Abstract
HER2 overexpression occurs in ~25% of breast cancers where it correlates with poor prognosis. Likewise, systemic inflammation in breast cancer correlates with poor prognosis although the process is not understood. In this study, we explored the relationship between HER2 and inflammation, comparing the effects of overexpressing wild-type or mutated inactive forms of HER2 in primary human breast cells. Wild-type HER2 elicited a profound transcriptional inflammatory profile, including marked elevation of IL-6 expression, which we established to be a critical determinant of HER2 oncogenesis. Mechanistic investigations revealed that IL-6 secretion induced by HER2 overexpression activated Stat3 and altered gene expression, enforcing an autocrine loop of IL-6/Stat3 expression. Both mouse and human in vivo models of HER2 amplified breast carcinoma relied critically on this HER2-IL-6-Stat3 signaling pathway. Our studies offer the first direct evidence linking HER2 to a systemic inflammatory mechanism that orchestrates HER2-mediated tumor growth. We suggest that the HER2-IL6-STAT3 signaling axis we have defined in breast cancer could prompt new therapeutic or prevention strategies for treatment of HER2-amplified cancers.
Keywords: HER2, IL-6, Stat3, inflammation, cytokines, breast cancer
Introduction
Breast cancer is a heterogeneous disease classified into subtypes based on gene expression profiles or biomarker expression (1). A subtype overexpressing HER2, accounts for ~25% of breast cancers, and therapeutics targeting HER2, such as trastuzumab and lapatinib, have demonstrated clinical efficacy (2). However, as many tumors are resistant either de novo or following therapy, it remains critical to fully understand the molecular and cellular changes elicted by HER2 overexpression during oncogenesis (2–5).
HER2 overexpression has been shown to activate multiple signaling complexes (2–4), which result in a striking dysregulation of the global transcriptome (5). While these studies have provided a framework for HER2-mediated signaling, the pathways and gene targets critical to HER2 oncogenesis remain incompletely understood. Recent studies have demonstrated that inflammatory pathways and genes (such as IL-6 and IL-8) are strongly upregulated by several different oncogenes and are critical to their transformative capacity (6–9). Of note, rat ErbB2 (HER2 homolog) transgenic animals develop tumors with inflammatory patterns by gene expression profiling (10;11), which correspond to the proinflammatory pattern of gene expression found in human tumors. Furthermore, clinical studies have demonstrated the activation of inflammatory genes within breast cancer biopsies, while several circulating inflammatory cytokines have been found in the serum of breast cancer patients (12–14), with high levels IL-6 and IL-8 associated with a poor prognosis (12;13;15–18).
To investigate if HER2-mediated signaling could elicit inflammation critical for oncogenesis, we compared gene expression patterns of cells overexpressing wild-type HER2 to a kinase inactivated HER2 (19). We documented that HER2 overexpression consistently elicited an inflammatory transcriptional signature, including marked elevation of IL-6 expression, which was required for HER2-mediated transformation. HER2-mediated secretion of IL-6 triggered JAK1-Stat3 signaling in an autocrine manner, resulting in amplified IL-6 activation of Stat3 in HER2+ cells and significantly enhanced HER2-mediated transformation. These findings were confirmed in the MMTV-neu mouse model and a human HER2 amplified breast carcinoma. In sum, we demonstrate that HER2 overexpression initiates a HER2-IL-6-Stat3 signaling loop required for HER2-mediated oncogenesis, providing a possible molecular basis for the clinical and pathologic inflammatory markers seen in breast cancer patients. This suggests that IL-6 targeted therapies could have significant impact on HER2 overexpressing cancer prevention or therapies.
Materials and Methods
Cell lines
Tumor cell lines MCF-10a, MCF-7, 4T1, and 3T3 were obtained from the American Tissue Culture Collection (Manasas, VA). KPL-4 cells were obtained from the originator, Dr. Kurebayashi (Kawasaki Medical School, Kurashiki, Japan) (20). HMECs were obtained from Dr. Jeffrey Marks (Duke University, Durham, NC). The 4T1 and 4T1-HER2 cells were obtained from Dr. Michael Kershaw (Cancer Immunology Program, Peter MacCallum Cancer Centre, Victoria, Australia) and all lines were validated and tested for contamination by the Duke University Tissue Culture Facility (21).
Adenoviral vector, plasmid, and cell line construction
Adenoviral vectors encoding HER2 and HER2ki were generated as previously described (19). HER2+ cell lines were created through retroviral infection with HER2 expressing vectors. Stat3-Luc reporter cell lines were created using a lentiviral reporter (SABiosciences- Frederick, MD). The NF-kB luciferase reporter was purchased from Stratagene (La Jolla, CA), while AP1 and C/EBP reporters were purchased from SABiosciences. Knock-down of JAK1, IL-6, and Stat3 genes was achieved using retro and lentiviral RNAi constructs purchased from Open Biosystems (Huntsville, AL). A human Stat3 knock-down GFP expressing lentivirus was kindly provided by Dr. Jaqueline Bromberg (Memorial Sloan-Kettering, NYC, NY).
Microarray and quantative rt-PCR Assessments
RNA was extracted using TRI-Reagent and RNAzol (Molecular Reagents Center, Madison, WI) and purified using a RNeasy kit (QIAGEN). Microarray analysis was conducted with Genespring 7.3 and GX10 (Affymetrix, Santa Clara, CA) using datasets deposited at NCBI’s Gene Omnibus Express (GEO) (accession numbers GSE13274 and GSE2528). Datasets were analyzed using the Database for Annotation Visualization and Integrated Discovery (DAVID) using standard methods (22). MicroRNA arrays were processed from TRI-Reagent cellular extracts as previously described (23). Real-time PCR was performed on an ABI 7300 system using standard methods and intron spanning primers. Expression differences were assessed using the comparative cycle threshold (CT) method against several control genes (GAPDH, Beta-Actin, HMBS, RN18S, and Rpl13).
In vitro assays and assessments
Proliferation was determined by MTT assay while soft agar assays were performed as described (19). Propidium Iodide (PI) staining was conducted by fixing cells in 95% EtOH, staining with Propidium Iodide, and assessing DNA content by flow cytometry on a FACScalibur (BD, Franklin Lakes, NJ). Luciferase experiments were conducted by transfecting reporters or using stable reporter cell lines and normalizing luminescence with a LacZ controls using a β-Galactosidase kit (Stratagene) or Renilla transfected controls using a Dual-Luciferase Assay (Promega, Madison, WI). ELISAs for IL-6 were performed using IL-6 ELISA kits from Biolegend (San Diego, CA). Kinase inhibitors were purchased from Enzo Life Sciences (Plymouth Meeting, PA) and Marligen (Rockville, MD). Western blotting used standard methods with antibodies from Cell Signaling Technology (Danvers, MA) and Abcam (Cambridge, MA).
