 28. Rabinovich et al. had previously validated that Gal-1 plays an immunoregulatory role through T-cell apoptosis using cultured peripheral blood mononuclear cells 29. It has also been shown that abrogating Gal-1 expression in Hs683 giloblastoma cells and B16F10 melanoma cells increased the sensitivity to chemotherapeutic agents 30,31. The role of host Gal-1 and tumor cell Gal-1 during tumor progression is yet to be determined.
Here we show that tumor Gal-1 is more critical in promoting tumor growth and metastasis and that host Gal-1 is less essential in our model. Depleting Gal-1 in the tumor resulted in a significant tumor growth delay and aborted the development of spontaneous lung metastasis in subcutaneously implanted tumors. Tumors expressing Gal-1 grew at the same rate and had similar numbers of lung metastases regardless of the host phenotype (Gal-1 WT and null mice), indicating that tumor Gal-1 is sufficient to promote tumor growth and distant spread in these mice. In contrast, a much smaller tumor growth delay effect was noted when host Gal-1 was removed and it was only observed when Gal-1 was down-regulated in the tumor. Since presumably the same protein was produced and secreted by the tumor and the host cells, a possible explanation for our findings is the difference in the level of Gal-1 protein generated and secreted between the tumor and the host normal tissues. Appreciably higher expression of Gal-1 mRNA has been reported for human lung cancers when compared to matched normal lung tissues 32. Similarly, we noted a considerably stronger expression of Gal-1 protein in the lung metastases from the Scr tumors than in the adjacent normal lungs of WT mice (Fig. S4). Moreover, our previous study has shown that hypoxia, which a common phenomenon in solid tumors, significantly enhanced the secretion of Gal-1 protein from tumor cells. Since hypoxia is not present in most normal tissues, hypoxia induced Gal-1 secretion from the tumor may also contribute to the differential effect noted for tumor and host Gal-1. Finally, the activity of this protein is enhanced when it forms homodimers, which are more likely to occur when it is secreted at high levels.
Several strategies presently exists for targeting Gal-1 activity; these include blocking antibodies to deplete Gal-1 and to interfere with its ligand binding, natural polysaccharides or synthetic glycodendrimers that complete with its glycan-binding ability or the peptide anginex, which mainly binds to Gal-1 in endothelial cells 24,33. If Gal-1 pro-angiogenic activity is the most crucial for tumor growth and metastasis, then targeting Gal-1 with anginex may be better than other approaches as it will be more specific with less normal tissue toxicity. On the other hand, if its immunomodulatory function is more crucial, then antibody or polysaccharide targeting approach may be more effective. Here we showed that Gal-1 immunomodulatory function may be more critical than its pro-angiogenic function in our tumor model. In immune-competent mice, there was significantly greater T-cell apoptosis for both CD4+ and CD8+ cells in Scr tumors than in shGal-1 tumors. This observation was consistent with previously published data for B16 melanoma cells 23. However, when either T- or B-cell functions are depleted, we no longer observed a difference in either the tumor growth rate or the MVD count between Scr and shGal-1 tumors. Together, these data suggest that Gal-1 immunomodulatory function governs its angiogenic effect and that targeting Gal-1 immunomodulatory property is an important avenue to explore for future therapy.
Interestingly, the growth rate for Scr and shGal-1 tumors when implanted in B6.129S2-Ign-6tm1Cgn/J (B-cell−/−) mice show no significant difference. Although Gal-1’s immunomodulatory function is primarily associated with its effects on T-cell, it has also been is shown to negatively regulate B-cell proliferation. Upon B-cell receptor activation, Gal-1 has been suggested to accelerate B-cell apoptosis via BIM upregulation 34-36. Moreover, past studies had demonstrated that these B-cell−/− mice possess reduced numbers of CD4+ and CD8+ splenic population compare to wild-type animals 37. They have smaller memory CD4+ pool and their CD4+ cells produced less IL2 upon antigen stimulation due to decreased CD4 expansion 38. These mice also failed to respond to T-cell dependent ovalbumin stimulation hence indicating abnormal T-cell function 37-39. Thus, the marginal T-cell function in these mice combined with Gal-1’s effects on B-cell function abolished the Gal-1 tumor growth promoting effect in the B-cell−/− mice.
In summary, using genetic models, we show tumor Gal-1 is more essential than host Gal-1 in enhancing tumor growth and metastasis, which appears to be mediated via its immunomodulatory function. These findings have broad implications in developing novel targeting strategies for Gal-1 in cancer.
Supplementary Material
Acknowledgments:
This investigation was supported by Public Health Service Grant Number P01-CA67166 (QTL, AB, HC, DB, AK, AJG) awarded by the National Cancer Institute, Department of Health and Human Services.