Mouse Experiments
Experiments using BALB/c, NOD CB17-Prkdc SCID/J and FVB/N-Tg(MMTVneu)202Mul/J (obtained from Jackson Labs, Bar Harbor, MA) were performed with Duke IACUC-approved protocols. For HER2 measurement of tumors, excised tumors were enzymatically digested as described below and measured using a HER2-PE labeled antibody (BD Biosciences, San Jose, CA). For xenograft experiments, cells were injected subcutaneously into the flank of NOD-SCID mice (at indicated concentrations) measured using calipers with volumes calculated by the formula (v=width*width*(length/2)). For live imaging experiments, mice were anesthetized using isoflurane, injected with 2.85 mg Luciferin (in 100ul of dH20), and monitored with a Xenogen IVIS 100 in vivo bioluminescence imaging system. Statistical differences were calculated using a mixed effects regression model using autoregressive covariance. Excised tumors were digested into single cell suspensions using a mix of Collagenase (1 mg/ml), DNAse (20U/ml), and Hyaluronidase (100 μg/ml) at 37°C for 3–5 hours, run through a cell strainer (80 μm, BD, Franklin Lakes, NJ) and cultured under standard conditions.
Results
Overexpression of HER2 elicits the activation of a broad inflammatory profile that includes IL-6
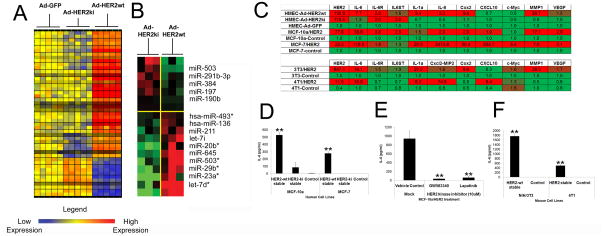
Although we have previously reported that global HER2-mediated gene expression changes were dependent on HER2 phosphorylation (19), we now report that a high proportion of these significantly affected genes (p<.05, >3 fold expression difference) are inflammatory-type genes (53 of 424 probesets, ~12.5%) (Fig 1A), that require HER2 overexpression and phosphorylation for their overexpression (Fig 1A). Concurrent examination of the microRNA profile also revealed significant differences in a cluster of miRNAs (Fig 1B), which included the expression of different let7 isoforms, recently demonstrated to impact IL-6 expression (Fig 1B) (7).
Figure 1. HER2 expression mediates an inflammatory response and expression of IL-6 across multiple cell types.
A) Microarray heat map of significantly dysregulated mRNA inflammatory genes (A) and miRNAs (B) (N=3–5, MOI=150, 16hpi). C) Quantitative real-time PCR analysis of inflammatory genes in Ad infected HMECs (as in A)) and HER2wt, HER2ki, and control stably infected cell lines (N=5, MOI=150, 16hpi). In all heat maps, red represents high expression and green represents low expression. D) IL-6 secretion from stably infected MCF-10a and MCF-7 cells (N=4). E) IL-6 secretion from HER2-stable MCF-10a cells treated with lapatinib, a lapatinib analog, or control vehicle (10 μM assessed at 24 hours post-treatment, N=4). E) IL-6 secretion from stably infected NIH/3T3 and 4T1 cells (N=4). For C–E, N=5, bars indicate SD, while * indicates a p<.05 from controls, and ** indicates a p<.01 from controls.
We also investigated HER2 induced inflammatory gene expression in immortalized (MCF-10a) and transformed (MCF-7) human breast cells. We found that HER2 overexpression significantly induced the expression of specific inflammatory genes across different types of human breast cells (Fig 1C) as well as murine fibroblasts (NIH/3T3) and transformed murine mammary tumor cells (4T1) (Fig 1C). As in human cells, expression of HER2 elicited significant activation of inflammatory gene expression, indicating that HER2 induction of inflammatory gene transcription is independent of species and cell type.
We next examined the impact of HER2 expression on IL-6 protein expression and secretion, demonstrating that supernatants from HER2 overexpressing human mammary cells contained high levels of IL-6 (Fig 1D), while treatment with small molecule HER2 inhibitors ablated IL-6 (Fig 1E). HER2 overexpression in NIH/3T3 and murine 4T1 cells induced similar elevations of IL-6 (Fig 1F).
HER2-mediated upregulation of IL-6 is dependent upon the parallel activation of multiple signaling pathways which activate several IL-6 transcription factors
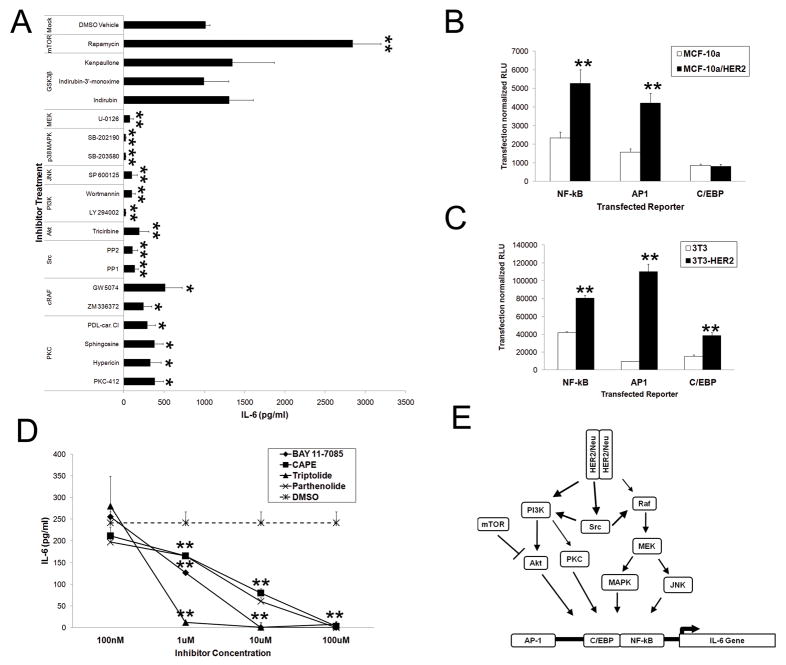
To identify HER2-IL-6 responsive pathways, we focused on known downstream kinases and transcription factors. We exposed HER2 expressing MCF-10a cells (MCF-10a-HER2) to a variety of specific kinase inhibitors and assessed IL-6 secretion, finding that specific inhibition of MAPK, JNK, PI3K, Akt and Src reduce secretion of IL-6 (Fig 2A). Although PKC inhibition reduced IL-6 secretion, inhibition of the mTOR pathway actually enhanced the HER2-mediated secretion of IL-6. Inhibition of other kinases, such as GSK3B, had no effect on the level of HER2-mediated IL-6 secretion (Fig 2A, data not shown). To investigate IL-6 transcriptional regulation, we utilized luciferase reporters for the dominant transcription factors present in the IL-6 promoter complex (NF-kB, AP-1, C/EBP). In MCF-10a cells, we found that while HER2 strongly induced NF-kB and AP-1 reporters, it had no effect on C/EBP expression (Fig 2B). However in 3T3 cells, HER2 expression induced the three dominant transcription factors (NF-kB, AP-1, and C/EBP), suggesting that HER2 induction of NF-kB and AP-1 is cell-type independent, but that C/EBP induction may be cell-type dependent (Fig 2C). As NF-kB was strongly induced in both cell types, we directly assessed the importance of NF-kB in HER2-mediated IL-6 secretion through pharmacologic disruption of NF-kB signaling in MCF-10a-HER2 cells and found a dose-dependent inhibition of IL-6 secretion (Fig 2D). Collectively, these results demonstrate that HER2 overexpression activates multiple pathways which synergistically result in the secretion of IL-6 through the activation of multiple transcription factors (Fig 2E).
Figure 2. Transcriptional regulation of HER2 mediated IL-6 expression.
A) Kinase inhibition (10 μM) of IL-6 secretion in MCF-10a-HER2 cells (N=3, 24 hours post-treatment, bars represent SD). B and C) NF-kB, AP-1, and C/EBP activity in MCF-10a and MCF-10a-HER2 cells (B) or NIH/3T3 or NIH/3T3-HER2 cells (C). In all samples, luciferase expression measured at 24 hours post-transfection (hpt), N=4, bars represent SD. D) NF-kB inhibition of IL-6 secretion in MCF-10a-HER2 cells (N=4, 24hpt, bars represent SD). Where indicated * and ** represent p<.05 and p<.01 respectively in comparison to controls. E) Schematic diagram of the signaling pathways regulating HER2-induced transcriptional upregulation of IL-6.
Secretion of IL-6 is required for HER2-mediated transformation and tumor growth in vivo
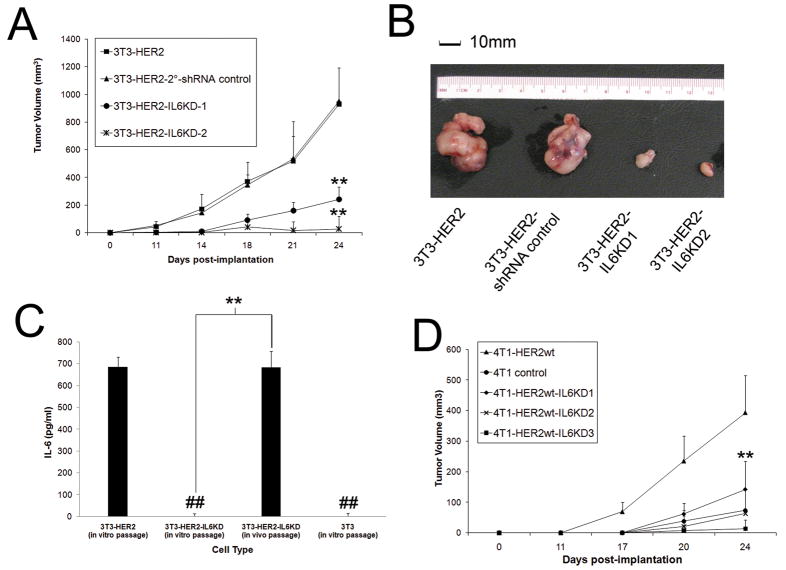
To determine if IL-6 secretion was required for HER2-mediated transformation, we inhibited IL-6 expression in 3T3-HER2 transformed cells by stable IL6KD (Fig S1) and assessed in vivo growth in NOD-SCID mice. IL-6 inhibition significantly attenuated in vivo tumor growth (Fig 3A-B) and 3T3-HER2-IL6KD tumors that eventually developed had re-acquired baseline IL-6 expression (compared to control 3T3-HER2 cells) (Fig 3C). In addition to the significant role IL-6 has in HER2-mediated transformation, we also investigated its role in the behavior of transformed mammary cells. In transformed 4T1 mammary carcinoma cells, we found that overexpression of HER2 (4T1-HER2) yielded a significant in vivo growth advantage compared to non-HER2 expressing 4T1 cells (Fig 3D, data not shown), which could be inhibited by blocking IL-6 expression (Fig S1), suggesting that IL-6 also plays a key role in HER2-facilitated growth in transformed cells.
Figure 3. IL-6 is required for HER2-mediated tumor growth in vivo.
A) 3T3-HER2 cells were modified with control and IL-6 KD lentiviruses and implanted (1×105 cells) via subcutaneous injection into SCID mice and tumor volume measured over time (N=5, bars represent SE). B) Visual representation of IL-6KD growth attenuation at day 24 of representative resected tumors. C) IL-6 secretion in tumor cells from resected tumors in comparison to in vitro passaged counterparts (N=5, bars indicate SD). D) 4T1-HER2 and 4T1 cells were modified with control and IL-6 KD lentiviruses and implanted (1×105 cells) via subcutaneous injection into SCID mice and tumor volume measured over time (N=5, bars represent SE). Where indicated * and ** represent p<.05 and p<.01 respectively in comparison to controls.