References:
- 1.Rabinovich GA, Ilarregui JM. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev. 2009;230:144–59. doi: 10.1111/j.1600-065X.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 2.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Baum LG, Tinari N, et al. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–20. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 4.Barondes SH, Castronovo V, Cooper DN, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–8. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 5.Cleves AE, Cooper DN, Barondes SH, et al. A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol. 1996;133:1017–26. doi: 10.1083/jcb.133.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seelenmeyer C, Stegmayer C, Nickel W. Unconventional secretion of fibroblast growth factor 2 and galectin-1 does not require shedding of plasma membrane-derived vesicles. FEBS Lett. 2008;582:1362–8. doi: 10.1016/j.febslet.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–85. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 8.Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572:285–93. doi: 10.1016/s0304-4165(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovich GA. Galectin-1 as a potential cancer target. Br J Cancer. 2005;92:1188–92. doi: 10.1038/sj.bjc.6602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camby I, Belot N, Rorive S, et al. Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 2001;11:12–26. doi: 10.1111/j.1750-3639.2001.tb00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Brule FA, Waltregny D, Castronovo V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J Pathol. 2001;193:80–7. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH730>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Cindolo L, Benvenuto G, Salvatore P, et al. galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int J Cancer. 1999;84:39–43. doi: 10.1002/(sici)1097-0215(19990219)84:1<39::aid-ijc8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Sanjuan X, Fernandez PL, Castells A, et al. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology. 1997;113:1906–15. doi: 10.1016/s0016-5085(97)70010-6. [DOI] [PubMed] [Google Scholar]
- 14.Szoke T, Kayser K, Baumhakel JD, et al. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology. 2005;69:167–74. doi: 10.1159/000087841. [DOI] [PubMed] [Google Scholar]
- 15.Vespa GN, Lewis LA, Kozak KR, et al. Galectin-1 specifically modulates TCR signals to enhance TCR apoptosis but inhibit IL-2 production and proliferation. J Immunol. 1999;162:799–806. [PubMed] [Google Scholar]
- 16.Liu SD, Whiting CC, Tomassian T, et al. Endogenous galectin-1 enforces class I-restricted TCR functional fate decisions in thymocytes. Blood. 2008;112:120–30. doi: 10.1182/blood-2007-09-114181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopitz J, von Reitzenstein C, Andre S, et al. Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem. 2001;276:35917–23. doi: 10.1074/jbc.M105135200. [DOI] [PubMed] [Google Scholar]
- 18.Tinari N, Kuwabara I, Huflejt ME, et al. Glycoprotein 90K/MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. Int J Cancer. 2001;91:167–72. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1022>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.van den Brule FA, Buicu C, Baldet M, et al. Galectin-1 modulates human melanoma cell adhesion to laminin. Biochem Biophys Res Commun. 1995;209:760–7. doi: 10.1006/bbrc.1995.1564. [DOI] [PubMed] [Google Scholar]
- 20.Wu MH, Hong TM, Cheng HW, et al. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res. 2009;7:311–8. doi: 10.1158/1541-7786.MCR-08-0297. [DOI] [PubMed] [Google Scholar]
- 21.Perillo NL, Pace KE, Seilhamer JJ, et al. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–9. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 22.Yang RY, Liu FT. Galectins in cell growth and apoptosis. Cell Mol Life Sci. 2003;60:267–76. doi: 10.1007/s000180300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–51. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 24.Thijssen VL, Postel R, Brandwijk RJ, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A. 2006;103:15975–80. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thijssen VL, Barkan B, Shoji H, et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70:6216–24. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 26.Le QT, Shi G, Cao H, et al. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932–41. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung CD, Patel VP, Moran M, et al. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J Immunol. 2000;165:3722–9. doi: 10.4049/jimmunol.165.7.3722. [DOI] [PubMed] [Google Scholar]
- 28.Camby I, Le Mercier M, Lefranc F, et al. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovich GA, Ramhorst RE, Rubinstein N, et al. Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ. 2002;9:661–70. doi: 10.1038/sj.cdd.4401009. [DOI] [PubMed] [Google Scholar]
- 30.Le Mercier M, Lefranc F, Mijatovic T, et al. Evidence of galectin-1 involvement in glioma chemoresistance. Toxicol Appl Pharmacol. 2008;229:172–83. doi: 10.1016/j.taap.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Mathieu V, Le Mercier M, De Neve N, et al. Galectin-1 knockdown increases sensitivity to temozolomide in a B16F10 mouse metastatic melanoma model. J Invest Dermatol. 2007;127:2399–410. doi: 10.1038/sj.jid.5700869. [DOI] [PubMed] [Google Scholar]
- 32.Kuo PL, Hung JY, Huang SK, et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. Journal of immunology. 2011;186:1521–30. doi: 10.4049/jimmunol.1002940. [DOI] [PubMed] [Google Scholar]
- 33.Giguere D, Bonin MA, Cloutier P, et al. Synthesis of stable and selective inhibitors of human galectins-1 and -3. Bioorg Med Chem. 2008;16:7811–23. doi: 10.1016/j.bmc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Siegel R, Roeder RG. Interaction of the B cell-specific transcriptional coactivator OCA-B and galectin-1 and a possible role in regulating BCR-mediated B cell proliferation. J Biol Chem. 2006;281:15505–16. doi: 10.1074/jbc.M509041200. [DOI] [PubMed] [Google Scholar]
- 35.Tsai CM, Chiu YK, Hsu TL, et al. Galectin-1 promotes immunoglobulin production during plasma cell differentiation. J Immunol. 2008;181:4570–9. doi: 10.4049/jimmunol.181.7.4570. [DOI] [PubMed] [Google Scholar]
- 36.Tabrizi SJ, Niiro H, Masui M, et al. T cell leukemia/lymphoma 1 and galectin-1 regulate survival/cell death pathways in human naive and IgM+ memory B cells through altering balances in Bcl-2 family proteins. J Immunol. 2009;182:1490–9. doi: 10.4049/jimmunol.182.3.1490. [DOI] [PubMed] [Google Scholar]
- 37.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–60. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–65. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 39.Melamed D, Miri E, Leider N, et al. Unexpected autoantibody production in membrane Ig-mu-deficient/lpr mice. J Immunol. 2000;165:4353–8. doi: 10.4049/jimmunol.165.8.4353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.