HER2 induced secretion of IL-6 can act in an autocrine fashion to elicit Stat3-mediated gene expression and signaling
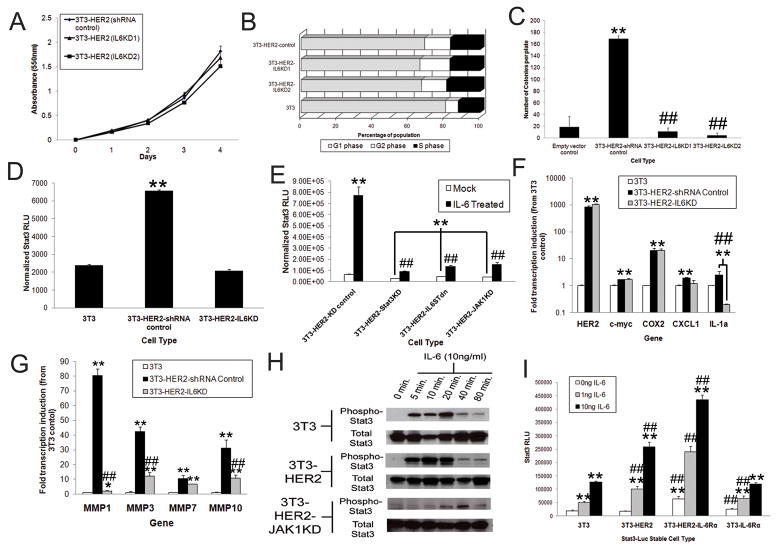
We next determined if IL-6 had autocrine effects on HER2-transformed cells in vitro. In vitro assessment of cellular proliferation revealed no difference in growth or cell cycle changes between control and IL-6KD 3T3-HER2 cells (Fig 4A), nor were any differences detected between these cell types in cell cycle regulation (Fig 4B). However, studies of anchorage-independent growth revealed significant growth attenuation by inhibition of IL-6 expression, thus signifying the importance of autocrine IL-6 signaling (Fig 4C). We thus focused on Stat3, the dominant transcription factor induced by IL-6. Using a lentiviral Stat3 luciferase reporter, we found that HER2 expression significantly induced the activation of Stat3 compared to control 3T3 cells and furthermore, that inhibition of IL-6 expression ablated Stat3 induction (Fig 4D). These results were specific for IL-6 induction of Stat3, as tandem investigations using transient transfection revealed that HER2-mediated activation of Stat3, but not Stat1, was dependent upon IL-6 secretion (Fig S2). To further elucidate and confirm that IL-6 activation of Stat3 was mediated by an IL-6-IL6R-IL6ST signaling complex via JAK kinases, we stably expressed a mutant IL6ST receptor and inhibited JAK1 expression in 3T3-HER2 Stat3-luciferase cells (Fig S3). In the absence of exogenous IL-6 stimulation, inhibition of IL6ST, JAK1, or Stat3 in 3T3-HER2 cells, all significantly inhibited Stat3 activation (Fig 4E), as previously demonstrated by the inhibition of IL-6 expression itself (Fig 4D). Notably, in the presence of exogenous IL-6 stimulation, we also found that that inhibition of these signaling nodes critically inhibited Stat3 induction (Fig 4E).
Figure 4. HER2 elicited IL-6 is critical for anchorage independent growth and autocrine-mediated Stat3 activation in vitro.
A) 3T3-HER2 were stably infected and growth monitored by MTT assay (N=6, bars represent SD). B) 3T3 and modified 3T3 cells were plated at equal densities and cell cycle phases assessed by Propidium Iodide (PI) at 24 hours post-plating. The average distribution of populations (~5×105 cells) is represented. C) Indicated 3T3 cells were plated in 0.3% agar at a concentration of 5×104 cells/ml and colony growth assessed at 3 weeks (N=3, bars indicate SD). D) 3T3-Stat3-Luc stable cells were modified to express HER2 and stably infected with IL-6 knock-down or control vectors. Equal populations of cells were then plated and Stat3 activation assessed by luciferase assay (N=6, bars represent SD). E) 3T3-Stat3-Luc-HER2 cells were modified to express a cytoplasmic domain truncated IL6ST (IL6STdn) or knocked-down for JAK1 or Stat3 expression, treated with IL-6 (10ng/ml) and luciferase activity assessed at 24 hours post-treatment (N=6, bars indicate SD, ## indicates a p<.01 in comparison to IL-6 treated controls). F and G) 3T3, 3T3-HER2, and 3T3-HER2-IL6KD cells were plated at equal densities and expression assessed by quantitative real-time PCR of inflammatory (F) and MMP genes (G) (N=4, bars indicate standard deviation, * and ** represent p<.05 and p<.01 from 3T3 controls while # and ## represent p<.05 and p<.01 between 3T3-HER2-IL6KD and 3T3-HER2 controls). H) 3T3, 3T3-HER2, and 3T3-HER2-JAK1KD cells were treated with IL-6 (10ng/ml) and Stat3 phosphorylation (pY705) analyzed at indicated time points. I) 3T3-Stat3-Luc, 3T3-Stat3-Luc-HER2, and 3T3-Stat3-Luc-HER2-IL6Rα, and 3T3-Stat3-Luc-IL6Rα modified cells were treated with IL-6 and Stat3 activation assessed by luciferase assay at 24 hours post-treatment (N=6, bars indicate SD, * and ** represent p<.05 and p<.01 respectively in comparison to untreated controls, and # and ## represent p<.05 and p<.01 from 3T3 control cells). Where indicated in experiments, * and ** represent p<.05 and p<.01 respectively in comparison to controls.
We next assessed the role of IL-6 on the expression of other inflammatory genes in 3T3, 3T3-HER2 and 3T3-HER2-IL6KD cells by quantitative RT-PCR and found that IL-6 inhibition did not affect certain genes such as c-myc and COX2, but found the expression of other genes weres significantly attenuated (Fig 4F). In particular, we had noted that MMP1 was significantly enhanced by IL-6 secretion, so we examined several other MMP genes known to play a role in oncogenesis (24) (Fig 4G). We found multiple MMP genes were significantly affected by inhibition of IL-6 secretion, thus demonstrating that HER2-mediated IL-6 secretion elicits autocrine activation of Stat3, perturbing cellular gene expression.
As previous studies have illustrated IL6ST-HER2 interactions in different cell types, we also sought to determine if HER2 expression could enhance autocrine IL-6 mediated signaling (3;25). Treatment of 3T3 and 3T3-HER2 cells revealed a nearly identical time course of activation, but at early time points, Stat3 appeared more phosphorylated in HER2 expressing cells in comparison to controls (Fig 4H). Identical IL-6 treatment of 3T3-HER2-JAK1KD cells confirmed that the enhanced Stat3 activation was being achieved through a JAK1-dependent pathway in 3T3-HER2 cells and not by alternative mechanisms (Fig 4H). To quantify Stat3 induction, we stably infected cells with Stat3-Luciferase reporters, selected 3T3 and 3T3-HER2 cells that had equivalent basal activation of Stat3 (Fig 4I) and found that IL-6 treatment activated Stat3 signaling to a significantly greater extent in HER2 expressing cells. As 3T3 cells had minimal expression of IL-6Rα (By FACS, data not shown), we hypothesized that HER2 amplification of IL-6-Stat3 signaling could be potentially abrogated by greater IL-6Rα expression. To test this hypothesis, we overexpressed IL-6Rα in both 3T3 and 3T3-HER2 Stat3-Luciferase cells and found that Stat3 activation was again enhanced in HER2 expressing counterparts (Fig 4I). Furthermore, we found that while IL-6Rα expression increased baseline Stat3 signaling in both cell types, it had a significantly greater effect in cells expressing HER2 upon IL-6 addition. These results suggest that, in addition to stimulating IL-6 secretion, HER2 expression enhances the activation of Stat3 signaling by IL-6. Collectively, these data demonstrate that HER2 expression plays a critical dual role in the activation of an autocrine IL-6-Stat3 signaling complex.
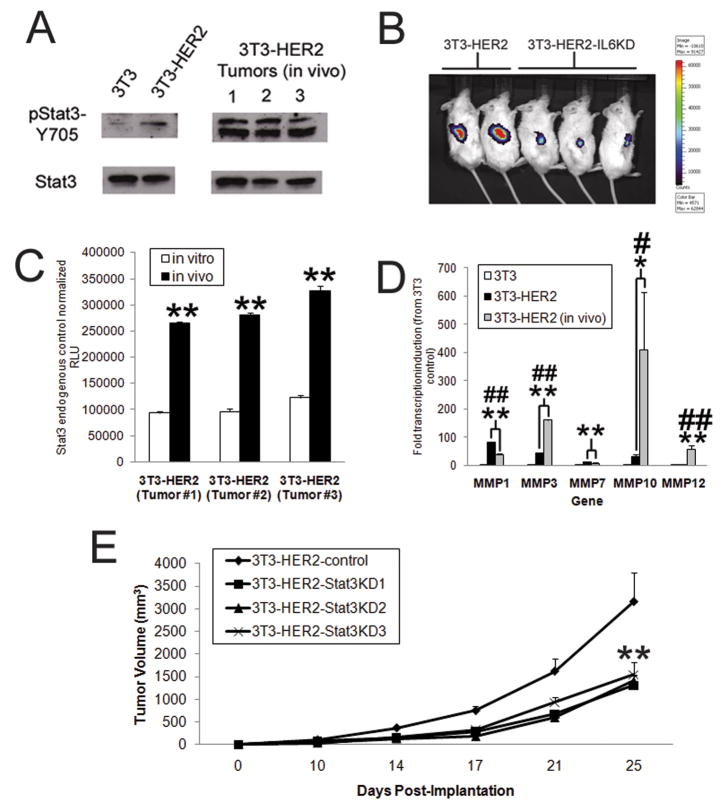
HER2- IL-6 activation of Stat3 significantly enhances tumor growth in vivo
To investigate the importance of HER2-IL-6-Stat3 signaling in vivo, we first assessed the level of phosphorylated Stat3 in extracted tumor tissue. We found that while 3T3-HER2 cells displayed modest phosphorylation of Y705 in vitro, in vivo samples from 3T3-HER2 tumor displayed much higher levels of phosphorylated Stat3 (Fig 5A). Notably, they also displayed a different pattern of activation, in multiple forms of phosphorylated Stat3 as well as different isoforms of unphosphorylated Stat3 (data not shown). Having observed significantly enhanced Stat3 phosphorylation in whole tumors in vivo, we next wanted to determine if IL-6 could mediate autocrine activation of Stat3 specifically within 3T3-HER2 tumor cells in vivo. We constructed 3T3-HER2 and 3T3-HER2-IL6KD cell lines with either a Stat3-Luc reporter or LacZ control reporter. When these cells were implanted in mice, striking differences were noted in the level of Stat3 activation at 14 days post-implantation using Xenogen imaging (Fig 5B, S4), consistent with difference seen in vitro. When tumors were excised and Stat3-mediated luciferase activation compared with identically in vitro passaged cells (all normalized with LacZ), we found that Stat3 was significantly more active in 3T3-HER2 cells under in vivo conditions compared to those same cells under in vitro conditions (Fig 5C). As these results suggested a more significant activation of Stat3 in vivo, we next assessed the expression of IL-6 affected MMP genes in vitro and in vivo. While certain genes (MMP1 and MMP7) were not strongly affected by the greater level of Stat3 activation in vivo, we did find that the expression of other MMP genes (MMP3, 10, 12) was significantly enhanced in vivo (Fig 5D). We then inhibited Stat3 expression in 3T3-HER2 cells (Fig S5) and compared in vivo growth with control 3T3-HER2 cells (Fig 5E). We found that Stat3 inhibition did significantly retard tumor growth, although not to the extent observed when IL-6 secretion was inhibited.
Figure 5. IL-6 activation of Stat3 is critical for HER2-mediated growth in vivo.
A) Total cell extracts from 3T3 and 3T3-HER2 (from in vitro cultures or xenografts biopsies) were subjected to western analysis to determine total and phosphorylated Stat3 expression (pY705). B) 3T3-Stat3-Luc-HER2 and 3T3-Stat3-Luc-HER2-IL6KD cells (1×105) were implanted in mice and Stat3 activation assessed by xenogen luciferase imaging at 11 days post-implantation (N=5, representative mice shown). C) Whole cell lysates from 3T3-Stat3-Luc-HER2-LacZ cells which were implanted in mice (sacrificed at 24dpi) or passaged (for corresponding 24 days) were assed for Stat3 activation, normalized to LacZ expression (samples from individual replicates shown, bars indicate SD). D) Quantitative rt-PCR from 3T3, 3T3-HER2, and 3T3-HER2 xenografts (sacrificed at 25dpi) to determine MMP gene expression (N=4, bars indicate SD, # and ## represent p<.05 and p<.01 between in vitro and in vivo conditions). E) The indicated types of Stat3KD and control 3T3-HER2 cells (1×105) were implanted via subcutaneous injection into SCID mice and tumor volume measured over time (N=5, bars represent SE). Where indicated * and ** represent p<.05 and p<.01 respectively in comparison to controls.
ErbB2 induction of IL-6 plays a critical role in an endogenous model of ErbB2-mediated oncogenesis
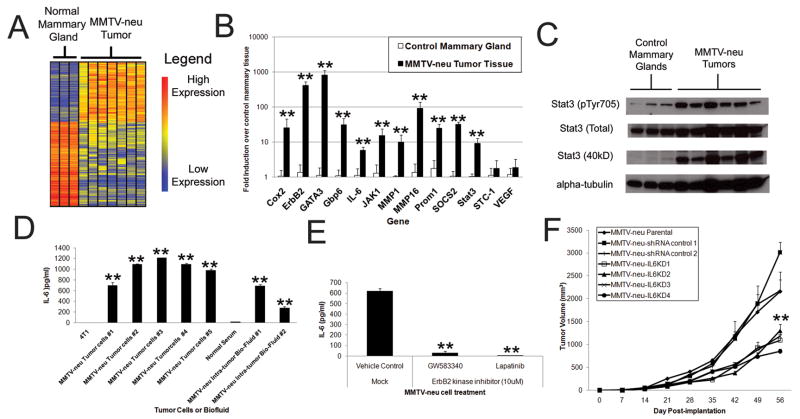
The MMTV-neu mouse model spontaneously develops mammary carcinomas dependent upon expression of activated ErbB2 (the rat homolog of HER2). Using published microarray datasets of developing MMTV-neu tumors(26), we found that a significant portion of genes were dysregulated in ErbB2+ tumors in comparison to control mammary gland tissue (Fig 6A, ~5% 309 probes with p<.05, >3 fold), of which ~10% (31 of 309 probesets) had immune-related functions. Quantitative rt-PCR analysis confirmed these findings (Fig 6B), revealing strong induction of several relevant inflammatory mediators including IL-6, Stat3, and SOCS2. Western blots of control and transformed MMTV-neu mammary tissue revealed tumor Stat3 activation, further confirming this IL-6 inflammatory phenotype (Fig 6C). Although interferon and inflammatory signatures have been reported in MMTV-neu tumors (10;11), we focused on IL-6 expression in tumor cells and biofluid from multiple MMTV-neu tumors and compared these to a transformed non-ErbB2 expressing murine breast cancer (4T1) (Fig 6D). MMTV-neu tumor cells secreted high levels of IL-6 and peri-tumoral fluid contained significant amounts of IL-6 (Fig 6D). Exposure of MMTV-neu tumor cells to ErbB2 inhibitors ablated IL-6 secretion (Fig 6E), and IL6KD MMTV-neu tumor cells were significantly growth attenuated compared to control infected or uninfected MMTV-neu cells (Fig 6F). Our findings thus demonstrate that endogenous ErbB2 expression supports an inflammatory phenotype, typified by IL-6 secretion, which plays an important role in MMTV-neu mammary tumor growth in vivo.
Figure 6. ErbB2-mediated induction of IL-6 plays a critical role in an endogenous ErbB2-mediated model of breast cancer.
A) Heatmap depiction of significantly dysregulated genes (1-way ANOVA with p<.05, >3 fold difference) in MMTV-neu tumors. B) Quantitative rt-PCR from MMTV-neu tumors and control mammary glands (N=5, bars indicate SD). C) Western blot analysis of lysates of MMTV-neu tumors and control mammary glands. D) Tumor cells from MMTV-neu tumors were isolated and cultured assess IL-6 secretion in comparison to a non-ErbB2 transformed mammary carcinoma cell line (at 24 hours post-plating). Fluid samples from tumors as well as control mice serum were also tested for IL-6 concentration by ELISA. In all samples N=3, bars represent SD. E) Tumor cells from MMTV-neu mice were passaged for 3 months, mock or ErbB2 kinase inhibitor treated, and IL-6 secretion assessed at 24 hours post-treatment (N=3, bars indicate SD). F) MMTV-neu tumor cells were modified by IL6KD or control lentiviral infection and implanted (1×106 cells) via subcutaneous injection into SCID mice and tumor volume measured over time (N=5, bars represent SE). Where indicated * and ** represent p<.05 and p<.01 respectively in comparison to controls.
ErbB2-mediated IL-6 expression in human tumor cells causes Stat3 activation and facilitates oncogenic growth
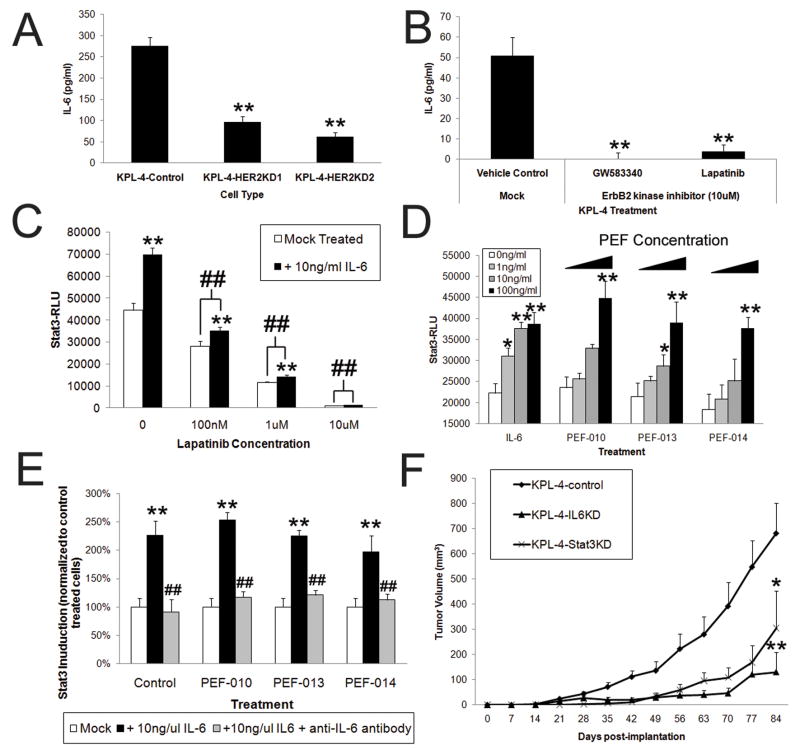
To ascertain the relationship between spontaneously amplified ErbB2 and IL-6 secretion in human cells, we utilized the human KPL-4 breast cancer line, which overexpresses HER2 and secretes IL-6. When HER2 was stably knocked-down, we found a significant, but not complete reduction of IL-6 secretion (Fig 7A and S7). As the high endogenous HER2 expression in KPL-4 cells was not completely knocked-down by shRNA (Fig S7), we next used pharmacologic inhibition of HER2 (Fig 7B), which resulted in a near complete ablation of IL-6 expression, demonstrating the importance of HER2 signaling in promoting IL-6 secretion in HER2 expressing tumor cells.
Figure 7. HER2 mediated secretion of IL-6 in human mammary carcinoma cells is critical for Stat3 activation and in vivo tumor growth.
A) KPL-4 cells (endogenously HER2+) were knocked-down for HER2 expression and IL-6 secretion assessed (N=6, bars represent SD). B) KPL-4 were treated with HER2 inhibitors (10 μM) or DMSO and IL-6 secretion assessed at 24 hours (N=4, bars represent SD). C) KPL-4-Stat3-Luc stable cells that were exposed to varying concentrations of IL-6 in the presence or absence of 10 μM Lapatinib for 24 hours and Stat3 activation assessed (N=6, bars represent SD, # and ## represent p<.05 and p<.01 from DMSO control treated KPL-4 and KPL-4+IL-6 counterparts). D) KPL-4-Stat3-Luc stable cells were exposed to varying concentration of IL-6 (as indicated) and Pleural effusion fluid (consisting of increasing concentrations of 0, 0.25%, 2.5%, and 25% of total media volume indicated by increasing bars) for 24 hours, after which Stat3 activity was quantified by luciferase assay (N=6, bars represent SD). D) KPL-4-Stat3-Luc cells were treated with 10ng/ml of IL-6 and PEF (at 10ng/ml or maximum IL-6 equivalent concentration, or 25%, 58%, or 100%), along with either mock treatment with 1 ng/ml of an anti-IL-6 neutralizing antibody or 1 μg/ml of a control IgG antibody. After a 24 hour incubation, Stat3 activity was quantified by luciferase assasy (N=6, bars represent SD, # and ## represent p<.05 and p<.01 from IL-6+IgG treated counterparts). F) The indicated types of modified KPL-4 cells (5×105) were implanted via subcutaneous injection into SCID mice and tumor volume measured over time (N=5, bars represent SE). Where indicated * and ** represent p<.05 and p<.01 respectively in comparison to untreated controls.
KPL-4 cells were then stably infected with Stat3-Lucifierase reporters and then treated with IL-6 in tandem with HER2 kinase inhibitors to assess Stat3 activation (Fig 7C). These studies revealed that HER2 inhibited cells had lower basal levels of Stat3 activation, correlating with their lower levels of IL-6 secretion (Fig 7C). More significantly, we found that high concentrations of IL-6 were not able to activate Stat3 in HER2 inhibited cells, suggesting that HER2 plays a prominent role in the IL-6 mediated activation of Stat3. These studies used levels of IL-6 (10 ng/ml) that approximated levels we found in pleural effusions from breast cancer patients (Fig S8). When KPL-4-Stat3-Luc cells were directly exposed to malignant pleural effusions, we again observed significant activation of Stat3 (Fig 7D), which was inhibited by addition of neutralizing IL-6 antibody (Fig 7E).
Finally, to determine if HER2-mediated expression of IL-6 was critical for the growth of human HER2+ breast carcinomas in vivo, both IL-6 and Stat3 were stably knocked down in KPL-4 cells which were then implanted in mice (Fig 7E, S9) and assessed for tumor growth. While the growth of Stat3KD cells was significantly inhibited, IL6KD cells displayed the most dramatically inhibited tumor growth, again suggesting that both autocrine and paracrine modes of IL-6 signaling likely play important roles in human tumor growth.
Discussion
Although oncogenes such as Ras, src, myc, and EGFR are known to trigger inflammatory pathways critical for oncogenesis (6–9;27), the relationship between HER2/neu and inflammation had previously been speculative (28). In the present study, we document that HER2 overexpression activates multiple inflammatory pathways, including the secretion of IL-6, which we identify as critical for HER2-mediated transformation. We found several pathways downstream of HER2 synergistically affected IL-6 expression and demonstrate that secreted IL-6 elicited autocrine Stat3 activation. We also found that Stat3 activation was enhanced in HER2 expressing cells and associated with cellular transcriptional changes, as well as anchorage-independent growth. Studies with endogenously arising ErbB2 tumors also revealed ErbB2-IL-6-Stat3 activation enhances tumor growth, signifying that these phenomena were not limited to a cellular model of HER2-mediated transformation. Likewise, investigation of a human breast carcinoma line with amplified HER2 also demonstrated that HER2-mediated IL-6 expression was critical for autocrine Stat3 activation, signaling amplification, as well as human tumor growth in vivo. In sum, these experiments reveal that HER2 activation and amplification of autocrine IL-6-Stat3 signaling are critical to its oncogenic capacity.
We found that inflammatory-related genes encompass ~10% of the most significant transcriptional changes induced by the overexpression of HER2 and that this inflammatory transcriptional response occurs in various cell types at different stages of transformation. The inflammatory effect on cellular properties is likely dependent upon cellular context as oncogene induced inflammatory pathways (such as IL-6) can lead to autocrine induced cellular senescence in non-immortalized cells (29), whereas inflammatory genes can enhance cellular oncogenicity in tumor cells (9;30–33). Additionally, inflammatory responses can influence other cells (such as fibroblasts, adipocytes, or immune cells) and modulate tumor-mediated immunity.
Our study is the first to demonstrate that overexpression of kinase active, but not inactive, HER2 induces IL-6 secretion and is thus dependent upon HER2 phsophorylation and preservation of multiple signaling pathways downstream of HER2. HER2 activation correlated with NF-kB and AP1 activation and NF-kB was critical to IL-6 expression. These findings are similar to those observed in the RAS mediated activation of IL-8 (9), which we also found to be induced by HER2, suggesting that oncogene-mediated cytokine gene expression is dependent on multiple coordinated signaling pathways. While this does not exclude the influence of other factors in the activation of IL-6 (such as let-7 involvement) (7), it demonstrates that interference with many signaling nodes downstream of HER2 can perturb IL-6 expression and thus implies the possibility of therapeutic intervention against HER2-mediated IL-6 secretion at multiple levels.
Our investigation also revealed that IL-6 secreted in response to HER2 expression was critical for HER2-mediated transformation and activation of Stat3 in vitro and in vivo, a finding corroborated by other studies that demonstrate IL-6 mediation of transformative properties in mammary epithelial and tumor cells (7;33). Collectively, these findings suggest that HER2-IL6-Stat3 activation is critical component of HER2-mediated oncogenesis, although a full evaluation of Stat3-mediated effects may vary based upon cell type. Notably, we found that HER2 plays an additional role in the IL-6-Stat3 signaling axis, through the amplification of Stat3 signaling after IL-6 treatment. While the exact nature of this role is unknown, previous studies have documented the involvement of HER2 with the IL6ST receptor (3;25), suggesting that HER2 expression on the cell surface could be an important part of the IL-6/IL6ST/IL-6Rα complex. As such, HER2 could play a critical dual role in this pathway acting as an initiator and amplifier of cellular IL-6 signaling. However, it should also be noted that in multiple contexts, our knockdown of Stat3 did not fully recapitulate the suppression of tumor growth achieved with IL-6 knock-down.
We found Stat3 was more highly activated in tumor cells in vivo in comparison to identical cells in vitro, consistent with the high levels of activated Stat3 reported in different types of tumor biopsies (34;35). While we found that tumor cell Stat3 activation was directly associated with tumor cell IL-6 expression in vitro and in vivo, stronger Stat3 activation in vivo could be a product of infiltrating cells as well as environmental stimuli that would provide additional sources or stimulations to permit Stat3 activation. For instance, the presence of high levels of soluble IL-6Rα in vivo (36) could permit IL-6 trans-signaling in tumor cells, as IL-6Rα could be a limiting activating factor in certain cell types. While it is unclear if pharmacologic HER2 inhibition could alleviate Stat3 activation in vivo, our data suggest that such an approach may provide Stat3 suppression through inactivation of HER2-mediated IL-6 secretion, as well as abrogation of HER2-mediated enhancement of IL-6-Stat3 signaling. In sum, our finding of enhanced Stat3 signaling in HER2+ tumor cells in vivo supports the importance of Stat3 activation in tumor cell populations in clinical settings.
Finally, while multiple studies have demonstrated IL-6 expression in breast cancer patients and linked expression with certain subsets and grades of malignancy (12;14;37;38), the source and mechanisms generating IL-6 in cancer patients has been undetermined. Likewise, other studies have determined that many breast cancers have activated Stat3, although the activators and significance of Stat3 in these tumors remains unknown (34;39). Our study demonstrates that HER2 overexpression activates a transcriptional inflammatory profile, which includes the significant secretion of IL-6 in multiple cell types, as well as in a mouse model of ErbB2 overexpression and in a human HER2+ breast carcinoma line. We further found that secreted IL-6 was critical for HER2-mediated oncogenesis and was mediated by autocrine activation of Stat3 in tumor cell populations, which was enhanced by cellular HER2 expression and in in vivo contexts. Thus, our findings demonstrate a potential origin and mechanism for IL-6 expression and its relevance to breast cancer progression. While further study of HER2-mediated inflammation is needed, these findings suggest that therapeutic targeting IL-6-Stat3 activation could augment existing prevention strategies and treatments of HER2+ cancers.
Supplementary Material
Acknowledgments
Supported by grants from the National Cancer Institute (NCI P50 CA89496-01 (H.K.L.), NCI R01 CA95447 (T.M.C.), Department of Defense Breast Cancer Research Program Clinical Translational Research Award (BC050221) (T.M.C.), and a Susan G. Komen Foundation Postdoctoral Fellowship Award (KG080627) (Z.C.H.).
Abbreviation footnote
- Ad
adenovirus
- ANOVA
Analysis of Variance
- FDR
Benjamini-Hochberg False Discovery Rate
- dpi
days post-infection
- ELISA
Enzyme-linked Immunosorbance Assay
- GO
Gene Ontology
- moi
multiplicity of infection
- wpi
weeks post-infection
Footnotes
The work was performed at: Duke University Medical Center, Durham, NC 27710.
Reference List
- 1.Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117(11):3155–63. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6(3):251–61. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Grant SL, Hammacher A, Douglas AM, Goss GA, Mansfield RK, Heath JK, et al. An unexpected biochemical and functional interaction between gp130 and the EGF receptor family in breast cancer cells. Oncogene. 2002;21(3):460–74. doi: 10.1038/sj.onc.1205100. [DOI] [PubMed] [Google Scholar]
- 4.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126(3):489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Staaf J, Ringner M, Vallon-Christersson J, Jonsson G, Bendahl PO, Holm K, et al. Identification of subtypes in human epidermal growth factor receptor 2--positive breast cancer reveals a gene signature prognostic of outcome. J Clin Oncol. 2010;28(11):1813–20. doi: 10.1200/JCO.2009.22.8775. [DOI] [PubMed] [Google Scholar]
- 6.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis 3. Genes Dev. 2007;21(14):1714–9. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117(12):3846–56. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Speers C, Tsimelzon A, Sexton K, Herrick AM, Gutierrez C, Culhane A, et al. Identification of novel kinase targets for the treatment of estrogen receptor-negative breast cancer. Clin Cancer Res. 2009;15(20):6327–40. doi: 10.1158/1078-0432.CCR-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8(5):R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103(5):642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 13.Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17(5):325–37. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–4. [PubMed] [Google Scholar]
- 15.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19(2B):1427–32. [PubMed] [Google Scholar]
- 16.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88(11):1721–6. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozcuk H, Uslu G, Samur M, Yildiz M, Ozben T, Ozdogan M, et al. Tumour necrosis factor-alpha, interleukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine. 2004;27(2–3):58–65. doi: 10.1016/j.cyto.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10(21):7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 19.Hartman ZC, Wei J, Osada T, Glass O, Lei G, Yang XY, et al. An adenoviral vaccine encoding full-length inactivated human Her2 exhibits potent immunogenicty and enhanced therapeutic efficacy without oncogenicity. Clin Cancer Res. 2010;16(5):1466–77. doi: 10.1158/1078-0432.CCR-09-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K, et al. Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Br J Cancer. 1999;79(5–6):707–17. doi: 10.1038/sj.bjc.6690114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kershaw MH, Jackson JT, Haynes NM, Teng MW, Moeller M, Hayakawa Y, et al. Gene-engineered T cells as a superior adjuvant therapy for metastatic cancer 1. J Immunol. 2004;173(3):2143–50. doi: 10.4049/jimmunol.173.3.2143. [DOI] [PubMed] [Google Scholar]
- 22.Huang dW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35(Web Server issue):W169–W175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Jima DD, Jacobs C, Fischer R, Gottwein E, Huang G, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood. 2009;113(19):4586–94. doi: 10.1182/blood-2008-09-178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu Y, Ravi L, Kung HJ. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393(6680):83–5. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 26.Landis MD, Seachrist DD, Montanez-Wiscovich ME, Danielpour D, Keri RA. Gene expression profiling of cancer progression reveals intrinsic regulation of transforming growth factor-beta signaling in ErbB2/Neu-induced tumors from transgenic mice. Oncogene. 2005;24(33):5173–90. doi: 10.1038/sj.onc.1208712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13(10):1211–8. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 28.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13(1):7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20(1):65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 32.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res. 2006;12(1):20–8. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25(5):241–53. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 37.Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002;2(4):311–5. doi: 10.3816/cbc.2002.n.008. [DOI] [PubMed] [Google Scholar]
- 38.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102(2):129–35. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 39.Berishaj M, Gao SP, Ahmed S, Leslie K, Al Ahmadie H, Gerald WL, et al. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9(3):R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.